Abstract
Ultrasound (US) of the thyroid has been used as a diagnostic tool since the late 1960s. US is the most important imaging tool for diagnosing thyroid disease. In the majority of cases a correct diagnosis can already be made in synopsis of the sonographic together with clinical findings and basal thyroid hormone parameters. However, the characterization of thyroid nodules by US remains challenging. The introduction of Thyroid Imaging Reporting and Data Systems (TIRADSs) has improved diagnostic accuracy of thyroid cancer significantly. Newer techniques such as elastography, superb microvascular imaging (SMI), contrast enhanced ultrasound (CEUS) and multiparametric ultrasound (MPUS) expand diagnostic options and tools further. In addition, the use of artificial intelligence (AI) is a promising tool to improve and simplify diagnostics of thyroid nodules and there is evidence that AI can exceed the performance of humans. Combining different US techniques with the introduction of new software, the use of AI, FNB as well as molecular markers might pave the way for a completely new area of diagnostic accuracy in thyroid disease. Finally, interventional ultrasound using US-guided thermal ablation (TA) procedures are increasingly proposed as therapy options for benign as well as malignant thyroid diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction and technical aspects
Ultrasound (US) of the thyroid has been used as a diagnostic tool since the late 1960s [1] and is nowadays well established for a variety of indications and applications. US is the most sensitive imaging test available for the examination of the thyroid gland and the estimation of its size, to detect and characterize focal lesions [2], accurately calculate their dimensions, identify the internal structure and vascularization using color Doppler imaging (CFD) [3] and evaluate diffuse changes in the thyroid parenchyma [4]. Thyroid US is able to confirm the presence of a thyroid nodule as well as other lesions or masses and is superior compared to the traditional physical examination using palpation [5]. In addition, US can be used for interventional procedures of the thyroid [6].
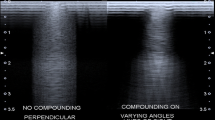
Over the last decades, ultrasound techniques have improved greatly: starting with low-resolution B-Mode ultrasound in the 1970 [7], the introduction of compound spatial sonography was an important step to optimize visualization of tissues such as the thyroid. Compound spatial sonography uses multiple sonograms obtained from different imaging angles to produce a single improved sonogram by obtaining images from several different imaging angles and combines them into a single image at real-time frame rates. The benefits of compound spatial thyroid sonography relate to reduction of image artifacts and noise and improved depiction of tissue boundaries [8]. The introduction of tissue harmonic imaging led to a further improved evaluation of tissue and especially nodule visualization on US [9]. Harmonic imaging exploits non-linear propagation of ultrasound through the body tissues. The high-pressure portion of the wave travels faster than the low-pressure portion resulting in distortion of the shape of the wave. This change in waveform leads to the generation of harmonics (multiples of the fundamental or transmitted frequency) from a tissue. At present, the 2nd harmonic is being used to produce the image because the subsequent harmonics are of decreasing amplitude and insufficient to generate a proper image [10]. Doppler ultrasound (CFD) is an imaging technique that exploits the shift in the frequency of US waves when they are reflected by moving blood (the Doppler effect) [11]. Doppler US can provide information on perithyroidal vessels and the vascularity of thyroid lesions and is widely used in daily practice for the evaluation of thyroid disease alongside gray-scale US [3]. US elastography (USE) can be used to assess the mechanical features of tissue elasticity [12]. USE has therefore been referred to as "electronic palpation" because it provides a reproducible assessment of tissue consistency. Depending on which physical quantities are measured, there are two main thyroid elastography methods in clinical practice: strain elastography (SE) and shear-wave elastography (SWE). They can be classified into different variants based on the excitation method (external force, internal force, and acoustic radiation force [ARF]) and how stiffness is expressed [13]. Elastography is ideal for measuring the tissue stiffness of the thyroid gland as well as focal lesions [14, 15]. Contrast enhanced ultrasound (CEUS) uses contrast agents consisting of microbubbles containing air or various gases within a shell [16]. When a US contrast agent is administered into the vasculature, it enhances the backscatter of the ultrasound waves by resonance within sonic windows. This results in a marked amplification of the signals from the blood flow and provides additional information about the microvasculature and perfusion. CEUS has been intensively studied in thyroid ultrasound, especially for the characterization of nodules [17, 18].
2 Epidemiology and indications for thyroid ultrasound
Abnormal thyroid function [19] and diffuse as well as focal thyroid abnormalities are common findings in the general population [20]. Ultrasonography is the most important imaging tool for diagnosing thyroid disease. In the majority of cases a correct diagnosis can already be made in synopsis of the sonographic together with clinical findings and basal thyroid hormone parameters and an appropriate therapy can be initiated thereafter. This applies in particular for goiter, Graves´ disease, Hashimoto´s disease, silent and postpartal thyroiditis etc.
Due to the expanding use of imaging techniques, the prevalence of thyroid nodules has also increased significantly: it is around 50–70% for ultrasound [21, 22]. However, the risk of malignancy for incidentally diagnosed thyroid nodules is low and only 0.3–5% [23, 24] – but significantly dependent on risk factors and preselection parameters. In addition, the risk of dying from thyroid cancer over the next 10 years is very low.
In clinical practice, the issue arises of the problem of the high number of ultrasound-diagnosed thyroid nodules with a quite low risk of malignancy. The greater availability of US-devices per se has already led to an increase in thyroid cancer rates, mostly low-risk cancers [25]. However, this is even more relevant for screening programs: ultrasound screening for thyroid cancer has resulted in a massive increase in the discovery of thyroid malignancies, generally papillary microcarcinomas (PTMC) [26]. Concurrent with the increased number of thyroid surgery was a rise in the number of surgical complications: 11% resulted in postoperative hypoparathyroidism, 2% in recurrent laryngeal nerve palsy. On the other hand, mortality resulting from thyroid malignancies remained unchanged – a typical situation resulting from over-diagnosis [27]. In addition, US screening had no impact not only on thyroid cancer mortality, but also not on detecting other subtypes or thyroid cancer [28]. Furthermore, in countries without screening programs such an increase in the diagnosis of thyroid carcinoma has not been observed [27]. Finally, the long-term prognosis seems to be similar between incidental and non-incidental diagnosed at least for PTMC of thyroid cancer, as long as there are no symptoms suspicious for metastases [29]. As a consequence, some authors recommended to stop over-reporting normal findings and update consensus guidelines [30] or even asked whether it is time to turn off the US machines in general [31].
Therefore, although it sounds almost too banal: there should be an indication for carrying out an US examination of the thyroid. The US Preventive Services Task Force (USPSTF) for example recommends against screening for thyroid cancer in asymptomatic adults (D recommendation) [32]. According to guidelines [33, 34], besides patients with abnormal thyroid function tests or local symptoms thyroid ultrasound is recommended in: a) All patients with a palpable thyroid nodule or with multinodular goiter; b) High-risk patients for thyroid malignancy e.g. a history of familial thyroid cancer, multiple endocrine neoplasia (MEN) type II or irradiated neck in childhood; c) Patients with palpable cervical adenopathy suspicious for malignancy; d) in the follow-up and monitoring of thyroid nodules. On the other hand, thyroid ultrasound is generally not recommended in patients with a normal thyroid on palpation, normal thyroid function and low risk of thyroid cancer as well as a screening test in the general population.
