Abstract
Ageing is the time-dependent gradual decline of the functional characteristics in an organism. It has been shown that it results in the loss of reproductive health and fertility. The age-dependent decline of fertility is a potential issue as the parenthood age is increasing in Western countries, mostly due to socioeconomic factors. In comparison to women, for whom the consequences of ageing are well documented and general awareness of the population is extensively raised, the effects of ageing for male fertility and the consequences of advanced paternal age for the offspring have not been widely studied. Studies with humans are welcome but it is hard to implement relevant experimental approaches to unveil the molecular mechanisms by which ageing affects male reproductive potential. Animal models have thus been extensively used. These models are advantageous due to their reduced costs, general easy maintenance in laboratory facilities, rigorous manipulation tools, short lifespan, known genetic backgrounds, and reduced ethical constraints. Herein, we discuss animal models for the study of male reproductive ageing. The most well-known and studied reproductive ageing models are rodents and non-human primates. The data collected from these models, particularly studies on testicular ageing, steroidogenesis, and genetic and epigenetic changes in spermatogenesis are detailed. Notably, some species challenge the currently accepted ageing theories and the concept of senescence itself, which renders them interesting animal models for the study of male reproductive ageing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ageing is a natural and currently unavoidable consequence of time for organisms, despite significant variations being found between different species. It is also known as senescence and is defined as the time-dependent and gradual decline of functional characteristics in an organism, leading to decreased fertility and an increasing probability of death [1,2,3]. Over the past decades, several theories have been proposed to explain the aetiology of ageing. Overall, there were two major theory categories: programmed ageing and damage accumulation. The most known and widely accepted programmed ageing theory, antagonistic pleiotropy, was proposed in 1957 by George Williams [4]. According to this theory, some genes that are beneficial for overall fitness earlier in life become deleterious with time. As natural selection tends to favour early life vigour and fitness, these genes would be casually selected and then responsible for the organism decline observed during ageing. In other words, mutations that would cause the increase of lifespan would simultaneously result in disadvantages during the early stages of life and vice versa. Another widely spread and accepted theory is the telomere theory. Telomeres are a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes where they play a role in protecting the terminal of chromosomes from being detected as double-strand breaks. Telomeres are gradually shortened in each cellular division, protecting cells from genomic instability and loss of genetic information [5]. The telomere theory defends that the shortening of telomeres leads to senescence, apoptosis, or even oncogenesis [6]. This concept led Leonard Hayflick to propose that there is a limited number of divisions that differentiated somatic cells would divide before they stop based on telomeres length, which was later known as “Hayflick limit” [7]. On the other side, the most widely accepted damage accumulation theories were the free radical and immunological ones. In brief, Gerschman proposed the free radical theory in the 1950s, later expanded by Harman, which argues that ageing is related to the accumulation of damage on DNA, proteins, and lipids by reactive oxygen species (ROS) [8, 9]. This unbalance on redox homeostasis was attributed to lower antioxidant defences and/or increased ROS production, which would result in accumulated oxidative stress-related damage on cells and tissues, including DNA fragmentation and accumulation of mutations. The immunologic theory, also known as autoimmune theory, was proposed in 1969 by Walford and hypothesizes that there is a time-dependent decline of the immune system which increases the vulnerability to infections and diseases whereas autoimmune-related damage is promoted [10, 11]. The result of accumulated damage, thus, caused by autoimmune and pathogen aetiology would result in the ageing of the organism. Currently, it is generally accepted that ageing is a multifactorial process that incorporates the mechanisms described by former theories. Based on the common mechanisms associated with ageing in different organisms, Lopéz-Otín et al. proposed in 2013 the nine cellular and molecular hallmarks of ageing, which are: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intracellular communication [12]. Together, these hallmarks are thought to contribute to the ageing process and determine the ageing phenotype.
One of the most common consequences of ageing is the decline of reproductive health and fertility. This age-dependent decline of fertility is a potential issue as the parenthood age is increasing in Western countries, mostly due to socioeconomic factors. According to the 2019 database of the annual live births in England and Wales, the proportion of men fathering live births over the age of 35 was 38.9% whereas that over the age of 40 was 15.3% [13]. In 2015, the proportion of men fathering live births over the age of 40 was found to be 12.7% in the United States [14]. In comparison to women, for whom the consequences of ageing are well documented and general awareness of the population is extensively raised, the effects of ageing for male fertility and the consequences of advanced paternal age for the offspring have not been widely studied. Nevertheless, it is well known that ageing negatively affects male fertility, leading to poorer sperm parameters, lower hormonal levels, impaired testicular function, and increased chromosome defects and DNA damage, which could also lead to harmful consequences for the offspring [7, 15]. Indeed, advanced paternal age is associated with a higher risk for genetic disorders [16], including achondroplasia [17], schizophrenia [18, 19], bipolar disorder [20], increased incidence of acute lymphoblastic leukaemia [21], and Down syndrome [22], or increased risk of miscarriage and stillbirth [15, 23].
Animal models have been extensively used to study the effects of ageing on male reproductive health. Animal models are advantageous due to their reduced costs, general easy maintenance in laboratory facilities, rigorous manipulation tools, short lifespans, known genetic backgrounds, and reduced ethical constraints [24]. The most widely used animal models, the mice (Mus musculus) and the rat (Rattus norvegicus) are routinely used due to their fully characterized genetic background, easy maintenance, and reduced costs. In this review, animal models for the study of male reproductive ageing will be discussed. The most well-known and studied reproductive ageing models are the rodents and non-human primate species. The data collected from these models, including testicular ageing, effects on steroidogenesis, and genetic and epigenetic changes in spermatogenesis will be detailed. Interestingly, some species challenge the currently accepted ageing theories and the concept of senescence itself, which renders them interesting animal models for the study of ageing. For instance, some jellyfish, worm, or even lobster species are considered to have negligible senescence [25,26,27]. More interestingly, the only mammal that is hypothesized to display negligible senescence is the naked mole-rat (Heterocephalus glaber) [28], which will be also discussed in this review.
2 Animal models for the study of male reproductive ageing
Animals have been extensively used in research and their contributions to the understanding of human physiology and diseases are countless. Within ageing research, various animal models, including rodents and primates, have been used to study the physiological pathways that regulate ageing and age-related diseases and the effects of life-prolonging interventions, such as caloric restriction. Laboratory mice (Mus musculus) and rats (Rattus norvegicus) are the preferred models for the study of ageing due to their well-known advantages, including their short lifespan and periods between generations, easy handling, availability and cost of maintenance, standardized husbandry, and extensive genomic and transcriptomic knowledge and manipulability. Nevertheless, the ageing phenotypes vary widely across species and conventional animal models can only provide limited information regarding ageing in humans [29]. Thus, data obtained from multiple species is necessary to better understand the processes involved in ageing and age-related diseases [30].
2.1 Rodent models of male reproductive ageing
The small size, ease of housing and handling, together with the short lifespan and high breeding capabilities has led to the widespread use of rodents as research models [31]. Rodents account for roughly 95% of all laboratory animals [32]. Mice and rats have several anatomical, physiological, and genetic similarities to humans, which makes them ideal models to study human physiology and diseases [31]. In ageing research, mutant mice were the first mammalian mutants to show increased [33] and decreased lifetimes [34], which led to the discovery of genes associated with longevity.
The decrease in men’s reproductive potential with advanced age is due to changes in their reproductive system. Aged men have lower testosterone levels than their younger counterparts [35]. Sperm concentration, motility, and morphology, also decrease with age [36, 37]. Several rat models are used to study male reproductive ageing, with distinct characteristics, advantages, and drawbacks (Table 1). The most well-studied rodent animal model that mimics human ageing is the Brown Norway rat (Fig. 1). Similarly to humans, aged Brown Norway rats exhibit decreased serum and testicular testosterone [38] and increased follicle-stimulating hormone (FSH), however, serum luteinizing hormone (LH) levels were found to be unaltered or decreased in these animals [39, 40]. Gonadotropin-releasing hormone (GnRH) secretion also diminishes with age [41]. Wang et al. reported an age-dependent decrease in serum inhibin [40] and Gruenewald et al. observed a decrease in prolactin and corticosterone and a decreasing trend in progesterone levels in aged Brown Norway rats [39]. Brown Norway rats also experience age-related alterations in the testes, including a reduction of the seminiferous tubules volume, impaired function of Leydig cells and impaired spermatogenesis, with a reduction or absence of spermatids [39, 41]. Wang et al. also reported a significant decrease in Sertoli cell number both in regressed testis from 22 months-old and in testis from 30 months-old Brown Norway rats [42]. Conversely, Wright et al. did not observe changes in the number of Sertoli cells, however, their older animals were younger (24 months-old) [43]. Altered function of Sertoli cells was also reported in older Brown Norway rats due to changes in the expression of transferrin and CP-2/cathepsin L mRNA [43]. Nevertheless, the data regarding ageing-related changes in Sertoli cell function and number in Brown Norway rats is scarce and further studies are needed. Wang et al. also observed a reduced sperm concentration and total sperm count in aged Brown Norway rats [40]. Sprague-Dawley rats are also used as models for testicular ageing. While an age-associated decrease in both serum and testicular testosterone is observed, contrarily to Brown Norway rats, pituitary adenomas, testicular tumours, and obesity are common in aged Sprague-Dawley rats which may confound the interpretation of results [38, 44]. Age-related testicular atrophy was also reported in these animals [45]. Additionally, in Sprague-Dawley rats, the LH levels remain constant throughout life [46]. Another rat model which develops decreased testicular function is the F344 rat. Bethea et al. reported a decrease in testicular weight and testosterone production in old F344 rats, whereas pituitary prolactin was increased. Unlike humans, the steroidogenic capacity of F344 rats’ Leydig cells is not altered with age but serum testosterone levels are lower due to reduced Leydig cells numbers [47]. Although some studies observed a reduced number of Leydig cells in the testis of older men, there is conflicting data and further studies are needed [48, 49]. Contradictory results, however, were found by Turek et al. which observed an increase in serum testosterone and prolactin levels in aged F344 rats [50]. These differences may be related to the heterogeneity found between testicular interstitial cell tumours, whose incidence is extremely high in F344 rats [51]. Gruenewald et al. reported an increase in testicular progesterone and estradiol secretion due to the development of age-dependent testicular Leydig cells tumours [50, 52]. In addition, GnRH, LH, and FSH concentrations are decreased in aged F344 rats [53]. Taken together, a higher risk of F344 rats to develop testicular and pituitary tumours and consequent aberrant hormones production interfere with testicular ageing studies [39, 47]. Some groups also used Wistar rats for reproductive ageing studies, as this animal model exhibits an age-dependent decrease in testosterone production without a decrease in LH levels [54]. In addition, age-dependent tubular atrophy was reported, mostly due to loss of germinative cells and Leydig cells hyperplasia [55, 56]. Similarly to F344 and Sprague-Dawley rats, Wistar rats also develop spontaneous pituitary tumours in old age [57].
