Abstract
Obesity is the second most common cause of preventable morbidity worldwide. Resting-state functional magnetic resonance imaging (fMRI) has been used extensively to characterise altered communication between brain regions in individuals with obesity, though findings from this research have not yet been systematically evaluated within the context of prominent neurobiological frameworks. This systematic review aggregated resting-state fMRI findings in individuals with obesity and evaluated the contribution of these findings to current neurobiological models. Findings were considered in relation to a triadic model of problematic eating, outlining disrupted communication between reward, inhibitory, and homeostatic systems. We identified a pattern of consistently increased orbitofrontal and decreased insula cortex resting-state functional connectivity in individuals with obesity in comparison to healthy weight controls. BOLD signal amplitude was also increased in people with obesity across studies, predominantly confined to subcortical regions, including the hippocampus, amygdala, and putamen. We posit that altered orbitofrontal cortex connectivity may be indicative of a shift in the valuation of food-based rewards and that dysfunctional insula connectivity likely contributes to altered homeostatic signal processing. Homeostatic violation signals in obesity may be maintained despite satiety, thereby ‘hijacking’ the executive system and promoting further food intake. Moving forward, we provide a roadmap for more reliable resting-state and task-based functional connectivity experiments, which must be reconciled within a common framework if we are to uncover the interplay between psychological and biological factors within current theoretical frameworks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The last two decades have seen the emergence of a field of neuroimaging aiming to characterize the brain function of individuals with obesity. This examination has provided insight into the neural mechanisms that drive overeating and aspires to ultimately inform the development of successful treatment interventions [1]. Broadly speaking, altered neural activity and functional connectivity (FC) in individuals with obesity, when compared to controls, have consistently converged upon regions and networks linked to three processes: (i) reward processing [2], (ii) inhibitory control [3] and (iii) homeostatic regulation [4]. The mechanisms by which these processes give rise to unhealthy food choices remain unclear, but may be partly explained by theoretical models such as ‘the competing neural systems model’ [5, 6].
1.1 Competing reward, inhibitory and homeostatic systems
According to the competing neural systems model, food-related reward seeking overpowers inhibitory systems mediating the control of food intake, leading to suboptimal decision-making characterised by impulsive choices [7, 8]. Studies using functional magnetic resonance imaging (fMRI) have found that impulsive reactions to food stimuli are associated with increased activity in subcortical regions, such as the nucleus accumbens, amygdala and ventral striatum [7, 9,10,11]. Moreover, individuals with obesity are more likely to direct attentional resources to food vs. non-food stimuli, with attentional biases to palatable foods being associated with heightened activation in the orbitofrontal cortex (OFC) [12, 13]. The OFC is known to selectively activate when processing of rewarding stimuli [14] and to drive overeating by assigning a higher value to somatic markers (e.g., ‘wanting’ hunger signals; [15, 16]. In contrast, inhibitory responses to reward are understood to be mediated by activity in lateral prefrontal and posterior parietal regions [7, 17]. These regions are incident to prefrontal and parietal networks which display increased activity and connectivity when carrying out higher-order executive functions, such as working toward a defined goal, weighing the future consequences of a specific action, or inhibiting an ongoing response [17]. For instance, activation in the dorsolateral prefrontal cortex (dlPFC), when making the choice to delay gratification for a larger later reward, has been found to predict the success of dietary interventions [18]. Relatedly, there is evidence supporting that impulsive decision making in individuals with obesity is related to reduced modulation of activation in brain regions linked to cognitive control [19]. Taken together, attentional biases to rewarding stimuli and reduced inhibitory control increase the likelihood of bypassing internal satiety signals and acting upon food cravings.
More recently, the involvement of an additional system has been proposed to explain overeating—a dysfunctional interoceptive-awareness system in which appetitive hormones (e.g., insulin, ghrelin, leptin) fail to properly balance physical homeostasis by communicating satiety signals [20]. For example, leptin (a hormone secreted in response to eating; [21, 22]) is produced by adipose cells and enterocytes in the small intestine to regulate energy balance by signalling the hypothalamus to inhibit hunger signalling [22]. In obesity, there is evidence suggesting that this signalling pathway may be disrupted within the hypothalamus or when information is relayed to the insula for hedonic evaluation [23, 24]. Subsequently, a disruptive interoceptive-awareness system may translate homeostatic violation signals into a stronger desire to eat.