3 Ultrasound for diagnosing thyroid nodules and thyroid cancer
In recent decades, numerous individual sonographic criteria for malignancy of thyroid nodules with varying sensitivity and specificity have evaluated and validated. These criteria include echogenicity, hypoechoic rim (halo), shape, margins, calcification, vascularization, size, number, composition as well as stiffness [35, 36]. However, since no single sonographic criterion is sufficiently sensitive and specific to evaluate malignancy, combinations or clusters of criteria have been investigated. Numerous classifications systems have been developed in order to establish standardized US-findings descriptions that improve the prediction of malignancy and provide uniform recommendations regarding situations in which a FNAB should be performed. To this end several so called “Thyroid Imaging Reporting and Data System” (TIRADS) has been developed. Among the numerous TIRADSs, the most commonly used are ATA [33], ACR-TIRADS [37], EU-TIRADS [38], Korean TIRADS [39] and AACE/AME/ETA [34]. As a recent systematic review has shown [40], the number of publications about TIRADSs/risk stratification systems (RSSs) has importantly increased over the time, being the Horvath TIRADS [41] the most evaluated one, which was one of the first TIRADS published in 2009.
Within all these TIRADSs, the high-risk categories of all currently available RSSs display strong associations to cytological diagnostic classes of "malignant/suspicious-for-malignancy" and the low-risk classes are clearly associated to "not neoplastic/benign" cytology [42]. The introduction of these systems has elevated the diagnostic performance of US to a level approaching that of fine-needle aspiration (FNA) cytology. The diagnostic accuracy of most of these TIRADSs is comparable with high sensitivity and negative predictive value for diagnosing thyroid cancer, even in regions with iodine deficiency [43, 44]. ACR- TIRADS has been shown to be superior to a non-categorial traditional risk stratification and offers a meaningful reduction in the number of thyroid nodules recommended for biopsy and significantly improve the accuracy of recommendations for nodule management [45]. However, internationally endorsed sonographic risk stratification systems vary widely in their ability to reduce the number of unnecessary thyroid nodule FNAs: in one study ACR-TIRADS outperformed the others, classifying more than half the biopsies as unnecessary with a false negative rate of 2.2% [46]. The establishment of a new "international TIRADS"-currently in progress-will be critical to guide us towards a new era.
Most TIRADSs are based exclusively on criteria of B-mode sonography. New tools for the sonographic assessment of thyroid nodules are the measurement of tissue stiffness using ultrasound, so-called elastography [47], and contrast-enhanced ultrasound (CEUS) [17]. In addition, TIRADS has some limitations. TIRADSs have mainly be evaluated to detect papillary thyroid cancer [48]. Furthermore, TIRADSs are not applicable in the presence of an autonomous adenoma [49]—this should be ruled out beforehand, e.g. by a scintigraphy. TIRAD systems also do not appear to be sufficiently accurate in the detection of medullary thyroid carcinomas [50, 51]. TIRADS therefore does not replace calcitonin measurement [52]. In addition to the sonographic criteria for malignancy, other risk factors such as age, sex, comorbidities and location of the nodules within the thyroid gland should always be taken into account [53].
Although there are clear recommendations when to perform a FNB according to nodule size and risk category in all TIRADS, there is only very limited evidence concerning the optimal US follow-up strategy of thyroid nodules classified as probably benign by TIRADS. While expert opinions currently recommends repeat evaluation of a sonographically and cytologically benign nodule at 1–2 years, one study demonstrated that this interval can be safely extended to 3 years without increased mortality or patient harm [54]. Nodule growth can be expected, though detection of malignancies was unchanged in this study. However, a recent scoping review found that evidence comparing different ultrasound follow-up intervals in patients with benign thyroid nodules is limited to one observational study, but suggests that the subsequent development of thyroid malignancies is very uncommon regardless of follow-up interval [55]. The authors also note that longer follow-up may be associated with more repeat biopsies and thyroidectomies, which could be related to more interval nodule growth that meets thresholds for further evaluation. Therefore, research is needed to clarify optimal ultrasound follow-up intervals for low to intermediate suspicion cytologically benign thyroid nodules and outcomes of discontinuing ultrasound follow-up for very low suspicion nodules.
4 Online calculators and artificial intelligence (AI) for the evaluation of thyroid nodules
Online calculators can simplify and help to characterize thyroid nodules by the use of programmed algorithms. Clinical data and ultrasound criteria are systematically queried and the risk of malignancy is calculated. As an example, the “Nodule App” (https://aace-thyroid.deontics.com) is commissioned by the task force and based on the updated 2016 AACE/ACE/AME clinical practice guideline.
Artificial intelligence (AI) is defined as the ability of machines to apply human-like reasoning to problem solving [56]. Recent years have seen a rapid growth of AI in many disciplines. AI encompasses two related computational techniques: machine learning (ML), in which computers learn by observing data provided by humans, and deep learning (DL), which employs neural networks that mimic brain structure and function to analyze data. Most AI platforms in thyroid disease have focused on malignancy risk stratification of nodules. Computer-aided diagnosis (CAD) systems are then being applied to the ultrasonographic diagnosis of malignant thyroid nodules. As examples, a comparative analysis of two ML-based diagnostic patterns with ACR-TIRADS showed that the ML-assisted dual modalities visual approach can assist doctors to diagnose thyroid nodules more effectively and considerably reduce the unnecessary FNB rate in the clinical management of thyroid nodules [57]. One study demonstrated that an ACR-TIRADS-based DL could improve the differentiation of malignant from benign thyroid nodules and had significant potential for clinical application on TR4 and TR5 [58]. In another study, a DL model to assist thyroid nodule diagnosis and management (ThyNet) assisted strategy significantly improved the diagnostic performance of US and helped reduce unnecessary fine needle aspirations for thyroid nodules [59].
In summary, although the results of some validation studies have been mixed, AI is a promising tool to improve and simplify diagnostics of thyroid nodules and there is evidence that AI can exceed the performance of some humans, particularly physicians with less experience [56].
Although CAD systems demonstrated good performance in diagnosing malignant thyroid nodules, experienced operators may still have an advantage over CAD systems during real-time diagnosis [60].