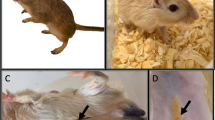
Schematic illustration of the ageing-related effects on the male reproductive function in the Brown Norway (BN) rat, the most similar male reproductive ageing animal model to the human condition. Chronic inflammation, increased reactive oxygen species (ROS) production, and oxidative stress-induced damage accumulation are the attributable causes for age-dependent decline in the male reproductive function, which is characterized by hormonal, morphological, and functional alterations that culminate in decreased testosterone production, testicular degeneration, and impaired spermatogenesis
Mice models have also been widely used in the study of male reproductive ageing, due to the existing parallelism between ageing in mice and humans (Table 2). Similar to humans, male C57BL/6 mice express age-related hormonal changes as well as alterations in the testes. Reduced testes size [58, 59], increased seminiferous tubules degeneration and germ cell depletion were reported in aged C57BL/6 mice [60]. Lacombe et al. also observed an age-related decrease in serum testosterone, however, FSH levels were found unaltered, and the LH levels showed a decreasing trend [60]. Aged male C57BL/6 mice also exhibit reduced fertility, with an increase in spermatozoa with abnormal morphology [58]. Male CBA/Ca mice manifest an age-related decrease in circulating testosterone levels, as well as smaller testes and reduced sperm parameters [61]. Similar to F344 rats, C57BL/6 and CBA/Ca mice commonly develop testicular interstitial cell tumours, which may interfere with the results of reproductive ageing studies [62]. Kotarska et al. evaluated the sperm quality of aged B10-BR mice with Y-chromosome long arm deletion (B10.BR-Ydel) and found a significant decrease in sperm parameters, accompanied by a reduction in the fertility of these animals [63]. Further studies, however, are still needed to disclosure age-related reproductive consequences on the aged B10-BR mice.
Accelerated ageing mice models have also been used for the study of reproductive ageing. Accelerated ageing models are easy to use, allow shorter experimental periods, and present a higher survival rate of the animal through the duration of the study, which constitutes advantages for their use in ageing studies compared to normally ageing models. D-galactose (D-gal) induced and senescence-accelerated prone (SAMP) mice are two types of accelerated ageing models. At high levels, D-gal is metabolized to aldose and hydroperoxide by galactose oxidase and this reaction results in the production of ROS. D-gal injections can mimic the ageing process by inducing increased oxidative stress, inflammatory processes, mitochondrial dysfunction, increased ageing markers, such as advanced glycation end products and their receptors, and cellular apoptosis [64]. Liao et al. optimized a mice model for reproductive ageing by administering D-gal injections to male C57BL/6 mice. D-gal-injected mice exhibited decreased testis weight and altered sperm parameters, which are analogous to the typical characteristics of old males [65]. SAMP strains of mice comprise a group of inbred strains in which the mice develop senescent phenotypes at an early age as well as shorter life spans. Mice from senescence-accelerated strains present a normal development until adulthood and achieve maturity of reproductive function as mice from normal senescence strains. While the ageing process starts at the same time in normal and accelerated senescence stains, the progression of the senescence process is faster in SAMP mice. SAMP mice show increased oxidative stress markers in several organs, including increased lipid peroxidation and protein carbonylation [66]. Mitochondrial dysfunction was also reported in SAMP mice, and a decrease in ATP production and higher redox state observed in these mice may compromise normal cell metabolism. SAMP mice displayed an accelerated age-associated increase in DNA damage and decreased DNA repair in various organs. Elevated oxidative stress, thus, is thought to be one of the mechanisms contributing to the faster ageing in these strains [66]. SAMP mice models have also been used in reproduction research [67]. Flood et al. described a significant age-related decrease in testosterone levels in older senescence-accelerated mouse prone 8 (SAMP8) mice, without morphological changes in the testes [68]. Although accelerated ageing animal models exhibit several advantages, it is not known the full extent of the reliability of the produced data and its comparability to the human ageing processes.
2.2 Non-human primate models of male reproductive ageing
Non-human primates constitute the best models for understanding human ageing, age-related diseases, as well as the effects of interventions to increase longevity due to their numerous similarities to humans [69]. In addition to their physiologic, genetic, cognitive, and social similarities, non-human primates also develop the same pathologies and ageing characteristics as humans. Non-human primates display various age-related changes in sexual behaviour and in the reproductive system, which are similar to some extent to the alterations observed in humans [70]. The use of primates in research, however, comes with some obstacles, as they cannot be manipulated in the same way as rodents and other smaller species due to technical and ethical concerns. Their size and weight, together with their long lifespan, pose difficulties in the housing and handling as well as in the duration of studies [71]. Non-human primate research, therefore, focuses on a handful of species and studies often include few subjects.
Rhesus macaque (Macaca mulatta) is the most extensively non-human primate model used in gerontology due to its extraordinary similarity with humans [69]. Rhesus macaques share around 93% genetic homology with humans, as several physiological, anatomical, and cognitive features are also parallel to those of humans. Old Rhesus macaques also show increased prevalence of diabetes mellitus, neoplasia, sarcopenia, bone loss, compromised immune function, and cognitive decline [72,73,74]. Rhesus macaques were also studied for reproductive ageing. Robinson et al. reported a significant decline in sexual activity in old Rhesus macaques, characterized by fewer intromissions and ejaculations [75]. Several authors found no age-related difference in testosterone and dihydrotestosterone levels [75,76,77]; however, Chambers et al. observed a negative correlation between the percentage of testosterone bound to testosterone-binding globulin and the sexual behaviour in old Rhesus macaques [76]. Kaler et al. found a distinct pattern of the pulsatile secretion of testosterone between young and older animals. The number of testosterone pulses increased at night in young Rhesus macaques, whereas no significant nocturnal increase in testosterone pulse number was observed in older animals. In addition, a diminution in both nocturnal testosterone concentration and pulse number was observed in older animals when compared to their younger counterparts [77]. Despite not being used as an ageing model per se, Japanese macaques (Macaca fuscata) also exhibit age-related testicular dysfunction. Similarly to Rhesus macaques, the sexual vigour of Japanese macaques (defined by the frequency of mounting and ejaculation) decreases with advancing age [78], and age-related degenerative changes of the reproductive organs have been observed [79]. Matsubayashi et al. determined that the weight of the reproductive organs, namely the testis, seminal vesicle, and prostate gland, showed a decreasing trend with age but no statistical significance was reported. Older animals exhibited a smaller diameter of the seminiferous tubules and hyperplasia of the interstitial tissue. In addition, while the thickness of the spermatogenic epithelium and the number of germ cells was diminished in aged Japanese macaques testes, spermatogenesis seemed to be conserved since sperm were present in the seminiferous tubules [79]. Hamada et al. found no differences in the testis volume of older and younger Japanese macaques, although their older animals were younger than the ones studied by Matsubayashi and collaborators [80].
Marmosets (Callithrix jacchus) are small primates with a maximum lifespan of 16.5 years that have also been used as ageing animal models. Marmosets have short generation times and relatively low maintenance costs, which makes them attractive laboratory models. Like Rhesus macaques, marmosets present age-related deterioration parallel to humans, including diabetes, chronic renal disease, amyloidosis, and cancer [69]. These characteristics make marmosets suitable models for the study of ageing and a promising translation approach to study human ageing and age-related diseases [81]. As a model for reproductive ageing, marmosets exhibit some interesting characteristics. Aged marmosets have lower circulating and urinary testosterone levels, which are related to hypothalamus-pituitary-gonad (HPG) axis dysfunction [82]. In the same study, it was also demonstrated that administration of synthetic GnRH led to increased circulating testosterone levels in aged marmosets, which suggests that the pituitary maintains its capacity to respond to GnRH stimulation, and Leydig cells retain their capacity to synthesize and release testosterone.
Another small, short-lived primate with potential interest for the study of ageing is the grey mouse lemur (Microcebus murinus). Mouse lemurs are the smallest and fastest developing and maturing primates. They have high breeding capabilities and more than 90% of sequence identity to the human genome. In addition, mouse lemurs share some ageing characteristics with humans, such as the greying of the fur and blindness due to cataracts [83]. Because some animals develop symptoms of premature ageing, such as cognitive and social decline, brain atrophy, amyloid plaques, and cytoskeletal Tau pathology, they have been proposed as models for ageing and Alzheimer’s disease [84]. While mouse lemurs are of potential interest for the study of ageing, their attractiveness as a reproductive ageing model is disputable. In effect, although testosterone levels decrease with age, the reproductive function is seasonal with a complete pause of gonadal hormones secretions in both sexes during the six months of the non-breeding season which could compromise conclusions [85].
3 Male reproductive ageing: lessons from animal models
3.1 Testicular ageing affects Leydig cell and steroidogenesis
In men, testosterone is mainly produced in the testes by Leydig cells (95%), whereas a small quantity is produced by the adrenal glands (about 5% of circulating testosterone) [86]. Testosterone biosynthesis is a complex multi-step process that starts with cholesterol mobilization into mitochondria. The mobilization of cholesterol, which is the rate-limiting step in steroidogenesis, can either occur from within the cell, from lipid droplets or plasma membrane, or the cholesterol present in the circulating plasma [87]. This process is potentiated by the action of the steroidogenic acute regulatory protein (StAR) and the translocator protein (TSPO). The action of both StAR and TSPO is dependent on the LH-induced activation of adenylyl cyclase, and consequent increase in cAMP and cAMP-dependent phosphorylation of proteins through protein kinase A (PKA) [88, 89]. Cholesterol is then converted into pregnenolone by the C27 cholesterol side-chain cleavage cytochrome P450 enzyme (CYP11A1), which is located on the matrix side of the inner mitochondrial membrane [90]. Pregnenolone is metabolized into testosterone in the smooth endoplasmic reticulum by the action of 3β-hydroxysteroid dehydrogenase (HSD3B), 17α-hydroxylase/17,20 lyase (CYP17A1), and type 3 17β-hydroxysteroid dehydrogenase (HSD17B) [91, 92]. Interestingly, LH can regulate both the expression of steroidogenic enzymes and the action of TSPO and StAR, the transmembrane proteins responsible for the transport of cholesterol into mitochondria [90]. Testosterone, in turn, regulates LH production and secretion by the pituitary through a negative feedback mechanism [93].