While homeostatic regulation of hunger is associated with activity in the lateral hypothalamus and insula [25, 26], self-control and reward processing involve large-scale networks in which self-control and reward-seeking systems may also be working against each other [7, 8, 27, 28]. Disrupted homeostatic signalling is understood to be a hallmark feature of obesity with one study finding that fasting increased FC between the homeostatic and reward systems in lean subjects, but not in those with obesity [29]. Additionally, reduced FC from the homeostatic to executive system may modulate food choices, with recent evidence demonstrating that individuals who binge eat present with lower FC between the regions in the homeostatic system and executive system [30]. A combination of an altered ability to accurately process metabolic cues, increased food-based reward reactivity, decreased inhibitory control, or a regime between these extremes may therefore contribute to overeating and poor food choices.
1.2 Insights from resting-state fMRI
Although task-based fMRI studies have proven useful in shedding light on the underlying differences in individuals with obesity, these may be complimented with task-negative (resting-state) fMRI, which provides precise measures into the functional synchronicity or “connectivity” (FC) of large-scale networks as well as key regions within these networks. Although some resting-state fMRI studies have found individuals with obesity to present with increased connectivity between regions involved with detecting and filtering salient information [31, 32], other studies have found no such differences in the salience network regions. Likewise, FC within the default mode network (DMN) has been found to reduce as a function of increased BMI [31, 33]. Given that FC in the default mode network has been purported to support attention and awareness of internal states, such as appetite or hunger signals [34,35,36], it is surprising that some intensive exercise interventions for individuals with obesity have resulted in reduction of default mode network functional connectivity [37]. Besides these large-scale resting-state networks alterations, increased FC has also been found in smaller reward-related regions such as the middle frontal gyrus, left ventromedial prefrontal cortex and lateral orbitofrontal cortex [38, 39] as well as greater resting-state FC between these reward-related regions and self-control regions in children with obesity [38]. Indeed, these findings and others suggest that FC may provide useful and complimentary information to task-based fMRI findings.
Further, the amplitude of spontaneous low-frequency fluctuations (ALFF) within the blood-oxygen-level-dependent (BOLD) signal [40, 41] can also be investigated using fMRI. ALFF is defined as the sum of amplitudes of each voxel's signal frequency spectrum within a low-frequency range. Given this measurement is inherently related to blood oxygenation, it can provide insight into altered oxygen metabolism within grey matter regions of interest [42]. A recent article showed associations between ALFF and BOLD in healthy adults, suggesting common underpinnings of resting-state activity and task-evoked BOLD response [43]. However, to date, FC and ALFF findings have not been collectively and systematically evaluated in obesity in order to determine the value they provide for broader causal theoretical frameworks.
To this end, a clearer understanding of resting-state FC alterations in individuals with obesity has the potential to aid in the development of objective markers of altered brain function, even before weight loss has manifested. These markers could be used in parallel with behavioural and cognitive measures to determine the effectiveness of weight-loss interventions in modifying the behaviour of individuals with obesity.
1.3 Our review
The aims of this systematic review are threefold: i) to examine the extent to which resting-state network FC findings support models advocating for competing neural systems and altered interoceptive awareness in individuals with obesity; ii) to evaluate the quality of methodological, acquisition, and processing protocols used in fMRI studies of individuals with obesity; and iii) to provide a roadmap of how future resting-state fMRI studies should address issues highlighted herein.
2 Method
2.1 Search strategy
This review was conducted in accordance with PRISMA guidelines [44]. Two electronic databases were searched (PubMed and Scopus) on the 26th of March 2020 (see Online Resource Fig. 1). There were no limits specified for year of publication using the search terms ("functional magnetic resonance imaging" OR "functional MRI" OR "fMRI" OR "resting-state" OR "functional connectivity") AND "obesity". Titles and abstracts were used to screen studies for relevancy after duplicates were removed. Reference lists of included studies were also screened for additional eligible studies.
2.2 Selection criteria
Studies that met the following criteria were as follows: (1) Original research published in a peer-reviewed journal, (2) Studies with a sample of participants with a BMI equal or > 30.0, (3) The use of resting-state fMRI (task negative) design or mixed task based/resting-state design with data reported for resting state condition. Studies that did not include healthy weight comparisons were included.