5 Ultrasound for follow-up of thyroid cancer
Sonography is one of the most important tools in postoperative follow-up for thyroid carcinoma and is recommended by all major medical societies. In older [61, 62] as well as more recent studies [63] US has shown its diagnostic performance for the detection of persistence or recurrence in different situations in the follow-up of thyroid carcinoma. Therefore, standardized protocols with documentation should be used in the follow-up of thyroid carcinoma by US. A full sonography should include examination of the thyroid bed [64, 65] and the cervical and lateral lymph node compartments [66]. The infrahyal muscles, trachea, esophagus, M. longus colli and the carotid artery as well as the cervical lymph node compartments serve as guiding structures. Due to postoperative changes in the context of wound healing, the sonographic assessment of the thyroid bed and possibly the central lymph node compartments in particular is often limited within the first 3 months postoperatively. Smaller lesions can be found in this location due to edema or accumulation of fluids. In the case of lesions > 3 months postoperatively, differential diagnostics include granulomas, neuromas, reactive lymph nodes and parathyroid glands (adenomas) [67]. More often, one also sees hyperechogenic suture material. A postoperative lesion in the thyroid bed will be classified as suspicious by sonography if it is hypoechogenic, cystic, poorly defined, more vascularized or progressive in size over time (> 50% of the volume) or shows calcifications [64, 68, 69]. Lymph node metastases can also be detected in the thyroid bed. In the first two years postoperatively, thyroid cancer is often a persistent disease rather than a recurrence [70].
A classification of lymph nodes into the categories normal, indeterminate, and suspicious of malignancy using evaluated sonomorphological criteria is recommended. In addition, the localization of the lymph nodes in the central and lateral compartments must be specified according to an evaluated classification [66]. An important point is the postoperative ultrasound for the sonomorphological characterization of the response to therapy as well as for risk stratification, which is recommended 6–12 months postoperatively. Retrospective studies show that risk stratification too early after total thyroidectomy or ablative radioiodine therapy has no advantage in this context [71, 72]. In one study, postoperative US 3–4 months after surgery was also the most sensitive method for detecting cervical LN metastases and superior to the TG value [63].
6 Doppler ultrasonography (CFD) and superb microvascular imaging (SMI) for thyroid disease
Color flow Doppler ultrasound (CFD) imaging allows the identification of blood flow within thyroid tissue and focal lesions (Fig. 1). Doppler US is widely used to assist in the diagnosis of thyroid nodules, metastatic cervical lymph nodes in patients with thyroid cancer, and thyroiditis, as well as for the monitoring of thyroid interventions. Moreover, there is no risk of exposure to ionizing radiation. All Doppler US techniques except CEUS are non-invasive. In older studies, peripheral vascularization of a thyroid nodule was supposed to be a sign of benignity, in contrast to an intranodular vascularization as a sign of malignancy [73]. In addition, two meta-analyses [36, 74] demonstrated that primarily intranodular vascularization—with or without perinodular vascularization – raises the likelihood of malignancy, whereas solely perinodular vascularization is considered a sign of benignity.
However, recent studies could not confirm these results [75, 76]. This is possibly due to differences in equipment and examiner-related aspects as well as device settings (particularly PRF). The diagnostic benefit of vascularization in predicting malignancy therefore remains controversial. Although CFD was widely used in the past to characterize thyroid nodules, recent guidelines do not recommend the routine use of Doppler US for US malignancy risk stratification of thyroid nodules [38]. In contrast, CFD plays an important role in the characterization of lymph nodes, where peripheral or diffusely increased vascularization is suspicious of malignancy [66] as wells as in parathyroid adenomas, where the detection of so called feeding vessels by CFD is an important indication of a parathyroid lesion [77].
As a new vascular imaging technology, superb microvascular imaging (SMI) can visualize low-velocity blood flow [78]. Technically, SMI is an innovative Doppler technique for vascular examination and uses an intelligent algorithm that efficiently separates low-speed flow signals from motion artifacts so that it can assess microvessels and the vessel distribution in more detail [79]. Compared with CFD, SMI can describe blood flow in more detail, and can obtain high-quality microvascular images without using contrast media, and it can also describe the blood flow around the sinus and in the nodule in more detail [80]. In malignant nodules, SMI depicted the presence of incomplete surrounding periphery microvasculature and of disordered heterogeneous internal microvasculature whereas benign nodules showed complete surrounding periphery microvasculature (ring sign) and homogeneity internal branching in this study. In recent years, the application of SMI was intensively studied in different organs, including thyroid nodules, and a recent meta-analysis concluded that the diagnostic efficiency of SMI for malignant thyroid nodules is superior to CFD, and SMI technology can provide significantly more information on vascularity, make up for the deficiency of CFD, and has better clinical application value [81]. However, these studies have some significant limitations: most of the published studies are from China, which may lead to potential regional bias. Second, most of the studies are retrospective using mostly devices from a single company. Due to the insufficient number of studies with nodule diameter < 1cm, the ability of SMI to differentiate and diagnose thyroid micronodules could not be evaluated. In addition, the methods of examination and diagnosis are not quantified, and there is no unified diagnostic standard or thresholds. Because of these limitations the diagnostic performance of SMI in differentiating benign and malignant thyroid nodules must be evaluated further and prospective a large-scale multi-center study are needed.
7 Elastography for thyroid disease
A classical criterion of malignancy of thyroid nodules is a hard consistency during palpation. The introduction of ultrasound-based methods for measuring tissue stiffness, i. e. elastography, has made a reproducible ultrasound- based method available. As mentioned above, depending on which physical quantities are measured, there are two main thyroid elastography methods in clinical practice: strain elastography (SE) and shear-wave elastography (SWE) that can be further classified into different variants based on the excitation method (external force, internal force, and acoustic radiation force [ARF]) and how stiffness is expressed [13]. The use of elastography (USE) to differentiate thyroid nodules has been the subject of numerous studies [82,83,84,85]. As one prospective multicenter study showed, B-mode ultrasound sensitivity for thyroid malignancy is increased by real-time elastography [86]. Taken together, elastography shows high sensitivity and specificity for thyroid nodules [87,88,89]. Importantly, in addition to a high sensitivity and specificity, a high negative predictive value (NPV) is necessary for investigations of diseases with low prevalence such as thyroid carcinoma [90]. With an NPV of 93–99%, elastography has shown high value in ruling out a malignancy, even in prospective multicenter studies [91]. In one meta-analysis, the NPV, i.e. exclusion of malignancy, for predominantly soft or primarily soft nodules (Fig. 2) was 97%; for entirely soft nodules, 99% [92]. On the other hand, the positive predictive value (PPV) of USE is significantly lower, i.e., a hard nodule, depending on the study, is malignant in only 30–50% of cases [93]. It is important, however, to emphasize additional limitations of USE: it is not valid for extensive macrocalcification and predominantly cystic nodules [94]. There should always be an overall clinical assessment including other criteria besides USE. Nevertheless, recent meta-analysis confirmed that USE is a useful imaging tool for thyroid nodule characterization and in accordance with recent guidelines and meta-analyses, the USE could be used daily in thyroid nodule malignancy risk stratification [95]. Guidelines recommend that elastography should not replace grayscale study, but it may be used as a complementary tool for assessing nodules for FNA, especially due to its high NPV [38].