Not only is testosterone essential for testicular function and spermatogenesis but also for the maintenance of secondary sexual characters and other physiological functions including the maintenance of muscle mass, bone density, cognitive function, sexual function, and immune system function [94]. Longitudinal studies have shown an age-dependent slow decline in testosterone serum levels in men even in the absence of disease, which may start as early as at the age of 30 years old [95]. This age-dependent decrease in testosterone levels occurs with a simultaneous increase in the levels of LH and FSH, which characterizes this condition as primary hypogonadism. Due to the age factor, this condition is also known as late-onset hypogonadism [96]. The low production of testosterone is responsible for a myriad of factors related to ageing, including loss of muscle mass, bone density, and increased fatigue and lipid accumulation in the adipose tissue [96, 97]. The decrease in testosterone levels in older males has also been associated with the age-related alterations in sexual behaviour, including a decline in sexual performance as observed in several mammalian species [98]. In rodents, an increased mount latency, intromission latency, ejaculation latency, postejaculatory interval, and intercopulatory interval were observed [99]. Animal models of reproductive ageing have been widely used to determine the aetiology of age-dependent decrease in testosterone production (Fig. 2). The data concerning ageing effects on Leydig cells and steroidogenesis is discussed in the following subtopics.
Schematic illustration of the mechanisms leading to age-dependent decrease in testosterone production by Leydig cells. A decrease in LH receptor expression and responsiveness due to ineffective coupling to Gs protein is hypothesized as one of the major causes for the decreased testosterone production by Leydig cells. Simultaneously, altered expression of steroidogenic enzymes, including StAR, TSPO, and CYP11A1, and alterations in regulatory pathways are also attributed to decrease testosterone production. Abbreviations: AA – arachidonic acid; AC – adenylyl cyclase; COX-2 - arachidonic acid/cyclooxygenase-2; ER - endoplasmic reticulum; LH - luteinizing hormone; PDE - phosphodiesterases; PKA – protein kinase A; StAR - steroidogenic acute regulatory protein; TSPO - translocator protein
3.1.1 Aged leydig cells are unresponsive to LH
As the LH levels remain unaltered or high in men, the unresponsiveness to LH or loss of steroidogenic capacity is mainly attributed to Leydig cells [100, 101], however, ageing effects on the HPG axis are also the result of alterations in the secretion patterns of both LH and testosterone [102]. While rodents are widely used as animal models for the study of ageing, the majority of strains undergo a decrease in testosterone production due to an age-dependent decrease in LH levels. As the alterations are at the hypothalamus-pituitary level, this condition is characterized as secondary hypogonadism and thus it is not similar to the human condition [97]. The only two exceptions seem to be the Wistar and Brown Norway rat. As aforementioned, Wistar rats are reported to decrease serum testosterone due to reduced LH receptor and steroidogenic gene expression while LH levels are maintained [54]. The Brown Norway rat displays an age-dependent decrease in testosterone levels with unaltered LH and increased FSH production, rendering this strain as a good model for the study of testicular ageing, particularly on Leydig cells and steroidogenesis [38]. However, Wang et al. reported conflicting results as a decreased in LH levels in older Brown Norway rats was observed [40]. Several studies have been conducted using the Brown Norway rat strain as a model for testicular ageing. Zirkin et al. perfused young (3–6 months old) and old (18–24 months old) Brown Norway rat testes with LH and observed that the older animals produced less testosterone [38]. These results were later confirmed by the same group, as they observed that isolated aged Leydig cells produce less testosterone than their counterparts from younger rats [103]. As the number of Leydig cells is thought to be constant during adulthood [104], the reduced testosterone levels have been hypothesized to result from the unresponsiveness of Leydig cells to LH. The number of LH receptors is reported to decline with age [105], although the binding of LH to 10% of LH receptors is sufficient to evoke a biological response and maintain testosterone levels [106]. One of the main hypotheses concerning the unresponsiveness to LH is the decline in cAMP production and consequent PKA activity in Leydig cells. The LH receptor is coupled to a G-protein, which is responsible for adenylyl cyclase activation and cAMP production. In turn, the cAMP/PKA signalling pathway is essential for the expression of steroidogenic enzymes and the transport of cholesterol into the mitochondria [87, 107]. Interestingly, Chen et al. demonstrated that Leydig cells isolated from older Brown Norway rats present a decreased production of cAMP when stimulated with LH, whereas those cells from both older and younger rats produce identical quantities of cAMP when treated with forskolin, a well-known adenylyl cyclase stimulator [105]. In a later study, the same group reported that culture of Leydig cells from older Brown Norway rats with dibutyryl cAMP, a membrane-permeable cAMP agonist that bypasses the LH receptor-adenylyl cyclase cascade, for three days, results in testosterone production similar to those from LH-stimulated young cells [108]. Taken together, these results led the authors to hypothesize that an LH receptor-G-protein coupling defect is the likely cause for the signalling cascade impairment. In support of these findings, Bakhtyukov et al. reported that the stimulating effect of both human chorionic gonadotropin (hCG) and TP03, an agonist of LH receptor, on testosterone production diminishes with age in Wistar rats, as well as adenylyl cyclase stimulation by gonadotropin and guanine nucleotide is also diminished, which led these authors to also hypothesize a weakening of the coupling of LH receptor and Gs protein [109]. Besides, Sokanovic et al. demonstrated that the expression of cAMP-degrading phosphodiesterases is increased in Leydig cells from older Wistar rats, which could further contribute to the observable age-dependent reduced cAMP levels [54]. In a later study, the same group observed that long-term treatment with sildenafil, a phosphodiesterase 5 inhibitor, significantly improves mitochondrial function and testosterone production in Leydig cells from older Wistar rats [110].
3.1.2 Cholesterol synthesis and translocation is impaired in aged leydig cells
Cholesterol translocation in Leydig cells relies upon the action of both StAR and TSPO. Both proteins are regulated by LH-induced activation of adenylyl cyclase, increasing cAMP and cAMP-dependent phosphorylation of PKA. Thus, it is not surprising that aged Leydig cells exhibit reduced levels of StAR, TSPO, and other steroidogenic enzymes including CYP11A1 [111,112,113]. Interestingly, it has been reported that testosterone biosynthesis is assured if enough cholesterol is translocated into the mitochondria even with the age-dependent reduction of steroidogenic enzymes levels [114], which highlights that defects in the cholesterol mobilization due to LH receptor unresponsiveness further contribute to the reduced testosterone production in aged Leydig cells.
The expression of StAR is responsive to LH, as Leydig cells treated with LH expeditiously increase StAR levels [115]. In addition, the knockout of StAR completely inhibits steroidogenesis [116]. As such, the decreased LH receptor-G-protein coupling efficiency has been found to impair StAR expression through the arachidonic acid/cyclooxygenase-2 (COX-2) pathway. Activation of LH receptor leads to the release of arachidonic acid in Leydig cells, which is metabolized by cellular lipoxygenases, epoxygenases or cyclooxygenases. The produced metabolites are known regulators of steroidogenesis, more precisely through the downregulation of StAR expression [117]. Wang et al. reported that Leydig cells from older male Brown Norway rats exhibit elevated COX-2 and decreased StAR protein levels in comparison to cells from younger rats. Interestingly, a 30-day treatment with a COX-2 inhibitor increased testosterone production and reverted serum testosterone levels [118]. Chen et al. also observed an increased COX-2 and decreased StAR expression in Leydig cells from older Brown Norway rats in comparison to cells from younger rats [119]. Further, inhibition of COX-2 resulted in increased testosterone production in Leydig cells isolated from both older and younger rats, which highlights that the age-dependent higher expression of COX-2 is the likely contributor for decreased StAR expression and consequent decreased testosterone production. In support of these results, Zhao et al. observed an age-dependent decrease in both StAR and CYP11A mRNA and protein levels in the SAMP8 mice strain [120]. In addition, an age-dependent increase in COX-2 protein levels was also reported. Bakhtyukov et al. also observed that Star and Cyp11a1 gene expression was decreased in elderly Wistar rats [109].
Similarly to StAR, TSPO levels were found reduced in aged Leydig cells [114]. TSPO functionally interacts with StAR and both together are responsible for cholesterol mobilization into the mitochondria of Leydig cells [121]. Chung et al. observed that incubation of isolated Leydig cells from older Brown Norway rats with two selective TSPO ligands (FGIN-1-27 and Ro5-4864) significantly increased testosterone production [122]. Additionally, serum testosterone was increased when both ligands were administrated to both old and young Brown Norway rats. In a later study by the same group, they observed that a single dose FGIN-1-27 administration maintained increased serum testosterone levels for over 10 h whereas it did not alter Star, Tspo, and several other steroidogenic enzymes gene expression [123]. Taken together, these authors hypothesized that the TSPO selective ligand FGIN-1-27 would be an interesting substitute for exogenous testosterone administration during testosterone replacement therapy. Chung et al. observed that administration of exogenous testosterone increased serum testosterone levels but significantly reduced serum LH, Leydig cells testosterone production, and intratesticular testosterone to below the concentration required to maintain spermatogenesis, whereas FGIN-1-27 administration increased serum testosterone levels without reducing intratesticular testosterone concentrations or altering testicular sperm counts [124]. Conversely, Tu et al. reported that TSPO knockout mice have normal steroidogenesis [125], which would question the function of TSPO in cholesterol mobilization into the mitochondria. These controversial results, however, were contested by some later studies [126,127,128]. Barron et al. studied the same TSPO knockout mice model and found that while testosterone levels were normal in younger animals, TSPO deficiency altered the steroidogenic flux and reduced total steroidogenic output [129]. In addition, these authors observed an exacerbated age-dependent decrease in testosterone in the TSPO knockout mice, which led the authors to postulate that potential compensatory mechanisms that are overwhelmed with ageing are actioned.