2.3 Exclusion criteria
Studies were excluded if: (1) Full-text article was not available, (2) Article was published in a language other than English, (3) Study was published only in abstract form or was an editorial, letter, comments or review article, and (4) Only first-level analyses available or single-subject/case study.
2.4 Data extraction
Two independent reviewers screened articles by title and abstract for relevance. These studies were then screened for eligibility for inclusion by full text evaluation. For each included article, two independent reviewers extracted data (LD, RC). Disagreements were collaboratively resolved within the team. Instructions detailing the type of information was also discussed amongst team members. The following information was extracted from each manuscript: (a) authors and year of publication; (b) sample characteristics (sample size, age group and sex distribution); (c) study design; (d) BMI range; (e) regions/networks of interest; (f) MRI acquisition parameters; (g) imaging findings; and (h) consideration of obesity related factors including comorbid diabetes, binge-eating, psychiatric disorders, medication, hypertension, hyperglycaemia, high cholesterol, insulin sensitivity, history of metabolic disorder, and sedentary lifestyle One additional reviewer (HA) then validated all the extracted data and the eligibility of each included article.
2.5 Neuroimaging data synthesis and coding
Two reviewers (NP, HA) screened each included article to identify the location and strength of reported FC (both within ROI and pairwise) effects. For each article, FC was manually localised into a common grey matter atlas (AAL; [45] based on MNI coordinates provided where possible. Any disagreement was discussed and resolved between team members. Additionally, a third independent reviewer (LD) validated the encoded data from each study, and each spatial localisation, for which any discrepancies were again discussed and addressed.
3 Results
3.1 Sample characteristics
Of the 1045 articles identified, 27 studies met inclusion criteria and were included for subsequent analyses (see Online Resource Fig. 1, Table 1). The following sample characteristics were derived from 22 studies (five were excluded due to insufficient demographics for a statistical comparison across groups or if percent ages were reported). In obesity groups, ages ranged between 14.67 and 61 years of age (M = 33.80, SD = 9.96), and BMI scores ranged from 29.26 to 43.2 (M = 36.77, SD = 3.75). Of these samples, seven consisted of females only, one with males only, and the remaining 16 including both males and females. Other than gender, most studies did not measure additional biopsychosocial sample characteristics related to obesity other than BMI (see Table 1). Five studies considered hunger levels at the time of scanning [32, 34, 39, 46, 47], three studies considered comorbid diabetes [30, 34, 48], three considered binge-eating [34, 49, 50] and only two considered comorbid psychiatric disorders [49, 51]. Factors including medication, hypertension, hyperglycaemia, high cholesterol, insulin sensitivity, history of metabolic disorder, and sedentary lifestyle were also seldom considered, with each factor only having been considered by no more than a single study (see Table 1 below).
3.2 Region-based between-groups FC
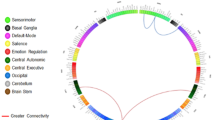
Overall, studies reported heterogeneous regions of interest and altered FC strength/direction between groups. However, several studies reported that the OFC presented consistently increased FC with other regions [33, 39, 47, 56, 62, 64] especially with the left middle frontal and temporal gyri [37, 53, 55] (Fig. 1). Other areas, such as the insula, presented decreased FC in individuals with obesity relative to controls [23, 31, 39, 53,54,55] (Fig. 1). Decreased pairwise FC was also reported involving the ACC in several studies [53,54,55,56] (Fig. 1).