It is important to realize that USE was studied primarily in papillary thyroid cancer (PTC) (Fig. 3). In studies, USE was of limited value in diagnosing medullary thyroid carcinoma [96] and does not replace measurement of calcitonin. USE might also not be very helpful in diagnosing follicular thyroid cancer (FTC) or for the differentiation of follicular adenomas from carcinomas, since FTC might be elastographically soft as well. Nevertheless, some studies assessed whether USE with strain ratio increases diagnostic accuracy of CFD in further characterization of cytologically Thy3 thyroid nodules and found USE an useful additional tool to CFD, since it improved characterization of thyroid nodules with indeterminate cytology [97]. Another study evaluated the value of shear wave elastography (SWE) in avoiding repeat fine-needle aspiration of thyroid nodules with nondiagnostic and undetermined cytology and found SWE a promising imaging method for reducing repeat FNAC for benign thyroid nodules with nondiagnostic and undetermined cytology when using standard deviation of the elasticity (ESD) as an index [98]. Finally, USE could not identify scintigraphically hyperfunctioning thyroid nodules as benign nodules reliably [99]. Autonomously functioning thyroid nodule may have variable elasticity at USE examination, being hard score associated with reduced/suppressed thyroid stimulating hormone [100].
The role of USE in other indications than nodules, for example diffuse thyroid diseases [101] or thyroiditis [102, 103] remains controversial. In clinical practice, USE is rarely useful for these indications.
8 Contrast enhanced ultrasound (CEUS) for thyroid disease
Contrast-enhanced ultrasound (CEUS) uses an intravenous agent that contains microbubbles. The contrast helps to see the flow of blood—and thus the perfusion—through organs and blood vessels. Numerous studies have used CEUS primarily to differentiate thyroid nodules, including nodules < 1 cm [104]. As for thyroid nodule malignancy risk stratification by US, for acceptable accuracy in malignancy a combination of several CEUS parameters should be applied: hypo-enhancement, heterogeneous, peripheral irregular enhancement in combination with internal enhancement patterns, and slow wash-in and wash-out curve lower than in normal thyroid tissue. In contrast, homogeneous, intense enhancement with smooth rim enhancement and “fast-in and slow-out” are indicative of the benignity of the thyroid nodule. [17, 105]. Studies have also shown an additional benefit of CEUS in addition to TIRADSs [106] and elastography [107].
Even though overlapping features require further standardization, CEUS may achieve reliable performance in detecting or excluding thyroid cancer. It might also play a role in guiding ablation procedures of benign and malignant thyroid nodules and metastatic lymph nodes, and providing accurate follow-up imaging to assess treatment efficacy [17]. It might also have an indication for the characterization of parathyroid adenoms, which typically show a early arterial hyperperfusion [108] (Fig. 4).
Hypoechogenic, taller than wide nodule (red arrow) in the left thyroid lobe (TG), close to the carotid artery (ACC) in a patient with known primary hyperparathyroidism and hypercalcemia; low MI B-mode. CEUS (right side) with early arterial hyperperfusion within the lesion (red arrow). Parathyroid adenoma with atypical localization within the thyroid (histologically proven). TR: trachea
However, CEUS is an invasive procedure using a sulfur-based contrast medium intravenously, it´s time consuming and CEUS is significantly more expensive than standard sonography with no reimbursement in most countries. In addition, CEUS for the characterization of thyroid nodules is not approved in clinical practice by the guidelines, but only approved for research. In addition, several adverse reactions to common contrast media (seen in up to 1 in 100 patients) including headache, nausea, rash and reactions at the injection site up to allergic shock have been reported [109]. SonoVue must not be injected into a vein in patients known to have right-to-left shunts, severe pulmonary hypertension, uncontrolled hypertension or adult respiratory distress syndrome (https://www.ema.europa.eu/ en/documents/overview/sonovue-epar-summary-public_en.pdf). It is therefore questionable whether CEUS will become established as a routine method for the assessment of thyroid nodules in the near future.
9 Multiparametric sonographic imaging (MPUS) of thyroid lesions: chances of B-mode, elastography and CEUS
As with risk stratification of thyroid nodules by US using TIRADS, the use of a combination of multiple US parameters might be useful to improve accuracy in detecting malignancy of thyroid nodules. This combined application of ultrasound techniques is called multiparametric ultrasound or MPUS. Multiparametric ultrasound consists of gray-scale B-mode, elastography and contrast enhanced ultrasound (CEUS) including Time-Intensity-Curve (TIC) analysis. MPUS has been applied in thyroid disease as well [110]. In a recent study, MPUS using a score based system of B-mode, shear-wave and CEUS malignancy criteria showed promising results in the detection of thyroid carcinomas and reached a sensitivity of 95% and specificity of 75.49% [111].
Although further and larger studies are needed to validate this, combining different US techniques with the introduction of new software, the use of AI, FNB as well as molecular markers might pave the way for a completely new area of diagnostic accuracy in thyroid disease [112].
10 Interventional ultrasound for thyroid disease
US-guided thermal ablation (TA) procedures are increasingly proposed as therapy options for benign as well as malignant thyroid diseases. Initially, interventional US was mainly used to treat cysts with ethanol [113, 114] or polidocanol [115, 116] ablation, but over the years its use has expanded to other indications, including solid thyroid lesions and there are numerous recommendations from different societies on this topic [6, 117,118,119,120]. As the European Thyroid Association stated [120], TA procedures are well tolerated, but a dedicated training of the operators is required and information on possible complications needs to be shared with the patients. In addition, several factors should be considered when weighing between observation, surgery, and TA for benign thyroid nodules: In solid non-hyperfunctioning nodules, TA induces a decrease in thyroid nodule volume, paralleled by improvement in symptoms. Nodule re-growth is possible over time and may necessitate repeat treatment, or surgery, in a dialogue with the patient. In autonomously functioning thyroid nodules (AFTN), radioactive iodine remains the first-line treatment, but TA may be considered in young patients with small AFTN due to higher probability of restoring normal thyroid function and avoidance of irradiation. TA may also be considered for cystic lesions that relapse after ethanol ablation (EA) or have a significant residual solid component following drainage and EA. The ETA recommends that TA should be restricted to benign lesions that cause symptoms or cosmetic concern. A meta-analysis confirms that both RFA and laser ablation are able to obtain a significant volume reduction in benign non-functioning solid thyroid nodules and a significant volume reduction is already evident at 6 months after thermal ablation and results are stable over the time [121].
However, also not recommended for the treatment of primary papillary thyroid microcarcinoma (PTMC) by the Korean and Italian guidelines there are numerous studies on TA in patients with PTMC. Although thyroidectomy is still the first-line treatment for PTMC in many countries, it often involves aggressive overtreatment. In the area of active surveillance of PTMC and hemithyroidectomy [122] and extensive long-term experience with this approach in some countries [123], TA has also been gradually used for the treatment of primary and recurrent PTMC in selected patients. TA has been shown to be an effective alternative method for surgery in the treatment of low-risk PTMC with no local tumor recurrence, lymph node metastasis, or distant metastasis during follow-up of 5 years and none delayed surgery with a pooled mean major complication rate of 1.2% [124]. Together, besides its effectiveness in treating PTMC, TA harbors the advantage of being minimally invasive, economical, having less bleeding and having a high postoperative quality of life [125]. Less data exists, however about long-term outcomes of RFA in comparison with surgery for unilateral multifocal PTMC. One recent study revealed 6-year comparable outcomes between RFA and surgery for unilateral multifocal PTMC [126]. RFA may therefore be a safe and effective alternative to surgery in selected patients with unilateral multifocal PTMC.