3.1.3 Inflammation and redox imbalance in aged leydig cells
Leydig cells and their steroidogenic activity are affected by the testicular environment and local factors produced by surrounding cells. An interesting study by Chen et al. demonstrated that Leydig cells could be restored in both young and old Brown Norway rats upon its deletion by a single injection of ethane dimethanesulfonate (EDS) and that testosterone production by the new Leydig cells from both young and old animals was similar 10 weeks after the treatment. Testosterone production, however, was decreased in older rats at 30 weeks post-treatment, suggesting that factors extrinsic to the new Leydig cells are responsible for the detected reduction in testosterone production [130]. One potential explanation for these observations is the incidence of inflammation and high oxidative stress, which have been linked to the age-dependent decrease in testosterone production by Leydig cells. Zhao et al. observed increased ROS levels in aged Leydig cells from the SAMP8 mice, a senescence-accelerated strain, which were associated with increased pro-inflammatory cytokines expression [120]. Interestingly, another study by the same group reported that moderate exercise significantly increased serum testosterone levels and decreased age-related inflammation and oxidative stress in the SAMP8 mice strain [67]. A chronic inflammation status is a well-known feature associated with ageing, leading some authors to define this condition as “inflammating”. The effects of chronic inflammation on the testis have been extensively studied, with a special focus on the effects of macrophage-derived inflammatory mediators on Leydig cells and steroidogenesis. Macrophages play an important and complex regulatory role for Leydig cells’ development and function. Macrophages produce growth and differentiation factors for Leydig cells, whose development is arrested if macrophages are absent from the testis [131]. Macrophages also produce inflammatory cytokines that can inhibit steroidogenesis (as reviewed by Rehman et al. [132]), and increase levels of serum proinflammatory cytokines; these increases, which occur in chronic inflammation scenarios, are correlated with low circulating testosterone [133, 134]. For instance, both macrophages and Leydig cells secrete Interleukin 1 beta (IL-1β), which induces COX-2 expression and subsequent downregulation of StAR [135]. Simultaneously, macrophages produce ROS [136]. Leydig cells also produce ROS during steroidogenesis due to the activity of mitochondrial P450 type enzymes [137]. ROS are essential for several cellular functions and signalling pathways. In turn, oxidative imbalance due to increased ROS production and/or impaired antioxidant system leads to oxidative damage. The accumulation of oxidative damage is one of the causes for ageing and an age-dependent decrease in testosterone production. Chen et al. reported that Leydig cells from aged Brown Norway rats produced more ROS than cells from younger rats despite the absolute volume of mitochondria in older cells being reduced [138]. Beattie et al. observed that Leydig cells isolated from both young and old Brown Norway rats produced ROS in response to LH stimulation, however this production peaked later and took longer to revert to the control levels in the aged Leydig cells [139]. Furthermore, Leydig cells from older rats were observed to suffer increased oxidative DNA damage in comparison to younger cells. Taken together, these results suggest an age-related diminution in antioxidant function in Leydig cells. Compelling evidence highlights that the age-related decrease in steroidogenesis by Leydig cells is related to an impaired antioxidant system. Cao et al. observed that the expression of glutathione (GSH), superoxide dismutase (Cu-Zn-SOD), superoxide dismutase 2 (SOD2), and glutathione peroxidase 1 (GPX-1) was reduced in Leydig cells isolated from old Sprague-Dawley rats. The only observable exception was the expression of catalase [140]. In support of these results, Lao et al. reported that total SOD and GPX activities were remarkably decreased in Leydig cells isolated from aged Brown Norway rats. In addition, Cu-Zn-SOD, SOD2, GPX, and GSH protein expression was decreased, although to a lesser extent as compared to the decrease in the activity of these enzymes [141]. Further evidence was obtained from studies conducted using antioxidant treatments. Chen et al. observed that the administration of the antioxidant vitamin E to Brown Norway rats, starting at six months and continuing until age twenty-five, attenuated the age-related reduction in testosterone production [142], whereas Aydın et al. reported that intraperitoneal injection of carnosine (250 mg/kg) 5 days a week for 2 months, reverted the increase in testicular ROS production and the oxidative stress-induced damage in a D-gal ageing model of Wistar rats [143]. In a later study, Chen et al. reported that treatment with buthionine sulfoximine (BSO), an inhibitor of GSH synthesis, decreased GSH content by 70% and testosterone production by 40% in Leydig cells isolated from adult Brown Norway rats. Similar results were observed in vivo as BSO injections twice a day for seven days into both young and aged Brown Norway rats led to a decrease in GSH content by 70%, in testosterone production by 50%, and a significant decrease in LH-stimulated cAMP production and steroidogenic enzymes [144]. Huang et al. studied the effects of testicular ageing in male C57BL/6J mice and observed a progressive reduction in the expression of two antioxidant enzymes (Mn-SOD and GPX-4) and sirtuin 1 (SIRT1). Simultaneously, the number of testicular macrophages were found to be higher in aged mice [145]. Chung et al. also observed that not only the expression of SIRT1 but also that of the nuclear factor erythroid 2–related factor 2 (NRF2) was decreased in Leydig cells from aged Brown Norway rats [146]. Interestingly, when aged Leydig cells were incubated with nicotinamide, a SIRT1 inhibitor, an increase in ROS levels and a decrease in testosterone production was observed. Furthermore, when nicotinamide was removed from the medium, there was an increase in testosterone production. On the other hand, activation of SIRT1 and NRF2 by honokiol and sulforaphane, respectively, maintained testosterone production when cells were exposed to oxidative stress, induced by H2O2 and NaIO3 [146]. NRF2 is a transcriptional factor that regulates the expression of several antioxidant enzymes and thus a major regulator of the cellular antioxidant system [147, 148]. An interesting study with a Nrf2 −/− C57BL/6 mouse model conducted by Chen et al. demonstrated that serum testosterone levels and Leydig cell testosterone production were significantly reduced from middle age (eight months) to accentuated reduction at old age (21–24 months) as compared to wild-type mice [149]. Taken together, ageing seems to be related to increased incidence of chronic inflammation and to impair the antioxidant system, leading to accumulation of ROS in the testis and consequent reduction of Leydig cells’ steroidogenesis.
3.2 Testicular ageing affects sertoli cells, germ cells, and spermatogenesis
Mounting evidence supports that human ageing is linked to male reproductive function decline and decreased sperm quality. Ageing is associated with alterations in the morphology and composition of the testis, decreased germ cell number, and impaired spermatogenesis which results in reduced sperm motility and abnormal morphology [2, 3]. In a similar fashion to humans, animal models of reproductive ageing display testicular alterations, impaired spermatogenesis and reduced sperm quality.
Studies in reproductive ageing models seek to determine whether the effects on spermatogenesis and sperm quality are due to continuous division of spermatogonial stem cells and consequent damage accumulation and/or from defects in the supporting Sertoli and myoid cells. Spermatozoa are continuously being generated in the seminiferous tubules of adult males. Spermatogenesis starts with the mitotic division of spermatogonia, that act as stem cells (also known as spermatogonial stem cells). Spermatogonial stem cells are present throughout the lifespan of the male organism and their viability and mitotic capacity are affected by ageing, exhibiting a reduction in numbers in an age-dependent manner [150]. Zhang et al. observed that the number and quality of spermatogonia decreased with age in ROSA26 mice. Interestingly, spermatogenesis was still arrested when spermatogonia isolated from younger mice were transplanted into the testis of older animals, suggesting that the supporting environment had also declined [151]. Kanatsu-Shinohara et al. isolated aged spermatogonial stem cells from male C57BL/6 mice and observed that they were hyperproliferative, which was related to short telomeres, decreased mitochondrial function, and higher glycolytic profile. In addition, an age-dependent loss of sperm-forming potential was observed. These phenotypes were later confirmed in both mouse (Klotho KO mice) and rat (Brown Norway) [152]. Levy et al. observed that the seminiferous tubules of older Brown Norway rats were devoid of germ cells and contained large intercellular spaces [153]. Alterations in aged Sertoli cells were also reported, including complete loss of cyclical variations of the organelles, irregularly shaped nuclei, and altered morphology of the endoplasmic reticulum and lysosomes. In addition, tight junctions between adjacent Sertoli cells that constitute the blood-testis barrier were rarely identified in older rats. Taken together, these results led the authors to suggest that the degeneration of germ cells were due to immunological aetiology or impaired Sertoli cell support. In support of these results, Syed and Hecht also observed impaired Sertoli cell-germ cell interactions in aged Brown Norway rats [154]. Wang et al. found an accentuated decrease in the total number of Sertoli cells per testis in old Brown Norway rats (30 months) [42]. In addition, an age-related decrease in seminiferous tubules diameter, length, volume and ultimately proportion of testis occupation was reported. The testicular sperm concentration and total sperm production were also significantly reduced in older rats. In another study by the same group, Wang et al. demonstrated that germ cell loss associated with ageing occurs via apoptosis, which was found to be independent of hypothalamic-pituitary dysregulation as LH administration did not decrease or delay testicular function decline [40]. Also in the Brown Norway rat, Syntin and Robaire observed an age-dependent incidence of morphologically abnormal spermatozoa and decreased percentage of motile spermatozoa in the cauda of the epididymis, suggesting defects during spermatogenesis and sperm maturation [155]. Rebourcet et al. observed that the size of the Sertoli cell population that forms during development in mice determines the number of germ cells and Leydig cells that will be present in the adult testis. In addition, the number of germ cells and Leydig cells that can be maintained in the adult depends directly on the size of the adult Sertoli cell population, which led these authors to develop a predictive model of the testis cell composition based on Sertoli cell population size [156]. Fabricant and Parkening observed an age-related increase in the percentage of morphologically abnormal spermatozoa in the C57BL/6 mice. In addition, the testis of older mice was smaller and presented a thicker tunica albuginea [157]. Nakano et al. reconstructed the seminiferous tubules of younger and older C57BL/6 mice and observed age-related alterations in spermatogenesis due to the formation of vacuoles in Sertoli cells [158]. Interestingly, these alterations were more pronounced close to the rete testis in comparison to the centre of the testis. Gosden et al. found that older CBA/Ca mice (20–23 months old) had substantially lower circulating levels of testosterone and smaller testis. Testicular and epididymal spermatozoa were reduced in number, had more morphologically abnormal spermatozoa and fewer exhibit progressive motility [159]. Overall, these studies indicate that not only does testicular ageing affect Sertoli cells and germ cells but also spermatogenesis.
3.2.1 Age-dependent oxidative stress and DNA damage accumulation
Oxidative stress is increased in the aged testis and consequently affects both steroidogenesis and spermatogenesis. Compelling evidence demonstrates oxidative stress is related to diminished male fertility, which is exacerbated with age due to increased ROS production and decreased antioxidant defences. Noblanc et al. found that both oxidative DNA damage and lipid peroxidation suffer an age-dependent increase in the epididymis of Sod1 knockout mice [160]. Weir and Robaire observed that antioxidant defences are impaired in spermatozoa from older Brown Norway rats. GPX expression and activity were decreased, and SOD activity was also diminished. In addition, excessive production of ROS was described in spermatozoa from older rats. This excessive production of ROS and decreased antioxidant capacity resulted in unbalanced redox homeostasis and led to increased lipid peroxidation [161]. Selvaratnam et al. found that germ cells from aged Brown Norway rats display an earlier decline in viability, elevated levels of ROS, and increased spermatocyte DNA damage as compared to young cells. The expression of DNA damage repair genes was also increased in germ cells from older rats exposed to oxidative stress, indicating that a redox dysfunction occurs in aged germ cells which results in greater DNA damage and, thus, an increased demand for DNA repair [162]. In another study by the same group, Selvaratnam et al. observed that mice overexpressing catalase did not exhibit age-dependent loss of spermatozoa or loss of testicular and Sertoli cells [163]. In addition, mice overexpressing catalase had lower levels of ROS and enhanced DNA repair mechanisms, although it appears to fade in advanced age. In turn, aged wild-type animals presented increased oxidative stress-induced DNA damage. Conversely, Paul et al. isolated pachytene spermatocytes and round spermatids from young (4 months old) and older (18 months old) Brown Norway rats and observed a decreased expression of genes related to DNA repair pathways, suggesting that aged germ cells and spermatozoa have an impaired DNA repair mechanisms [164]. In a later study, the same group reported that aged Brown Norway rats had deteriorated spermatogonial stem cells with downregulation of genes related to mitosis and DNA repair, which would compromise spermatogenesis [165].