Aggregated resting-state fMRI findings in obese vs. healthy weight individuals. Figure 1a depicts altered pairwise FC in individuals with obesity reported by all studies, and only those featuring the OFC, insula and ACC. Figure 1b shows the number of studies reporting altered FC represented as a ratio. Figure 1c shows the number of studies reporting altered ALFF findings represented as a ratio
3.3 BOLD signal measurements
Measurements derived from BOLD signals were seldom investigated relative to seed-based FC. However, among these findings, increased ALFF was reported pertaining mostly to subcortical regions including in the left amygdala [57], bilateral amygdala [58], putamen and insula [37, 57], and hippocampus [57, 58]. Contrastingly, one study reported decreased ALFF in cortical regions including the medial prefrontal cortex, posterior cingulate cortex, OFC and superior frontal gyrus [57]. As such, a modest pattern emerged whereby subcortical regions presented increased ALFF. Only a single study investigated ALFF after bariatric surgery, where individuals with obesity presented with decreased ALFF in the middle frontal and postcentral gyri [51]. Others also found decreased fALFF after bariatric surgery albeit in the bilateral superior frontal gyrus, OFC, gyrus rectus [52]. Other measures of activity such as degree centrality were also explored, with Zhang et al., [64] reporting increased degree centrality in the left striatum/caudate, and decreased degree centrality in the right OFC. Although consistent alterations in OFC derived from BOLD signals coincided with alterations in region-based pairwise FC involving the OFC, there is only a modest pattern of increased ALFF in subcortical regions in obese individuals.
3.4 Longitudinal interventions
Most study designs in this review were cross-sectional, with nine being longitudinal [30, 44, 49, 51, 52, 56, 59,60,61]. Of these, four investigated changes in fMRI measures after a lifestyle intervention [44, 49, 56, 59], three after bariatric surgery [52, 60, 61] and two after laparoscopic surgery [52, 60]. All lifestyle intervention studies found decreased FC, mostly pertaining to key default mode network regions such as the posterior cingulate and temporal cortices [49, 56, 59]. Other than the consistently decreased direction of FC strength relative to controls, there was no discernible pattern among the regions identified. These studies reported decreased FC within default mode, cognitive control and motor networks [49, 59], between the hippocampus and frontal gyrus [56], and reporting increased nodal global efficiency in frontal regions [44].
Importantly, these changes may not be limited to individuals with obesity, as exercise interventions in otherwise healthy individuals have also elicited this pattern [62,63,64,65]. After bariatric surgery, decreased FC was reported in frontal cortical regions coupled with increased FC in the insula [59] and striatal (caudate) regions implicated in self-regulation [60]. However, Olivo et al., [30] found Increased FC between the precuneus and anterior superior temporal gyrus, which decreased in individuals with obesity at one month and one-year follow up respectively. As such, there is emerging evidence that these functional changes may decrease over time [61] and further research is needed to determine if surgical interventions indeed translate into sustained changes in FC.
4 Discussion
Cumulatively, resting-state fMRI evidence suggests individuals with obesity exhibit increased OFC FC and decreased insula FC incident, albeit with some variation in the direction of these FC strengths across studies. These alterations could be reflective of increased reward sensitivity and lower interoceptive awareness in individuals with obesity. Hereafter, we discuss (i) the extent to which these findings support neurobiological models of obesity advocating for competing neural systems and altered interoceptive awareness, as well as (ii) pervasive methodological issues in obesity resting-state fMRI studies included here, such as sample homogeneity, region of interest selection and fMRI acquisition and analysis, and (iii) we discuss how future resting-state fMRI studies could address the issues highlighted herein and provide suggestions for moving forward.
4.1 How do resting-state fMRI findings support current neurobiological frameworks of obesity?
Our finding across studies of consistently decreased insula cortex FC may reflect a dysfunctional interpretation of homeostatic signals in obesity, whereby homeostatic violation signals (e.g., hunger) may be maintained despite reaching satiety. The insula plays a vital role in integrating food-related interoceptive signals [66] where information on energy and nutrient availability is received from the brainstem and thalamus via the vagal pathways [66,67,68]. Reduced FC of the insula cortex therefore may reflect dysfunctional satiety signalling, particularly when linked with reward-related regions [39] including the ventral striatum and OFC (connected via the cortico-ventral basal ganglia circuit). This may translate to reduced reward derived from eating and satisfaction after eating, both of which have been implicated in reward learning, reward expectancy and valuation [69, 70]. Moreover, alterations in this interoceptive-awareness system may override inhibitory signals from the executive system, further enabling the reward-seeking system [20, 22] as outlined in the competing neural systems model [7, 10, 25,26,27]. However, these findings were seldom compared with changes in BMI or behavioural measures, which are essential in order to establish a causal link between alterations in insula FC and eating behaviours.