Data availability
The data that support the findings of this review are openly available at http://doi.org.
Abbreviations
- AI:
-
Artificial intelligence
- ARFI:
-
Acoustic radiation force imaging
- B-mode:
-
Brightness mode
- CAD:
-
Computer-aided diagnosis
- CEUS:
-
Contrast enhanced ultrasound
- CFD:
-
Color flow Doppler
- DL:
-
Deep learning
- EA:
-
Ethanol ablation
- ETA:
-
European Thyroid Association
- FNB:
-
Fine needle biopsy
- FTC:
-
Follicular thyroid carcinoma
- ML:
-
Machine learning
- MPUS:
-
Multiparametric ultrasound
- MTC:
-
Medullary thyroid carcinoma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PRF:
-
Pulse repetition frequency
- PTC:
-
Papillary thyroid carcinoma
- PTMC:
-
Papillary thyroid microcarcinoma
- RFA:
-
Radiofrequency ablation
- SE:
-
Strain elastography
- SMI:
-
Superb microvascular imaging
- SWE:
-
Shear-wave elastography
- TA:
-
Thermal ablation
- THI:
-
Tissue harmonic imaging
- TIC:
-
Time-Intensity-Curve
- TIRADS:
-
Thyroid Imaging Reporting and Data Systems
- US:
-
Ultrasound
- USE:
-
Ultrasound elastography
References
Fujimoto Y, Oka A, Omoto R, et al. Ultrasound scanning of the thyroid gland as a new diagnostic approach. Ultrasonics. 1967;5:177–80. https://doi.org/10.1016/S0041-624X(67)80065-9.
Dighe M, Barr R, Bojunga J, et al. Thyroid ultrasound: State of the art. Part 2 - Focal thyroid lesions. Med Ultrason. 2017;19. https://doi.org/10.11152/mu-999.
Chung J, Lee YJ, Choi YJ, et al. Clinical applications of doppler ultrasonography for thyroid disease: Consensus statement by the korean society of thyroid radiology. Ultrasonography. 2020;39:315–30. https://doi.org/10.14366/usg.20072.
Dighe M, Barr R, Bojunga J, et al. Thyroid ultrasound: State of the art Part 1 - thyroid ultrasound reporting and diffuse thyroid diseases. Med Ultrason. 2017;19:79–93.
Wiest PW, Hartshorne MF, Inskip PD, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–96. https://doi.org/10.7863/jum.1998.17.8.487.
Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44. https://doi.org/10.1016/j.ultrasmedbio.2017.08.1889.
Crocker EF, McLaughlin AF, Kossoff G, et al. The gray scale echographic appearance of thyroid malignancy. J Clin Ultrasound. 1974;2:305–6. https://doi.org/10.1002/JCU.1870020411.
Shapiro RS, Simpson WL, Rausch DL, et al. Compound spatial sonography of the thyroid gland: evaluation of freedom from artifacts and of nodule conspicuity. AJR Am J Roentgenol. 2001;177:1195–8. https://doi.org/10.2214/AJR.177.5.1771195.
Szopinski KT, Wysocki M, Pajk AM, et al. Tissue harmonic imaging of thyroid nodules: initial experience. J Ultrasound Med. 2003;22:5–12. https://doi.org/10.7863/JUM.2003.22.1.5.
Uppal T. Tissue harmonic imaging. Australas J Ultrasound Med. 2010;13:29–31. https://doi.org/10.1002/J.2205-0140.2010.TB00155.X.
Roguin A. Christian Johann Doppler: the man behind the effect. Br J Radiol. 2002;75:615–9. https://doi.org/10.1259/BJR.75.895.750615.
Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. https://doi.org/10.1055/s-0033-1335205.
Zhao C-K, Xu H-X. Ultrasound elastography of the thyroid: principles and current status. Ultrason (Seoul, Korea). 2019;38:106–24. https://doi.org/10.14366/usg.18037.
Cosgrove D, Barr R, Bojunga J, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: Part 4. Thyroid. Ultrasound Med Biol. 2016. https://doi.org/10.1016/j.ultrasmedbio.2016.06.022.
Săftoiu A, Gilja OH, Sidhu PS, et al. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: Update 2018. Ultraschall der Medizin - Eur J Ultrasound. 2019. https://doi.org/10.1055/a-0838-9937.
Ajmal S. Contrast-enhanced ultrasonography: Review and applications. Cureus. 2021;13:e18243. https://doi.org/10.7759/CUREUS.18243.
Radzina M, Ratniece M, Putrins DS, et al. Performance of contrast-enhanced ultrasound in thyroid nodules: Review of current state and future perspectives. Cancers (Basel). 2021;13:5469. https://doi.org/10.3390/CANCERS13215469.
Trimboli P, Castellana M, Virili C, et al. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Medica. 2020;125:406–15. https://doi.org/10.1007/s11547-019-01129-2.
Madariaga AG, Santos Palacios S, Guillén-Grima F, et al. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99:923–31. https://doi.org/10.1210/JC.2013-2409.
Russ G, Leboulleux S, Leenhardt L, et al. Thyroid incidentalomas: Epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J. 2014;3:154–63. https://doi.org/10.1159/000365289.
Meisinger C, Ittermann T, Wallaschofski H, et al. Geographic variations in the frequency of thyroid disorders and thyroid peroxidase antibodies in persons without former thyroid disease within Germany. Eur J Endocrinol. 2012;167:363–71. https://doi.org/10.1530/EJE-12-0111.
Reiners C, Wegscheider K, Schicha H, et al. Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid. 2004;14:926–32. https://doi.org/10.1089/thy.2004.14.926.
Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–7. https://doi.org/10.1210/jc.2006-0690.
Grussendorf M, Ruschenburg I, Brabant G. Malignancy rates in thyroid nodules: a long-term cohort study of 17,592 patients. Eur Thyroid J. 2022;11. https://doi.org/10.1530/ETJ-22-0027.
Haymart MR, Banerjee M, Reyes-Gastelum D, et al. Thyroid ultrasound and the increase in diagnosis of low-risk thyroid cancer. J Clin Endocrinol Metab. 2019;104:785–92. https://doi.org/10.1210/jc.2018-01933.
Lee J-H, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet (London, England). 2014;384:1848. https://doi.org/10.1016/S0140-6736(14)62242-X.
Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–7. https://doi.org/10.1056/NEJMp1604412.
Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016;26:1535–40. https://doi.org/10.1089/thy.2016.0075.
Reinke R, Mathiesen JS, Larsen SR, et al. Incidental and non-incidental papillary thyroid microcarcinoma in Denmark 1996–2015: A national study on incidence, outcome and thoughts on active surveillance. Cancer Epidemiol. 2019;60:46–50. https://doi.org/10.1016/J.CANEP.2019.03.011.