Compelling evidence demonstrates that several genes and ROS-related pathways play a function in testicular ageing. Nakamura et al. studied the effects of Nrf2 gene deletion on testicular oxidative stress and fertility. NRF2 is a regulator of the cellular antioxidant system, by modulating the expression of several antioxidant enzymes. Deletion of the Nrf2 gene led to a decrease in testicular and epididymal antioxidant levels, whereas lipid peroxidation and germ cell apoptosis were increased. Testicular and epididymal sperm counts and epididymal sperm motility suffered an age-dependent reduction in Nrf2−/− mice. Thus, transcription factor NRF2 seems to play an important role in preventing age-related testicular oxidative stress and its deleterious effects on spermatogenesis [166]. Ozkosem et al. studied spermatozoa from Prdx6 knockout mice and observed an age-dependent reduction in sperm quality. Peroxiredoxin 6 (PRDX6) is an antioxidant enzyme that has both GPX and Ca2+-independent phospholipase A2 activities and protects the cells from oxidative stress-mediated damage. Sperm DNA fragmentation and oxidation were increased, whereas sperm DNA protamination and compaction were decreased in spermatozoa from aged males Prdx6−/− mice. The absence of PRDX6 also caused an age-dependent impairment in sperm maturation and diminished sperm motility. In addition, Prdx6−/− aged males revealed a decrease in fertility, indicated by fewer litters and smaller litter sizes, when compared to wild-type. These results suggest an important role of PRDX6 in the management of age-associated oxidative stress [167]. Smith et al. found that thioredoxin domain-containing proteins (TXNDC) 2 and 3, which participate in the regulation of the redox status of spermatozoa, play an important role in the protection against oxidative stress in sperm from aged mice. TXNDC deficient spermatozoa were found to suffer from an age-dependent increase in ROS production, DNA damage and lipid-aldehyde-protein adducts, a reduction in sperm motility, as well as an impairment in sperm chromatin protamination [168]. Taken together, an age-related increase in oxidative stress, which is associated with an impaired antioxidant system, is responsible for diminished reproductive potential and accumulation of DNA damage.
3.3 Age-induced epigenetic alterations in spermatozoa
The epigenome is a record of nucleic acid and histone alterations in an organism, including DNA methylation, histone modifications, chromatin accessibility, and alterations in small RNA expression. The epigenome can regulate gene expression which can have a transgenerational effect, i.e. can be passed onto the next generation following fertilization [169]. Lifestyle habits, diet, external environment, exposure to drugs and pollutants, and other biological processes including ageing are known to induce epigenetic alterations in spermatozoa which can be transmitted to the offspring [170]. Spermatozoa are described as a unique cell type with a highly specialized epigenome (as reviewed by Jenkins et al. [171]). Although the epigenome is very sensitive to several factors, the epigenetic alterations and their regulatory consequences for the cell or its transgenerational effects are still poorly understood. The incidence of advanced paternal age is growing in the Western culture due to socioeconomic pressures, which significantly affects sperm parameters and induce epigenetic signatures that may affect the offspring. While the number of studies focusing on epigenetic alterations in human spermatozoa is steadily increasing due to growing scientific interest and potential translational applications, transgenerational studies are nearly unfeasible in humans due to the time gap between generations, confusion with genetic, ecological and cultural inheritance, and several others ethical and scientific issues [172]. Thus, transgenerational studies are mainly conducted in animal models. The main advantages are the short time gap between generations and the possibility to study multiple generations, strict environmental and dietary control, decreased economic weight and ethical issues [173, 174].
DNA methylation patterns are a notably unique feature of the sperm epigenome, which reflects and modulates sperm function [171]. Methylation occurs at cytosine residues found at cytosine phosphate guanine dinucleotides (CpGs), and its occurrence or absence can regulate the transcription of genes [175]. Moreover, compelling evidence suggests that DNA methylation signatures result in the propagation of heritable characteristics. For instance, advanced paternal age is reported to confer methylation profiles that increase the risk of incidence of some forms of cancer [176,177,178]. In addition, advanced paternal age is also associated with neuropsychiatric disorders in the offspring. There is evidence that older fathers are positively linked to an increased risk of schizophrenia in the offspring [179, 180]. Autism is also positively correlated with increased paternal age [181, 182]. Interestingly, some animal models were able to replicate these neuropsychiatric phenotypes. Milekic et al. compared the DNA methylation profile from young and old male 129SvEv/Tac mice and observed a significant loss of methylation related to transcriptional regulation in the older group. Besides, the offspring had similar DNA methylation alterations in the brain that were associated with behaviour changes and transcriptional dysregulation of developmental genes implicated in autism and schizophrenia [183]. In support of these results, a previous study by Smith et al. also observed reduced social and exploratory behaviour in the offspring of older male C57BL/6J mice as compared to younger fathers [184].
Beyond the potential neuropsychiatric consequences, advanced paternal age is also linked with several other transgenerational effects. Xie et al. observed a reduced lifespan and an exacerbated development of ageing traits in the offspring of older male C57BL/6JRj mice as compared to the offspring mice of young fathers [185]. In addition, several differentially methylated promoters of genes involved in the regulation of evolutionarily conserved longevity pathways and increased activity of the mTORC1 pathway were observed. Interestingly, mTOR inhibition by rapamycin mitigated the observed development of ageing traits in the offspring of older mice [185]. Oakes et al. compared the methylation profile in spermatozoa and liver of young and old Brown Norway rats and observed age-dependent hypermethylation of ribosomal DNA, which led the authors to suggest that spermatozoa are vulnerable to age-dependent DNA methylation alterations that could lead to abnormalities in fertility and progeny outcomes [186].
Although few studies are found in the literature, age-related alterations in miRNA expression have also been reported. In a recent study, Han et al. performed a transcriptome analysis of testes in C57BL/6 mice (3, 6, 12, and 18 months old) and observed that the expression of 1571 mRNAs and 715 lncRNAs changed during testicular ageing, from which 46 mRNAs and 34 lncRNAs were identified as stringently related to the terminal stage of male reproductive ageing [187]. Interestingly, some of the identified RNAs are related to hormonal regulation. In Wistar rats, Suvorov et al. detected an altered expression of 249 miRNA, 908 piRNA and 227 tRNA-derived RNA in aged as compared to younger animals [188]. Curiously, Matsushima et al. found no differences between miRNAs and tRNAs in spermatozoa from aged as compared to young house sparrow [189]. Even fewer studies addressed the functional or regulatory activity of these RNAs. Ma et al. observed that miR-574 was upregulated in the sperm of aged C57BL/6 mice, which was linked to subpar sperm motility potentially due to suppressed mitochondrial function and reduced ATP production [190]. Wu et al. identified 10 differently expressed miRNA in spermatozoa from younger as compared to aged bulls, which were related to embryo development and metabolism and protein synthesis in blastocysts [191]. Still, further research in age-related epigenetic alterations is needed to clarify the role of these RNAs and identify potential biomarkers of age-related dysfunctions.
4 Negligible senescence animal models: the future for reproductive ageing studies?
Most of our current knowledge and the research focused on the mechanisms of reproductive ageing have been performed using the aforementioned classical models such as the mouse (Mus musculus) or rat (Rattus norvegicus). As aforementioned discussed, the use of these animal models is inherent to their well-known advantages. Yet, these models exhibit several drawbacks – the narrowing of the ageing process to fewer species, which impairs a broader understanding of the mechanisms that determine ageing, and mice and rats have a considerably short life expectancy and age faster than theoretically expected, representing negative outliers to the maximum longevity theories that correlate lifespan and body size [192]. Besides, whether the molecular ageing mechanisms described in these rodent species can be extrapolated to humans is unclear, as life-prolonging effects developed for short-lived species do not exhibit the same effects in the long-lived ones [193].
Negligible senescence was first introduced by the biogerontologist Caleb Finch to connote organisms that do not demonstrate senescence, i.e., reduction of their reproductive capability, functional decline, or rising death rates with age [194, 195]. As some of these organisms maintain their reproductive capability for longer, the characterization of the molecular mechanisms underlying these processes and its comparison to the classical ageing models are of high interest. For instance, older lobsters of the genus Homarus, which are estimated to reach 50 years in the wild and up to 100 years in captivity [196], are thought to be more fertile than their younger counterparts [197]. Interestingly, their telomerase activity is high in adult specimens and all tissues [27]. Another interesting species is the ocean quahog of the North Atlantic (Arctica islandica), whose highest reported lifespan was of 507 years. This long-lived bivalve clam has the characteristic of reaching sexual maturation rapidly, without exhibiting significant biological ageing for decades [198]. A high antioxidant capacity is reported in this species which stabilizes at the age of 30 and do not decline further with age [199, 200].
Among mammals, mole-rats from the Fukomys and Heterocephalus genera attract gerontologists the most due to their high longevity. Fukomys species present a lifespan of more than 20 years [201, 202], which has been posited to be due to the great proteasome activity and high stability of gene expression during ageing [203, 204], particularly among genes related to mitonuclear balance, protein synthesis, autophagy, inflammation, and resistance to hypoxia or cancer [205, 206]. A unique feature among Fukomys species is the fact that breeders live about twice as long as their non-reproductive conspecifics, which brings the opportunity to study intra-species comparisons [201, 207]. On the other hand, naked mole-rats (Heterocephalus glaber) are the rodents with the highest lifespan, exceeding 30 years [208]. It is reported that ageing and age-related complications are virtually absent for 80% of their lifespan [209]. Notably, naked mole-rats exhibit a sustained fecundity and an age-independent mortality rate, leading some authors to suggest that this species is the first known mammal with negligible senescence [28]. Some authors, nevertheless, consider these assumptions premature [210]. Similarly to the Fukomys species, naked mole-rats exhibit higher cancer resistance [211], efficient DNA damage repair [212, 213], higher autophagy rates of damaged cells [214], proteasome activity [215], and telomere and epigenome maintenance [216, 217]. Compelling evidence highlights that naked mole-rats challenge the free radical theory of ageing, as ROS production in this species is unexpectedly high [218]. Mitochondrial protein expression and function, and ROS production are maintained during their lifespan [219,220,221]. Consequently, oxidation levels of lipid, protein and DNA are higher than those observed in mice [222, 223], although naked mole-rats have a more pronounced antioxidant system [218]. In addition, there is evidence that suggests that ROS do not cause damage on cell membranes and arteries from naked mole-rats [224, 225], which renders these mammals interesting models for the study of protection against oxidative stress.