The OFC is specifically related to evaluating external stimuli based on the body’s present state [71], and the combination of decreased insula and increased OFC/ventral striatum FC may indicate that satiety is less rewarding for individuals with obesity. Therein, this finding lends support to the ‘reward-based eating’ model of obesity [24]. This theory is supported by findings whereby participants with obesity, unlike their healthy weight counterparts, did not show increased FC between the insula and OFC as hunger increased [39], which would be demonstrative of adaptive seeking of food-reward when hungry. In contrast, a negative relationship between insula-ventral striatum FC and hunger (growing satiety increases FC) was found in individuals with obesity, indicating that satiety may contribute to food-related reward-seeking [39]. However, the question remains as to whether altered resting-state FC is related to activity of these same circuits during the execution of paradigms and of in-vivo food-related decision making. A recent study by Li et al., (2020) shed light on this link; reporting that resting-state activity in the left hippocampus/amygdala was positively correlated with activation induced by high-calorie food cues, with both measures correlating with BMI.
4.2 Alternate resting-state fMRI measures
Only one study specifically investigated ALFF and response to food cues in individuals with obesity [58] reporting that the relationship between BMI and hippocampus/amygdala responses to high calorie food cues was mediated by resting-state activity. Increased hippocampal ALFF may therefore reflect an altered baseline activity of brain regions. Other measures of activity, such as an increased baseline neuronal firing rate, have been reported to be related to a faster initiation of eating after fasting in rats [72]. Other than alterations in these specific brain regions, there was widespread heterogeneity in both regions of interest and FC findings within/between these regions. As such, the findings from most ALFF studies showed little overlap. This inconsistency may be explained by a lack of homogeneity between obese samples, fMRI reliability issues clouding effects between groups, and variation in region of interest, as well as atlas/parcellation choice resulting in incompatible region comparison and incongruent findings.
4.3 Sample homogeneity
Sample size and homogeneity are some of the most important issues harbouring the reliability of resting-state fMRI findings in obesity, especially given that there are significant psychological and behavioural factors that may contribute to, or be intertwined with overeating [73,74,75]. For example, both, anxiety and increased reward-seeking can prompt overeating. This type of eating is driven by the desire to reduce negative emotional states, whereas reward-related eating occurs to increase expected positive emotional states [76, 77] and as such, the underlying neural mechanisms of avoiding negative states and seeking positive states are fundamentally distinct [78]. Moreover, there is evidence suggesting that individuals with obesity display deficits in regulating negative emotional states [79] and that this impairment may be mediated by altered connectivity in the ventromedial prefrontal cortex and the insula [80, 81].
Another key issue relating to samples is a lack of reporting hunger levels, which may be directly influential to insula FC or BOLD related measures [82, 83]. Other research has found BMI to be positively correlated with excitatory influence of the anterior insula over the hypothalamus, as well as reduced inhibitory influence of the ventromedial prefrontal cortex on the anterior insula, regardless of hunger-levels [84]. This finding may be indictive of reduced sensitivity to or interpretation of energy homeostasis; lending support to our interpretation of insula-related dysfunctional signalling of calorie and nutrient satiety. Moreover, most studies only identified the weight category of individuals, without considering hunger states, psychiatric comorbidities, insula resistance, and other metabolic conditions that can modulate brain function. Without properly linking these conditions to meaningful differences in behavioural measures (e.g., eating patterns, dietary intake, psychological factors, cognitive functioning), it is challenging to interpret the impact of differences in resting-state FC.
4.4 Region of interest selection
We found that few studies investigated whole-brain connectivity and instead focused on contrasting resting-state networks and subregions. The parcellation of regions within these networks was also largely heterogenous, making comparison of findings problematic. The reporting of the laterality and coordinates of these regions was also lacking, with many studies not reporting this information at all. Although the resting-state fMRI measurements used were varied within this review, the specific MRI data analysis methods were often unclear in the included studies. For instance, many studies referring to using “seed-based” analyses, without reporting the adequate information for readers to fully understand the analyses technique used, i.e., which regions were used as seeds and targets [23, 46, 48]. This issue harkens back to a decade-long issue in which highly dimensional fMRI studies often do not report key information in order to replicate findings [85].