Hofman MS. Thyroid nodules: time to stop over-reporting normal findings and update consensus guidelines. BMJ. 2013;347: f5742.
Cronan JJ. Thyroid nodules: is it time to turn off the US machines? Radiology. 2008;247:602–4. https://doi.org/10.1148/RADIOL.2473072233.
Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for thyroid cancer. JAMA. 2017;317:1882. https://doi.org/10.1001/jama.2017.4011.
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. https://doi.org/10.1089/thy.2015.0020.
Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22. https://doi.org/10.4158/EP161208.GL.
Bojunga J. Ultrasound of thyroid nodules. Ultraschall der Medizin - Eur J Ultrasound. 2018;39:488–511. https://doi.org/10.1055/a-0659-2350.
Remonti LR, Kramer CK, Leitão CB, et al. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015;25:538–50. https://doi.org/10.1089/thy.2014.0353.
Hoang JK, Middleton WD, Tessler FN. Update on ACR TI-RADS: Successes, challenges, and future directions, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216:570–8. https://doi.org/10.2214/AJR.20.24608.
Russ G, Bonnema SJ, Erdogan MF, et al. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur Thyroid J. 2017;6:225–37. https://doi.org/10.1159/000478927.
Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–95.
Trimboli P, Ferrarazzo G, Deandrea M, et al. Interest of researchers in ultrasound systems for risk stratification of thyroid nodules (TIRADS): a systematic review. Clin Transl Imaging. 2022;10:185–90. https://doi.org/10.1007/s40336-021-00472-7.
Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–51. https://doi.org/10.1210/jc.2008-1724.
Trimboli P, Durante C. Ultrasound risk stratification systems for thyroid nodule: between lights and shadows, we are moving towards a new era. Endocrine. 2020;69. https://doi.org/10.1007/s12020-020-02196-6.
Seifert P, Schenke S, Zimny M, et al. Diagnostic performance of Kwak, EU, ACR, and Korean TIRADS as well as ATA guidelines for the ultrasound risk stratification of non-autonomously functioning thyroid nodules in a region with long history of iodine deficiency: A German multicenter trial. Cancers (Basel). 2021;13. https://doi.org/10.3390/CANCERS13174467.
Kim PH, Suh CH, Baek JH, et al. Diagnostic performance of four ultrasound risk stratification systems: A systematic review and meta-analysis. Thyroid. 2020. https://doi.org/10.1089/thy.2019.0812.
Hoang JK, Middleton WD, Farjat AE, et al. Reduction in thyroid nodule biopsies and improved accuracy with American college of radiology thyroid imaging reporting and data system. Radiology. 2018;287:185–93. https://doi.org/10.1148/radiol.2018172572.
Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: Toward the “Right” TIRADS. J Clin Endocrinol Metab. 2019;104:95–102. https://doi.org/10.1210/jc.2018-01674.
Cosgrove D, Barr R, Bojunga J, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: Part 4. Thyroid. Ultrasound Med Biol. 2017;43. https://doi.org/10.1016/j.ultrasmedbio.2016.06.022.
Trimboli P, Castellana M, Piccardo A, et al. The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta-analysis Rev Endocr Metab Disord. 2021;22:453–60. https://doi.org/10.1007/S11154-020-09592-3.
Castellana M, Virili C, Paone G, et al. Ultrasound systems for risk stratification of thyroid nodules prompt inappropriate biopsy in autonomously functioning thyroid nodules. Clin Endocrinol (Oxf). 2020;93:67–75. https://doi.org/10.1111/cen.14204.
Matrone A, Gambale C, Biagini M, et al. Ultrasound features and risk stratification systems to identify medullary thyroid carcinoma. Eur J Endocrinol. 2021;185:193–200. https://doi.org/10.1530/EJE-21-0313.
Ferrarazzo G, Camponovo C, Deandrea M, et al. Suboptimal accuracy of ultrasound and ultrasound-based risk stratification systems in detecting medullary thyroid carcinoma should not be overlooked. Findings from a systematic review with meta-analysis. Clin Endocrinol (Oxf). 2022;97:532–40. https://doi.org/10.1111/CEN.14739.
Trimboli P, Valderrabano P, Pitoia F, et al. Appropriate and mindful measurement of serum calcitonin in patients with thyroid nodules. A white paper Endocrine. 2023. https://doi.org/10.1007/S12020-023-03485-6.
Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: Consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31:183–92. https://doi.org/10.1089/thy.2020.0330.
Medici M, Liu X, Kwong N, et al. Long- versus short-interval follow-up of cytologically benign thyroid nodules: a prospective cohort study. BMC Med. 2016;14:11. https://doi.org/10.1186/s12916-016-0554-1.
Chou R, Dana T, Mayson SE, et al. Ultrasound follow-up of benign thyroid nodules: A scoping review. Thyroid. 2023;33. https://doi.org/10.1089/THY.2022.0692.
Tessler FN, Thomas J. Artificial intelligence for evaluation of thyroid nodules: a primer. Thyroid. 2023;33:150–8. https://doi.org/10.1089/THY.2022.0560.
Zhao C-K, Ren T-T, Yin Y-F, et al. A comparative analysis of two machine learning-based diagnostic patterns with thyroid imaging reporting and data system for thyroid nodules: Diagnostic performance and unnecessary biopsy rate. Thyroid. 2020. https://doi.org/10.1089/thy.2020.0305.
Wu GG, Lv WZ, Yin R, et al. Deep learning based on ACR TI-RADS can improve the differential diagnosis of thyroid nodules. Front Oncol. 2021;11. https://doi.org/10.3389/FONC.2021.575166.
Peng S, Liu Y, Lv W, et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicentre diagnostic study. Lancet Digit Heal. 2021;3:e250–9. https://doi.org/10.1016/S2589-7500(21)00041-8.
Xu L, Gao J, Wang Q, et al. Computer-aided diagnosis systems in diagnosing malignant thyroid nodules on ultrasonography: a systematic review and meta-analysis. Eur Thyroid J. 2020;9:186–93.
Pacini F, Molinaro E, Castagna MG, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–73. https://doi.org/10.1210/jc.2002-021925.
Torlontano M, Crocetti U, Augello G, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–3. https://doi.org/10.1210/jc.2005-1185.
Matrone A, Gambale C, Piaggi P, et al. Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. J Clin Endocrinol Metab. 2017;102:893–902. https://doi.org/10.1210/jc.2016-2860.
Ko M-S, Lee JH, Shong YK, et al. Normal and abnormal sonographic findings at the thyroidectomy sites in postoperative patients with thyroid malignancy. AJR Am J Roentgenol. 2010;194:1596–609. https://doi.org/10.2214/AJR.09.2513.
Chua WY, Langer JE, Jones LP. Surveillance neck sonography after thyroidectomy for papillary thyroid carcinoma: Pitfalls in the diagnosis of locally recurrent and metastatic disease. J Ultrasound Med. 2017;36:1511–30.
Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2:147–59. https://doi.org/10.1159/000354537.