While the maintenance and breeding of mole-rats in captivity are relatively easy, it has some drawbacks. Generation times are fairly long and these species produce small litters. Mean litter sizes are of 2–3 animals, which only reach full maturity in the second year of life. In addition, the complex social system and the loss of the queen may lead to longer periods of social instability without reproduction, which could lead to the death of many individuals due to fights for successorship [174]. The captivity and manipulation of these species, therefore, are more challenging when compared with the classical mice or rat models.
5 Conclusion and future perspectives
Studying the biological and physiological processes concerning human ageing has several clinical and socioeconomic potential advantages. The population of Western countries is getting older due to decreased natality rates, lifestyle, and crescent preoccupation and social pressure for career-driven life choices. Thus, the average age is slowly increasing and its study will bring novel therapies and improve healthcare, increasing the quality of life for elder people. At the same time, paternal age is increasing. Advanced paternal age has consequences for the offspring and the risk of development of several neuropsychiatric conditions has been documented. Nevertheless, the full extent of advanced paternal age-related consequences is unknown. More than molecular and chemical, age-related genetic and epigenetic alterations in the germ cell line demand further research.
Most of the current knowledge on male reproductive ageing is collected from ageing animal models, the majority from rodent species. The study of these animal models has several advantages, including standardization, ease of manipulation, lower time between generations, and reduced costs. The Brown Norway rat is considered the cornerstone for the male reproductive ageing study as it is most proximal to the human male reproductive ageing condition and its breeding costs are low. Albeit rodents and humans share a high genetic similarity [226], significant differences exist. While human studies have also been conducted, the lack of standardization and unfeasibility to control genetic backgrounds and invasiveness of the methods hamper the construction of solid conclusions. For this purpose, studies with a large number of subjects will provide valuable data.
An interesting approach for the male reproductive ageing study is to investigate and characterize the tail of the coin. Animals with negligible senescence do not undergo age-related reproductive decline. Yet, several questions arise and would result in compelling results: why does it occur? What are the molecular mechanisms that regulate their steady reproductive capability? How do they cope with damage accumulation and oxidative stress? The naked mole-rat, the only potential mammal with negligible senescence could become a very attractive ageing (or anti-ageing) animal model that will provide valuable data for ageing studies. Fortuitously, novel therapeutic targets that are likely to result in innovative therapies against advanced paternal age and age-related male reproductive consequences are also expected to be set in the future.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- BSO:
-
buthionine sulfoximine
- COX-2:
-
arachidonic acid/cyclooxygenase-2
- D-gal:
-
D-galactose
- EDS:
-
ethane dimethanesulfonate
- FSH:
-
follicle-stimulating hormone
- GnRH:
-
gonadotropin-releasing hormone
- GPX:
-
glutathione peroxidase
- GSH:
-
glutathione
- hCG:
-
human chorionic gonadotropin
- HPG:
-
hypothalamus-pituitary-gonad
- HSD17B:
-
17β-hydroxysteroid dehydrogenase
- HSD3B:
-
3β-hydroxysteroid dehydrogenase
- IL-1β:
-
interleukin 1 beta
- LH:
-
luteinizing hormone
- NRF2:
-
nuclear factor erythroid 2–related factor 2
- PKA:
-
protein kinase A
- ROS:
-
reactive oxygen species
- SAMP:
-
senescence-accelerated mouse prone
- SIRT1:
-
sirtuin 1
- SOD:
-
superoxide dismutase
- StAR:
-
steroidogenic acute regulatory protein
- TSPO:
-
translocator protein
- PRDX6:
-
peroxiredoxin 6
- TXNDC:
-
thioredoxin domain-containing proteins
References
Gilbert SF. Ageing: the biology of senescence, in Developmental Biology. Sinauer Associates: Sunderland (MA); 2000.
Johnson L, et al. Increased germ cell degeneration during postprophase of meiosis is related to increased serum follicle-stimulating hormone concentrations and reduced daily sperm production in aged men. Biol Reprod. 1990;42(2):281–7.
Johnson L, et al. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod. 1984;31(4):785–95.
Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11(4):cp13–3.
Lopes AC, Oliveira PF, Sousa M. Shedding light into the relevance of telomeres in human reproduction and male factor infertilitydagger. Biol Reprod. 2019;100(2):318–30.
Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14(1):28–34.
Nguyen-Powanda P, Robaire B. Oxidative Stress and Reproductive Function in the Aging Male. Biology (Basel). 2020;9(9):282.
Gerschman R, et al. Studies on oxygen poisoning: protective effect of beta-mercaptoethylamine. Proc Soc Exp Biol Med. 1954;85(1):75–7.
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300.
Effros RB. Roy Walford and the immunologic theory of aging. Immun Ageing. 2005;2(1):7.
Diggs J. Autoimmune Theory of Aging. Encyclopedia of Aging and Public Health. Boston: Springer US; 2008. pp. 143–4.
López-Otín C, et al. Hallm Aging Cell. 2013;153(6):1194–217.
Office-for-National-Statistics. Birth characteristics in England and Wales: 2019. Editor: UK-Statistics-Authority; 2019.
Khandwala YS, et al., The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod, 2017. 32(10): p. 2110–2116.
Sharma R, et al. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13(1):35.
Toriello HV, et al. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10(6):457–60.
Kovac JR, et al. The effects of advanced paternal age on fertility. Asian J Androl. 2013;15(6):723–8.
Jaffe AE, et al. Paternal age, de novo mutations and schizophrenia. Mol Psychiatry. 2014;19(3):274–5.
Torrey EF, et al. Paternal age as a risk factor for schizophrenia: how important is it? Schizophr Res. 2009;114(1–3):1–5.
Frans EM, et al. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65(9):1034–40.
Nybo Andersen AM, Urhoj SK. Is advanced paternal age a health risk for the offspring? Fertil Steril. 2017;107(2):312–8.
Girirajan S. Parental-age effects in Down syndrome. J Genet. 2009;88(1):1–7.
Abbas HA, et al. Effects of Advanced Paternal Age on Reproduction and Outcomes in Offspring. NeoReviews. 2015;16(2):e69–83.
Taormina G, et al. Longevity: Lesson from Model Organisms. Genes (Basel). 2019;10(7):518.
He J, et al. Life Cycle Reversal in Aurelia sp.1 (Cnidaria, Scyphozoa). PLoS ONE. 2015;10(12):e0145314.
Tan TC, et al. Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms. Proc Natl Acad Sci U S A. 2012;109(11):4209–14.
Klapper W, et al. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett. 1998;439(1–2):143–6.
Ruby JG, Smith M, Buffenstein R. Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. elife. 2018;7:e31157.
Jones OR, et al. Diversity of ageing across the tree of life. Nature. 2014;505(7482):169–73.
Cohen AA. Aging across the tree of life: The importance of a comparative perspective for the use of animal models in aging. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt A):2680–9.
Bryda EC. The Mighty Mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207–11.
Hickman DL, et al., Commonly Used Animal Models. Principles of Animal Research for Graduate and Undergraduate Students, 2017: p. 117–175.
Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225.
Hasty P, et al. Aging and genome maintenance: lessons from the mouse? Science. 2003;299(5611):1355–9.
McBride JA, Carson CC 3rd, and Coward RM. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8(1):47–60.
Johnson L, Petty CS, Neaves WB. Influence of age on sperm production and testicular weights in men. J Reprod Fertil. 1984;70(1):211–8.
Harris ID, et al. Fertility and the aging male. Rev Urol. 2011;13(4):e184-90.
Zirkin BR, et al. Testicular Steroidogenesis in the Aging Brown Norway Rat. J Androl. 1993;14(2):118–23.
Gruenewald DA, et al. The Brown Norway Rat as a Model of Male Reproductive Aging: Evidence for Both Primary and Secondary Testicular Failure. J Gerontol. 1994;49(2):B42–50.
Wang C, et al. Reproductive aging in the Brown Norway rat is characterized by accelerated germ cell apoptosis and is not altered by luteinizing hormone replacement. J Androl. 1999;20(4):509–18.
Wang C, et al. Male Reproductive Ageing: Using the Brown Norway Rat as a Model for Man. Novartis Found Symp. 2002;242:82–95. discussion 95.
Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology. 1993;133(6):2773–81.
Wright WW, Fiore C, Zirkin BR. The effect of aging on the seminiferous epithelium of the brown Norway rat. J Androl. 1993;14(2):110–7.
Taylor I, Mowat V. Comparison of longevity and common tumor profiles between Sprague-Dawley and Han Wistar rats. J Toxicol Pathol. 2020;33(3):189–96.
James R, Heywood R. Age-related variations in the testes of sprague-dawley rats. Toxicol Lett. 1979;4(4):257–61.
Kaler LW, Neaves WB. The androgen status of aging male rats. Endocrinology. 1981;108(2):712–9.
Bethea CL, Walker RF. Age-related changes in reproductive hormones and in Leydig cell responsivity in the male Fischer 344 rat. J Gerontol. 1979;34(1):21–7.
Petersen PM, Seierøe K, Pakkenberg B. The total number of Leydig and Sertoli cells in the testes of men across various age groups - a stereological study. J Anat. 2015;226(2):175–9.
Mularoni V, et al. Age-related changes in human Leydig cell status. Hum Reprod. 2020;35(12):2663–76.
Turek FW, Desjardins C. Development of Leydig cell tumors and onset of changes in the reproductive and endocrine systems of aging F344 rats. J Natl Cancer Inst. 1979;63(4):969–75.
Goodman DG, et al. Neoplastic and nonneoplastic lesions in aging F344 rats. Toxicol Appl Pharmacol. 1979;48(2):237–48.
Gruenewald DA, et al. Excessive testicular progesterone secretion in aged male Fischer 344 rats: a potential cause of age-related gonadotropin suppression and confounding variable in aging studies. J Gerontol. 1992;47(5):B164-70.
Gruenewald DA, Matsumoto AM. Age-related decreases in serum gonadotropin levels and gonadotropin-releasing hormone gene expression in the medial preoptic area of the male rat are dependent upon testicular feedback. Endocrinology. 1991;129(5):2442–50.
Sokanovic SJ, et al. Age related changes of cAMP and MAPK signaling in Leydig cells of Wistar rats. Exp Gerontol. 2014;58:19–29.
Tucker MJ. Diseases of the Wistar Rat. CRC Press; 1997.
Auroux M, Nawar NN, Rizkalla N. Testicular aging: vascularization and gametogenesis modifications in the Wistar rat. Arch Androl. 1985;14(2–3):115–21.
Barsoum NJ, et al. Morphofunctional investigations on spontaneous pituitary tumors in Wistar rats. Toxicol Pathol. 1985;13(3):200–8.
Fabricant JD, Parkening TA. Sperm morphology and cytogenetic studies in ageing C57BL/6 mice. J Reprod Fertil. 1982;66(2):485–9.
Lessard-Beaudoin M, et al. Characterization of age-associated changes in peripheral organ and brain region weights in C57BL/6 mice. Exp Gerontol. 2015;63:27–34.