4.5 Correlation reliability
Resting-state fMRI findings in clinical cohorts in general are often harboured by reliability issues, prompting the use of advanced acquisition and analysis techniques [84, 86] and these applications in obese samples are no exception. The less-than-ideal MRI sequences that are often used will in turn reduce both the reliability of resting-state networks [87, 88] and the reliability of the subsequent correlations [84, 89] respectively. For instance, many studies reported relatively high repetition times, a low number of volumes, and low scan duration [39, 44, 59] (Online Resource Fig. 2) and some did not report the number of volumes or scan duration at all [49, 90] (Online Resource Fig. 2F). However, some studies used relatively shorter repetition times, higher number of volumes and longer scan durations [46, 47, 49]. Together, these methodological issues may partly explain the variability observed in findings across studies.
4.6 A roadmap forward for elucidating neural mechanisms underpinning obesity
Resting-state fMRI is valuable and complimentary tool, in addition to task-based fMRI, to elucidate the neural underpinnings of large-scale network and region-based alterations in obesity. Despite this, resting-state FC measures are not well understood in the context of other fMRI measurements and few studies have used multimodal fMRI. Hereafter, we present proposals for establishing best practices with respect to functional MRI acquisition, study design, complimentary post-processing techniques, and detail how these can be reconciled into a practical framework. We next provide MRI acquisition and data analysis and methodological suggestions.
Across many studies included in this review, it was difficult to ascertain the specific MRI parameters used, with studies underreporting critical information [85, 87], highlighting the need to report all fMRI related information (i.e., all MRI acquisition parameters and pre-processing specifications) at least in supplementary data [91]. Furthermore, many of the data acquired in these studies are limited in terms of temporal sensitivity, and correlation reliability, warranting rapid fMRI acquisition sequences (multiband factor ~ 3; [92]) coupled with longer scan-times (above 13 min; [89, 93]). To further bolster the reliability of fMRI findings in obesity, we suggest the use of multiband echo planar imaging, permitting the acquisition of multiple volumes at once, resulting in more volumes per scan [93, 94]. This method should be used with caution, given multiband acquisitions are inherently more susceptible to motion artefact [95, 96]—an important consideration in samples with obesity, who may find scanning to be uncomfortable. Therefore, ensuring that scanning sessions are not excessive in length is imperative to maintain comfort and subsequently reduce the intrusion of motion artefact in clinical populations during longer MRI scans [89, 97]. With this, we also suggest studies considering specific guidelines proposed by Frank et al. [59] who provide a very detailed recommendations relating to demographics, covariates, comorbidities, and technical study design in populations with eating disorders. This study offers regarding how to report demographic data, hunger-related variables, hormonal effects, comorbidities, and medications.
Presently, it is not clear how measures such as ALFF relate to task-based, resting-state or dynamic FC [98, 99]. This warrants the inclusion of these post-processing measures within a single appropriate methodology (see Fig. 2). These measures must be considered both over time and in response to weight loss or surgical interventions in order to determine whether FC alterations are reversable, whether eating behaviours are more strongly associated with brain connectivity or activity measures, and whether fMRI measurements can provide an objective marker of future successful weight-loss. Herein, the design of lifestyle interventions must be considered. Intensive lifestyle interventions may have a higher chance of eliciting significant weight loss [48, 51, 60], however interventions that are highly multifaceted (see [100]), although effective, may impede the ability to link specific interventions to fMRI measurements. Hence, one may wish to opt for traditional interventions such as diet or exercise to limit heterogeneity of effects across individuals.
A schematic design to elucidate neural mechanisms underpinning obesity. Panel A provides recommended acquisition parameters. B shows recommended sample sizes (ie. greater than 50; [101] as well as how these samples can be grouped. Panel C shows possible task paradigms and hunger states that should be contrasted. Panel D shows post-processing techniques that should be utilised together. Lastly, panel E indicates important covariates that must either be controlled for or serve as exclusion criteria
One of the most significant barriers in current methodologies pertains to the lack of empirical information on psychological and physiological factors within samples, which are crucial in order to incorporate inter-subject variability and understand the extent to which competing neural systems may be modulated by important covariates [91]. These include but are not limited to, medication, hypertension, hyperglycaemia, high cholesterol, insulin sensitivity, history of metabolic disorder, and sedentary lifestyle. Given the high number of these variables, studies must decide which are used as covariates and as exclusion criteria. We suggest that psychological disorders and untreated metabolic conditions be considered as exclusion criteria given these are heterogenous in nature and may therefore have a higher propensity to contaminate findings. On the other hand, factors such as hunger, hyperglycaemia, high cholesterol, insulin sensitivity and sedentary lifestyle are more likely to be comorbid in people with obesity, and as such may present as more realistic covariate inclusions. As a hypothetical research question, one might consider the following: can the neural mechanisms underpinning obesity and overeating be explained when considering satiety states, behavioural variables and resting-state fMRI measurements? (see Fig. 2).