Kobaly K, Mandel SJ, Langer JE. Clinical review: Thyroid cancer mimics on surveillance neck sonography. J Clin Endocrinol Metab. 2015;100:371–5.
Shin JH, Han B-K, Ko EY, et al. Sonographic Findings in the Surgical Bed After Thyroidectomy. J Ultrasound Med. 2007;26:1359–66. https://doi.org/10.7863/jum.2007.26.10.1359.
Kamaya A, Gross M, Akatsu H, et al. Recurrence in the thyroidectomy bed: Sonographic findings. Am J Roentgenol. 2011;196:66–70. https://doi.org/10.2214/AJR.10.4474.
Bates MF, Lamas MR, Randle RW, et al. Back so soon? Is early recurrence of papillary thyroid cancer really just persistent disease? Surgery. 2018;163:118–23. https://doi.org/10.1016/j.surg.2017.05.028.
Hong CM, Lee WK, Jeong SY, et al. Superiority of delayed risk stratification in differentiated thyroid cancer after total thyroidectomy and radioactive iodine ablation. Nucl Med Commun. 2014;35:1119–26. https://doi.org/10.1097/MNM.0000000000000183.
Castagna MG, Maino F, Cipri C, et al. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165:441–6. https://doi.org/10.1530/EJE-11-0466.
Ivanac G, Brkljacic B, Ivanac K, et al. Vascularisation of benign and malignant thyroid nodules: CD US evaluation. Ultraschall der Medizin - Eur J Ultrasound. 2007;28:502–6. https://doi.org/10.1055/s-2007-963023.
Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253–63. https://doi.org/10.1210/jc.2013-2928.
Moon HJ, Kwak JY, Kim MJ, et al. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255:260–9. https://doi.org/10.1148/radiol.09091284.
Maddaloni E, Briganti SI, Crescenzi A, et al. Usefulness of color doppler ultrasonography in the risk stratification of thyroid nodules. Eur Thyroid J. 2021;10:339–44. https://doi.org/10.1159/000509325.
Rickes S, Sitzy J, Neye H, et al. High-resolution ultrasound in combination with colour-Doppler sonography for preoperative localization of parathyroid adenomas in patients with primary hyperparathyroidism. Ultraschall Med. 2003;24:85–9. https://doi.org/10.1055/s-2003-38667.
MacHado P, Segal S, Lyshchik A, et al. A novel microvascular flow technique: Initial results in thyroids. Ultrasound Q. 2016;32:67–74. https://doi.org/10.1097/RUQ.0000000000000156.
Fu Z, Zhang J, Lu Y, et al. Clinical applications of superb microvascular imaging in the superficial tissues and organs: a systematic review. Acad Radiol. 2021;28:694–703. https://doi.org/10.1016/J.ACRA.2020.03.032.
Lu R, Meng Y, Zhang Y, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging. 2017;17. https://doi.org/10.1186/S12880-017-0241-5.
Jiang L, Zhang D, Chen Y-N, et al. The value of conventional ultrasound combined with superb microvascular imaging and color Doppler flow imaging in the diagnosis of thyroid malignant nodules: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14. https://doi.org/10.3389/FENDO.2023.1182259.
Friedrich-Rust M, Sperber A, Holzer K, et al. Real-time elastography and contrast-enhanced ultrasound for the assessment of thyroid nodules. Exp Clin Endocrinol Diabetes. 2010;118:602–9. https://doi.org/10.1055/s-0029-1237701.
Friedrich-Rust M, Romenski O, Meyer G, et al. Acoustic Radiation Force Impulse-Imaging for the evaluation of the thyroid gland: A limited patient feasibility study. Ultrasonics. 2012;52. https://doi.org/10.1016/j.ultras.2011.06.012.
Bojunga J, Dauth N, Berner C, et al. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PLoS One. 2012;7. https://doi.org/10.1371/journal.pone.0042735.
Asteria C, Giovanardi A, Pizzocaro A, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18:523–31. https://doi.org/10.1089/thy.2007.0323.
Trimboli P, Guglielmi R, Monti S, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab. 2012;97:4524–30. https://doi.org/10.1210/jc.2012-2951.
Liu B-J, Li D-D, Xu H-X, et al. Quantitative Shear Wave Velocity Measurement on Acoustic Radiation Force Impulse Elastography for Differential Diagnosis between Benign and Malignant Thyroid Nodules: A Meta-analysis. Ultrasound Med Biol. 2015;41:3035–43. https://doi.org/10.1016/j.ultrasmedbio.2015.08.003.
Zhan J, Jin J-M, Diao X-H, et al. Acoustic radiation force impulse imaging (ARFI) for differentiation of benign and malignant thyroid nodules-A meta-analysis. Eur J Radiol. 2015;84:2181–6. https://doi.org/10.1016/j.ejrad.2015.07.015.
Bojunga J, Herrmann E, Meyer G, et al. Real-time elastography for the differentiation of benign and malignant thyroid nodules: A meta-analysis. Thyroid. 2010;20. https://doi.org/10.1089/thy.2010.0079.
Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. Inhal Toxicol. 2014;26:811–28. https://doi.org/10.3109/08958378.2014.955932.
Friedrich-Rust M, Vorlaender C, Dietrich CF, et al. Evaluation of strain elastography for differentiation of thyroid nodules: Results of a prospective DEGUM multicenter study. Ultraschall Med. 2016;37:262–70. https://doi.org/10.1055/s-0042-104647.
Nell S, Kist JW, Debray TPA, et al. Qualitative elastography can replace thyroid nodule fine-needle aspiration in patients with soft thyroid nodules. A systematic review and meta-analysis. Eur J Radiol. 2015;84:652–61. https://doi.org/10.1016/j.ejrad.2015.01.003.
Vorländer C, Wolff J, Saalabian S, et al. Real-time ultrasound elastography–a noninvasive diagnostic procedure for evaluating dominant thyroid nodules. Langenbeck’s Arch Surg/Dtsch Gesellschaft für Chir. 2010;395:865–71. https://doi.org/10.1007/s00423-010-0685-3.
Bhatia KSS, Rasalkar DP, Lee YP, et al. Cystic change in thyroid nodules: a confounding factor for real-time qualitative thyroid ultrasound elastography. Clin Radiol. 2011;66:799–807. https://doi.org/10.1016/j.crad.2011.03.011.
Cantisani V, De Silvestri A, Scotti V, et al. US-elastography with different techniques for thyroid nodule characterization: Systematic review and meta-analysis. Front Oncol. 2022;12. https://doi.org/10.3389/FONC.2022.845549.
Andrioli M, Trimboli P, Amendola S, et al. Elastographic presentation of medullary thyroid carcinoma. Endocrine. 2014;45:153–5. https://doi.org/10.1007/S12020-013-0062-4.
Cantisani V, Maceroni P, D’Andrea V, et al. Strain ratio ultrasound elastography increases the accuracy of colour-Doppler ultrasound in the evaluation of Thy-3 nodules. A bi-centre university experience Eur Radiol. 2015. https://doi.org/10.1007/s00330-015-3956-0.