Lacombe A, et al. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc Natl Acad Sci U S A. 2006;103(10):3793–8.
Gosden RG, et al. Structure and gametogenic potential of seminiferous tubules in ageing mice. J Reprod Fertil. 1982;64(1):127–33.
Brayton C. Spontaneous Diseases in Commonly Used Mouse Strains. In: Fox JG et al, editors. The Mouse in Biomedical Research. Burlington: Academic Press; 2007. pp. 623–717.
Kotarska K, et al. Aging deteriorates quality of sperm produced by male mice with partial Yq deletion. Syst Biol Reprod Med. 2017;63(6):360–3.
Azman KF, Zakaria R. D-Galactose-induced accelerated aging model: an overview. Biogerontology. 2019;20(6):763–82.
Liao CH, et al. Optimizing a Male Reproductive Aging Mouse Model by D-Galactose Injection. Int J Mol Sci. 2016;17(1):98.
Hosokawa M. A higher oxidative status accelerates senescence and aggravates age-dependent disorders in SAMP strains of mice. Mech Ageing Dev. 2002;123(12):1553–61.
Zhao X, et al. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp Gerontol. 2013;48(9):869–80.
Flood JF, et al. Age-related decrease of plasma testosterone in SAMP8 mice: replacement improves age-related impairment of learning and memory. Physiol Behav. 1995;57(4):669–73.
Mattison JA, Vaughan KL. An overview of nonhuman primates in aging research. Exp Gerontol. 2017;94:41–5.
Black A, Lane MA. Nonhuman primate models of skeletal and reproductive aging. Gerontology. 2002;48(2):72–80.
Phillips KA, et al. Why primate models matter. Am J Primatol. 2014;76(9):801–27.
Ramsey JJ, Laatsch JL, Kemnitz JW. Age and gender differences in body composition, energy expenditure, and glucoregulation of adult rhesus monkeys. J Med Primatol. 2000;29(1):11–9.
Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age (Omaha). 1997;20(1):1–13.
Ngwenya LB, et al. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci. 2015;9:102.
Robinson JA, et al. Effects of age and season on sexual behavior and plasma testosterone and dihydrotestosterone concentrations of laboratory-housed male rhesus monkeys (Macaca mulatta). Biol Reprod. 1975;13(2):203–10.
Chambers KC, Hess DL, Phoenix CH. Relationship of free and bound testosterone to sexual behavior in old rhesus males. Physiol Behav. 1981;27(4):615–20.
Kaler LW, et al. The androgen status of aging male rhesus macaques. Endocrinology. 1986;119(2):566–71.
Eaton GG. Longitudinal Studies of Sexual Behavior in the Oregon Troop of Japanese Macaques, in Sex and Behavior. Springer US; 1978. pp. 35–59.
Matsubayashi K, Enomoto T, Nakano M. Preliminary Report on Histological Characteristics of Male Reproductive Organs in Senile Japanese Macaques. Anthropol Sci. 1994;102(Supplement):199–205.
Hamada Y, et al. Changes in testicular and nipple volume related to age and seasonality in Japanese macaques (Macaca fuscata), especially in the pre- and post-pubertal periods. Primates. 2005;46(1):33–45.
Abbott DH, et al. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53(4):339–50.
Tardif SD, et al., Reproduction and aging in marmosets and tamarins, in Primate reproductive aging. 2008, Karger Publishers. p. 29–48.
Lavery WL. How relevant are animal models to human ageing? J R Soc Med. 2000;93(6):296–8.
Ezran C, et al. The Mouse Lemur, a Genetic Model Organism for Primate Biology, Behavior, and Health. Genetics. 2017;206(2):651–64.
Languille S, et al. The grey mouse lemur: a non-human primate model for ageing studies. Ageing Res Rev. 2012;11(1):150–62.
Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8(1):1–28.
Aghazadeh Y, Zirkin BR, Papadopoulos V. Pharmacological regulation of the cholesterol transport machinery in steroidogenic cells of the testis. Vitam Horm. 2015;98:189–227.
Midzak A, et al. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem. 2011;286(11):9875–87.
Midzak A, Zirkin B, Papadopoulos V. Translocator protein: pharmacology and steroidogenesis. Biochem Soc Trans. 2015;43(4):572–8.
Wang Y, et al. Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction. 2017;154(4):R111–22.
Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–70.
Beattie MC, et al. Leydig cell aging and hypogonadism. Exp Gerontol. 2015;68:87–91.
Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod. 2001;64(3):735–42.
Huhtaniemi IT. Andropause–lessons from the European male ageing study. in Annales d’endocrinologie. Elsevier; 2014.
Harman SM, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31.
Braga PC, et al. Late-onset hypogonadism and lifestyle-related metabolic disorders. Andrology. 2020;8(6):1530–8.
Carrageta DF, et al. Obesity and male hypogonadism: Tales of a vicious cycle. Obes Rev. 2019;20(8):1148–58.
Wu D, Gore AC. Changes in androgen receptor, estrogen receptor alpha, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58(2):306–16.
Smith ER, et al. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992;26(1):110–35.
Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434.
Veldhuis JD, et al. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302(1):E117-22.
Pincus SM, et al., Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proceedings of the National Academy of Sciences, 1996. 93(24): p. 14100–14105.
Chen H, et al. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15(6):551–7.
Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124(2):762–70.
Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143(5):1637–42.
Hsueh AJ, Dufau ML, Catt KJ. Gonadotropin-induced regulation of luteinizing hormone receptors and desensitization of testicular 3’:5’-cyclic AMP and testosterone responses. Proc Natl Acad Sci U S A. 1977;74(2):592–5.
Liao C, Reaven E, Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J Steroid Biochem Mol Biol. 1993;46(1):39–47.
Chen H, et al. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004;145(10):4441–6.
Bakhtyukov AA, et al., Decrease in the Basal and Luteinizing Hormone Receptor Agonist–Stimulated Testosterone Production in Aging Male Rats. Advances in Gerontology, 2019. 9(2): pp. 179–85.
Sokanovic SJ, et al. Aging-Related Increase of cGMP Disrupts Mitochondrial Homeostasis in Leydig Cells. J Gerontol A Biol Sci Med Sci. 2021;76(2):177–86.
Luo L, Chen H, Zirkin BR. Are Leydig cell steroidogenic enzymes differentially regulated with aging? J Androl. 1996;17(5):509–15.
Luo L, Chen H, Zirkin BR. Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl. 2001;22(1):149–56.
Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl. 2005;26(1):25–31.
Culty M, et al. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 2002;23(3):439–47.
Clark BJ, et al. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269(45):28314–22.
Lin D, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267(5205):1828–31.
Castillo AF, et al. cAMP increases mitochondrial cholesterol transport through the induction of arachidonic acid release inside this organelle in Leydig cells. FEBS J. 2006;273(22):5011–21.
Wang X, et al. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology. 2005;146(10):4202–8.
Chen H, et al. Cyclooxygenases in rat Leydig cells: effects of luteinizing hormone and aging. Endocrinology. 2007;148(2):735–42.
Zhao Y, et al. The roles of p38 MAPK --> COX2 and NF-kappaB --> COX2 signal pathways in age-related testosterone reduction. Sci Rep. 2019;9(1):10556.
Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281(50):38879–93.
Chung JY, et al. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology. 2013;154(6):2156–65.
Chen F, et al. Acute effects of the translocator protein drug ligand FGIN-1-27 on serum testosterone and luteinizing hormone levels in male Sprague-Dawley ratsdagger. Biol Reprod. 2019;100(3):824–32.
Chung JY, et al. Effects of pharmacologically induced Leydig cell testosterone production on intratesticular testosterone and spermatogenesisdagger. Biol Reprod. 2020;102(2):489–98.
Tu LN, et al. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289(40):27444–54.
Fan J, et al. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci U S A. 2015;112(23):7261–6.
Papadopoulos V, et al. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol. 2015;408:90–8.
Chung JY, et al. Cholesterol accumulation, lipid droplet formation, and steroid production in Leydig cells: Role of translocator protein (18-kDa). Andrology. 2020;8(3):719–30.
Barron AM, et al. Steroidogenic abnormalities in translocator protein knockout mice and significance in the aging male. Biochem J. 2018;475(1):75–85.
Chen H, et al. Steroidogenic fate of the Leydig cells that repopulate the testes of young and aged Brown Norway rats after elimination of the preexisting Leydig cells. Exp Gerontol. 2015;72:8–15.
Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57(1–2):3–18.
Rehman A, Pacher P, Hasko G. Role of Macrophages in the Endocrine System. Trends Endocrinol Metab. 2021;32(4):238–56.
Maggio M, et al., The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest, 2005. 28(11 Suppl Proceedings): p. 116–119.
Frungieri MB, et al. Ageing and inflammation in the male reproductive tract. Andrologia. 2018;50(11):e13034.
Matzkin ME, et al. Cyclooxygenase-2 in testes of infertile men: evidence for the induction of prostaglandin synthesis by interleukin-1beta. Fertil Steril. 2010;94(5):1933–6.
Herb M, Schramm M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxid (Basel). 2021;10(2):313.
Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38(1–2):171–96.
Chen H, et al. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36(8):1361–73.
Beattie MC, et al. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013;88(4):100.
Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88(1):61–7.
Luo L, et al. Aging and the brown Norway rat leydig cell antioxidant defense system. J Androl. 2006;27(2):240–7.
Chen H, et al. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40(8–9):728–36.
Aydin AF, et al. Carnosine prevents testicular oxidative stress and advanced glycation end product formation in D-galactose-induced aged rats. Andrologia. 2018;50(3):e12939.
Chen H, et al. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008;149(5):2612–9.
Huang D, et al. Steroidogenesis decline accompanied with reduced antioxidation and endoplasmic reticulum stress in mice testes during ageing. Andrologia. 2018;50(1):e12816.
Chung J-Y, Chen H, Zirkin B. Sirt1 and Nrf2: regulation of Leydig cell oxidant/antioxidant intracellular environment and steroid formation. Biol Reprod. 2021;105(5):1307–16.
Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48(2):91–104.
Cho HY, et al. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med. 2005;38(3):325–43.
Chen H, et al. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Mol Cell Endocrinol. 2015;409:113–20.
Suzuki N, Withers HR. Exponential decrease during aging and random lifetime of mouse spermatogonial stem cells. Science. 1978;202(4373):1214–5.
Zhang X, et al. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74(1):119–24.
Kanatsu-Shinohara M, et al. Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proc Natl Acad Sci U S A. 2019;116(33):16404–9.
Levy S, et al. The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. J Androl. 1999;20(3):356–65.
Syed V, Hecht NB. Disruption of germ cell-Sertoli cell interactions leads to spermatogenic defects. Mol Cell Endocrinol. 2002;186(2):155–7.