Chen L, Shi Y-X, Liu Y-C, et al. The values of shear wave elastography in avoiding repeat fine-needle aspiration for thyroid nodules with nondiagnostic and undetermined cytology. Clin Endocrinol (Oxf). 2019;91:201–8. https://doi.org/10.1111/cen.13992.
Ruhlmann M, Stebner V, Görges R, et al. Diagnosis of hyperfunctional thyroid nodules: impact of US-elastography. Nuklearmedizin. 2014;53:173–7. https://doi.org/10.3413/NUKMED-0660-14-04.
Trimboli P, Paone G, Zatelli MC, et al. Real-time elastography in autonomously functioning thyroid nodules: relationship with TSH levels, scintigraphy, and ultrasound patterns. Endocrine. 2017;58:488–94. https://doi.org/10.1007/s12020-017-1277-6.
Yang Z, Zhang H, Wang K, et al. Assessment of diffuse thyroid disease by strain ratio in ultrasound elastography. Ultrasound Med Biol. 2015;41:2884–9. https://doi.org/10.1016/j.ultrasmedbio.2015.07.012.
Menzilcioglu MS, Duymus M, Gungor G, et al. The value of realtime ultrasound elastography in chronic autoimmune thyroiditis. Br J Radiol. 2014;87. https://doi.org/10.1259/bjr.20140604.
Cepeha CM, Paul C, Borlea A, et al. The value of strain elastography in predicting autoimmune thyroiditis. Diagnostics (Basel, Switzerland). 2020;10. https://doi.org/10.3390/DIAGNOSTICS10110874.
Li X, Gao F, Li F, et al. Qualitative analysis of contrast-enhanced ultrasound in the diagnosis of small, TR3-5 benign and malignant thyroid nodules measuring ≤1 cm. Br J Radiol. 2020;93:20190923. https://doi.org/10.1259/bjr.20190923.
Liu Q, Cheng J, Li J, et al. The diagnostic accuracy of contrast-enhanced ultrasound for the differentiation of benign and malignant thyroid nodules A PRISMA compliant meta-analysis. Med (United States) 2018;97.
Xu Y, Qi X, Zhao X, et al. Clinical diagnostic value of contrast-enhanced ultrasound and ti-rads classification for benign and malignant thyroid tumors one comparative cohort study. Med (United States). 2019;98. https://doi.org/10.1097/MD.0000000000014051.
Xi X, Gao L, Wu Q, et al. Differentiation of thyroid nodules difficult to diagnose with contrast-enhanced ultrasonography and real-time elastography. Front Oncol. 2020;10. https://doi.org/10.3389/fonc.2020.00112.
Agha A, Hornung M, Stroszczynski C, et al. Highly efficient localization of pathological glands in primary hyperparathyroidism using contrast-enhanced ultrasonography (CEUS) in comparison with conventional ultrasonography. J Clin Endocrinol Metab. 2013;98:2019–25. https://doi.org/10.1210/jc.2013-1007.
Hu C, Feng Y, Huang P, et al. Adverse reactions after the use of SonoVue contrast agent: Characteristics and nursing care experience. Medicine (Baltimore). 2019;98: e17745. https://doi.org/10.1097/MD.0000000000017745.
Cantisani V, D’Ambrosio F, Nielsen MB. Multiparametric ultrasound of thyroid nodules: Where do we stand? Ultraschall Med. 2017;38:357–9. https://doi.org/10.1055/S-0043-111682.
Brandenstein M, Wiesinger I, Künzel J, et al. Multiparametric sonographic imaging of thyroid lesions: Chances of B-mode, elastography and CEUS in relation to preoperative histopathology. Cancers (Basel). 2022;14:4745. https://doi.org/10.3390/CANCERS14194745.
Fresilli D, David E, Pacini P, et al. Thyroid nodule characterization: how to assess the malignancy risk. Update of the literature. Diagnostics (Basel, Switzerland). 2021;11. https://doi.org/10.3390/DIAGNOSTICS11081374.
Hahn SY, Shin JH, Na DG, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean society of thyroid radiology. Korean J Radiol. 2019;20:609–20. https://doi.org/10.3348/kjr.2018.0696.
Deandrea M, Trimboli P, Creanza A, et al. Long-term follow-up of cystic thyroid nodules treated with percutaneous ethanol injection (PEI) using two different approaches. Eur J Endocrinol. 2020;183:489–95. https://doi.org/10.1530/EJE-20-0213.
Gong X, Zhou Q, Chen S, et al. Efficacy and safety of ultrasound-guided percutaneous polidocanol sclerotherapy in benign predominantly cystic thyroid nodules: a prospective study. Curr Med Res Opin. 2017;33:1505–10. https://doi.org/10.1080/03007995.2017.1325732.
Gong X, Wang F, Du H, et al. Comparison of ultrasound-guided percutaneous polidocanol injection versus percutaneous ethanol injection for treatment of benign cystic thyroid nodules. J Ultrasound Med. 2018;37:1423–9. https://doi.org/10.1002/jum.14482.
Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–25. https://doi.org/10.3348/KJR.2012.13.2.117.
Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperth. 2019;36:376–82. https://doi.org/10.1080/02656736.2019.1575482.
Feldkamp J, Grünwald F, Luster M, et al. Non-surgical and non-radioiodine techniques for ablation of benign thyroid nodules: Consensus statement and recommendation. Exp Clin Endocrinol Diabetes. 2020. https://doi.org/10.1055/a-1075-2025.
Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9:172–85. https://doi.org/10.1159/000508484.
Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67:35–43. https://doi.org/10.1007/S12020-019-02019-3.
Chung SR, Baek JH, Choi YJ, et al. Thermal ablation for the management of papillary thyroid microcarcinoma in the era of active surveillance and hemithyroidectomy. Curr Oncol Rep. 2022;24:1045–52. https://doi.org/10.1007/s11912-022-01268-2.
Miyauchi A, Ito Y, Fujishima M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023;33. https://doi.org/10.1089/THY.2023.0076.
Cho SJ, Baek SM, Na DG, et al. Five-year follow-up results of thermal ablation for low-risk papillary thyroid microcarcinomas: systematic review and meta-analysis. Eur Radiol. 2021;31:6446–56. https://doi.org/10.1007/S00330-021-07808-X.
Chen Z, Zhang W, He W. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: A systematic review. Clin Endocrinol (Oxf). 2023;98:296–305. https://doi.org/10.1111/CEN.14857.
Yan L, Yang Z, Li Y, et al. Five-year outcome between radiofrequency ablation vs. surgery for unilateral multifocal papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2023. https://doi.org/10.1210/CLINEM/DGAD360.
Funding
None.
Author information
Authors and Affiliations
Contributions
JB literature search, writing and editing of the manuscript; PT writing and editing of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
N/A.
Informed consent
N/A.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bojunga, J., Trimboli, P. Thyroid ultrasound and its ancillary techniques. Rev Endocr Metab Disord 25, 161–173 (2024). https://doi.org/10.1007/s11154-023-09841-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09841-1