Syntin P, Robaire B. Sperm structural and motility changes during aging in the Brown Norway rat. J Androl. 2001;22(2):235–44.
Rebourcet D, et al. Sertoli Cell Number Defines and Predicts Germ and Leydig Cell Population Sizes in the Adult Mouse Testis. Endocrinology. 2017;158(9):2955–69.
Fabricant JD, Parkening TA. Sperm morphology and cytogenetic studies in ageing C57BL/6 mice. Reproduction. 1982;66(2):485–9.
Nakano T, et al. Three-dimensional morphological analysis of spermatogenesis in aged mouse testes. Sci Rep. 2021;11(1):23007.
Gosden RG, et al. Structure and gametogenic potential of seminiferous tubules in ageing mice. Reproduction. 1982;64(1):127–33.
Noblanc A, Klaassen A, Robaire B. The Exacerbation of Aging and Oxidative Stress in the Epididymis of Sod1 Null Mice. Antioxid (Basel). 2020;9(2):151.
Weir CP, Robaire B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J Androl. 2007;28(2):229–40.
Selvaratnam J, Paul C, Robaire B. Male Rat Germ Cells Display Age-Dependent and Cell-Specific Susceptibility in Response to Oxidative Stress Challenges. Biol Reprod. 2015;93(3):72.
Selvaratnam J, Robaire B. Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production. Exp Gerontol. 2016;84:12–20.
Paul C, Nagano M, Robaire B. Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the Brown Norway rat. Biol Reprod. 2011;85(6):1269–78.
Paul C, Nagano M, Robaire B. Aging results in molecular changes in an enriched population of undifferentiated rat spermatogonia. Biol Reprod. 2013;89(6):147.
Nakamura BN, et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49(9):1368–79.
Ozkosem B, et al. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015;5:15–23.
Smith TB, et al. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic Biol Med. 2013;65:872–81.
Bernstein BE, Meissner A, Lander ES. The mammalian epigenome Cell. 2007;128(4):669–81.
Jenkins TG, Aston KI, Carrell DT. Sperm epigenetics and aging. Transl Androl Urol. 2018;7(Suppl 3):S328–35.
Jenkins TG, et al. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst Biol Reprod Med. 2017;63(2):69–76.
Horsthemke B. A critical view on transgenerational epigenetic inheritance in humans. Nat Commun. 2018;9(1):2973.
Phillips NLH, Roth TL. Animal Models and Their Contribution to Our Understanding of the Relationship Between Environments, Epigenetic Modifications, and Behavior. Genes (Basel). 2019;10(1):47.
Holtze S, et al. Alternative Animal Models of Aging Research. Front Mol Biosci. 2021;8:660959.
Moore LD, Le T, Fan G. DNA methylation and its basic function Neuropsychopharmacology. 2013;38(1):23–38.
Oksuzyan S, et al. Birth weight and other perinatal characteristics and childhood leukemia in California. Cancer Epidemiol. 2012;36(6):e359-65.
Murray L, et al. Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer. 2002;86(3):356–61.
Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35(6):1495–503.
Miller B, et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull. 2011;37(5):1039–47.
Naserbakht M, et al. Advanced paternal age is a risk factor for schizophrenia in Iranians. Ann Gen Psychiatry. 2011;10(1):15.
Idring S, et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol. 2014;43(1):107–15.
Frans EM, et al. Autism risk across generations: a population-based study of advancing grandpaternal and paternal age. JAMA Psychiatry. 2013;70(5):516–21.
Milekic MH, et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry. 2015;20(8):995–1001.
Smith RG, et al. Advancing paternal age is associated with deficits in social and exploratory behaviors in the offspring: a mouse model. PLoS ONE. 2009;4(12):e8456.
Xie K, et al. Epigenetic alterations in longevity regulators, reduced life span, and exacerbated aging-related pathology in old father offspring mice. Proc Natl Acad Sci U S A. 2018;115(10):E2348–57.
Oakes CC, et al. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc Natl Acad Sci U S A. 2003;100(4):1775–80.
Han G, et al. Transcriptome Analysis of Testicular Aging in Mice. Cells. 2021;10(11):2895.
Suvorov A, et al. Aging Induces Profound Changes in sncRNA in Rat Sperm and These Changes Are Modified by Perinatal Exposure to Environmental Flame Retardant. Int J Mol Sci. 2020;21(21):8252.
Matsushima W, et al. Mature sperm small-RNA profile in the sparrow: implications for transgenerational effects of age on fitness. Environ Epigenet. 2019;5(2):dvz007.
Ma J, et al. Mitochondria-related miR-574 reduces sperm ATP by targeting ND5 in aging males. Aging. 2020;12(9):8321–38.
Wu C, et al. Sperm miRNAs- potential mediators of bull age and early embryo development. BMC Genomics. 2020;21(1):798.
de Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci. 2007;62(2):149–60.
Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55(3):B117-23.
Finch CE. Longevity, senescence, and the genome. Chicago: University of Chicago Press; 1994.
Finch CE. Update on slow aging and negligible senescence–a mini-review. Gerontology. 2009;55(3):307–13.
Polinski JM, et al. The American lobster genome reveals insights on longevity, neural, and immune adaptations. Sci Adv. 2021;7(26):eabe8290.
Bodnar A. Marine invertebrates as models for aging research. Exp Gerontol. 2009;44(8):477–84.
Gruber H, et al. Age-related cellular changes in the long-lived bivalve A. islandica. Age (Dordr). 2015;37(5):90.
Munro D, Blier PU. The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging Cell. 2012;11(5):845–55.
Abele D, et al. Imperceptible senescence: ageing in the ocean quahog Arctica islandica. Free Radic Res. 2008;42(5):474–80.
Dammann P, Burda H. Sexual activity and reproduction delay ageing in a mammal. Curr Biol. 2006;16(4):R117-8.
Begall S, et al. Life expectancy, family constellation and stress in giant mole-rats (Fukomys mechowii). Philos Trans R Soc Lond B Biol Sci. 2021;376(1823):20200207.
Sahm A, et al. Higher gene expression stability during aging in long-lived giant mole-rats than in short-lived rats. Aging. 2018;10(12):3938–56.
Sahm A, et al. Increased longevity due to sexual activity in mole-rats is associated with transcriptional changes in the HPA stress axis. Elife. 2021;10:e57843.
Davies KT, et al. Family Wide Molecular Adaptations to Underground Life in African Mole-Rats Revealed by Phylogenomic Analysis. Mol Biol Evol. 2015;32(12):3089–107.
Sahm A, et al. Long-lived rodents reveal signatures of positive selection in genes associated with lifespan. PLoS Genet. 2018;14(3):e1007272.
Schmidt CM, Jarvis JUM, Bennett NC. The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis). Afr Zool. 2013;48(1):193–6.
Grimes KM, et al. Getting to the heart of the matter: age-related changes in diastolic heart function in the longest-lived rodent, the naked mole rat. J Gerontol A Biol Sci Med Sci. 2012;67(4):384–94.
Edrey YH, et al. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J. 2011;52(1):41–53.
Dammann P, et al. Comment on ‘Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age’. Elife. 2019;8:e45415.
Shepard A, Kissil JL. The use of non-traditional models in the study of cancer resistance-the case of the naked mole rat. Oncogene. 2020;39(28):5083–97.
Evdokimov A, et al. Naked mole rat cells display more efficient excision repair than mouse cells. Aging. 2018;10(6):1454–73.
MacRae SL, et al. DNA repair in species with extreme lifespan differences. Aging. 2015;7(12):1171–84.
Zhao S, et al. High autophagy in the naked mole rat may play a significant role in maintaining good health. Cell Physiol Biochem. 2014;33(2):321–32.
Rodriguez KA, et al. A cytosolic protein factor from the naked mole-rat activates proteasomes of other species and protects these from inhibition. Biochim Biophys Acta. 2014;1842(11):2060–72.
Tan L, et al. Naked Mole Rat Cells Have a Stable Epigenome that Resists iPSC Reprogramming. Stem Cell Reports. 2017;9(5):1721–34.
Adwan Shekhidem H, et al. Telomeres and Longevity: A Cause or an Effect? Int J Mol Sci. 2019;20(13):3233.
Munro D, et al. The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell. 2019;18(3):e12916.
Kim EB, et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479(7372):223–7.
Vyssokikh MY, et al. Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program. Proc Natl Acad Sci U S A. 2020;117(12):6491–501.
Holtze S, et al. Study of Age-Dependent Structural and Functional Changes of Mitochondria in Skeletal Muscles and Heart of Naked Mole Rats (Heterocephalus glaber). Biochem (Mosc). 2016;81(12):1429–37.
Andziak B, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5(6):463–71.
Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5(6):525–32.
Hulbert AJ, Faulks SC, Buffenstein R. Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. J Gerontol A Biol Sci Med Sci. 2006;61(10):1009–18.
Labinskyy N, et al. Comparison of endothelial function, O2–· and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291(6):H2698–704.
Yue F, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515(7527):355–64.
Gilad GM, Gilad VH. Age-related reductions in brain cholinergic and dopaminergic indices in two rat strains differing in longevity. Brain Res. 1987;408(1–2):247–50.
Cameron TP, et al. Longevity and reproductive comparisons for male ACI and Sprague-Dawley rat aging colonies. Lab Anim Sci. 1982;32(5):495–9.
Amador A, et al. Testicular LH receptors during aging in Fisher 344 rats. J Androl. 1985;6(1):61–4.
Kunstyr I, Leuenberger HG. Gerontological data of C57BL/6J mice. I. Sex differences in survival curves. J Gerontol. 1975;30(2):157–62.
Festing MF, Blackmore DK. Life span of specified-pathogen-free (MRC category 4) mice and rats. Lab Anim. 1971;5(2):179–92.
Takeda T, Higuchi K, Hosokawa M. Senescence-accelerated Mouse (SAM): With Special Reference to Development and Pathological Phenotypes. ILAR J. 1997;38(3):109–18.
Funding
This work was supported by the Portuguese Foundation for Science and Technology: D.F. Carrageta (SFRH/BD/136779/2018), M.G. Alves (PTDC/MEC-AND/28691/2017), UMIB (UIDB/00215/2020 and UIDP/00215/2020), ITR (LA/P/0064/2020) and QOPNA (UID/QUI/00062/2019) co-funded by FEDER funds (POCI/COMPETE 2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
The authors have no conflicts of interest to declare relevant to this article’s content.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David F. Carrageta and Bárbara Guerra-Carvalho contributed equally to this article.
Rights and permissions
About this article
Cite this article
Carrageta, D.F., Guerra-Carvalho, B., Spadella, M.A. et al. Animal models of male reproductive ageing to study testosterone production and spermatogenesis. Rev Endocr Metab Disord 23, 1341–1360 (2022). https://doi.org/10.1007/s11154-022-09726-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09726-9