4.7 Conclusion
Increased OFC, and decreased Insula FC may reflect increased reward sensitivity and lower interoceptive awareness in individuals with obesity. Whether findings we report here are a cause or consequence of obesity is still unknown, and therefore FC in obesity must be investigated before, during, and after food consumption in order to understand the exact interplay between theoretical frameworks with psychological and biological factors (see Fig. 2a–c). More specifically, multimodal functional MRI including dynamic resting-state FC coupled with task-based BOLD activity and FC analyses may elucidate how these regions relay information related to food choices and the desire to eat (see Fig. 2d). This approach has the potential to deliver empirical markers that could predict response to specific interventions. That is, the successful alteration of FC within and between these regions may inform the treatment programs on an individual basis—ultimately improving treatment outcomes for individuals with obesity.
Availability of data and material
Raw data can be made available upon request.
Code availability
A MATLAB file containing analyses steps can be made available upon request.
Abbreviations
- AAL:
-
Automatic Anatomical Labelling
- ALFF:
-
Amplitude of Low Frequency Fluctuation
- BMI:
-
Body Mass Index
- BOLD:
-
Blood Oxygenated Level Dependency
- DEBQ:
-
Dutch Eating Behaviour Questionnaire
- dlPFC:
-
Dorsolateral Prefrontal Cortex
- DMN:
-
Default Mode Network
- FC:
-
Functional Connectivity
- fMRI:
-
Functional Magnetic Resonance Imaging
- FPN:
-
Frontoparietal Network
- BES:
-
Binge Eating Scale
- HADS:
-
Hospital Anxiety and Depression Scale
- ICA:
-
Independent Component Analysis
- MNI:
-
Montreal Neurological Institute
- OFC:
-
Orbitofrontal Cortex
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ROI:
-
Region of Interest
- SN:
-
Salience Network
- SPSRQ:
-
The Sensitivity to Punishment and Sensitivity to Reward Questionnaire
- YFAS:
-
Yale Food Addiction Scale
References
Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, Alonso-Alonso M, Audette M, Malbert C-H, Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015;8:1–31.
Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46.
Steward T, Miranda-Olivos R, Soriano-Mas C, Fernández-Aranda F. Neuroendocrinological mechanisms underlying impulsive and compulsive behaviors in obesity: a narrative review of fMRI studies. Rev Endocr Metab Disord. 2019;20:263–72.
Makaronidis JM, Batterham RL. Obesity, body weight regulation and the brain: insights from fMRI. Br J Radiol. 2018;91:20170910.
Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428.
Stoeckel LE, Birch LL, Heatherton T, Mann T, Hunter C, Czajkowski S, Onken L, Berger PK, Savage CR. Psychological and neural contributions to appetite self-regulation. Obesity. 2017;25:S17–25.
Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63.
Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90:S85–91.
He Q, Turel O, Bechara A. Brain anatomy alterations associated with Social Networking Site (SNS) addiction. Sci Rep. 2017;7:1–8.
He Q, Turel O, Brevers D, Bechara A. Excess social media use in normal populations is associated with amygdala-striatal but not with prefrontal morphology. Psychiatry Res Neuroimaging. 2017;269:31–5.
Turel O, He Q, Xue G, Xiao L, Bechara A. Examination of neural systems sub-serving Facebook “addiction.” Psychol Rep. 2014;115:675–95.
Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–703.
Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34:1494–500.
Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology. 2010;211:87–98.
Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604.
Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–94.
McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7.
Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, Haynes J-D. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015;109:318–27.
Miranda-Olivos R, Steward T, Martínez-Zalacaín I, et al. The neural correlates of delay discounting in obesity and binge eating disorder. J Behav Addict. 2021. https://doi.org/10.1556/2006.2021.00023.
Chen R, Li DP, Turel O, Sørensen TA, Bechara A, Li Y, He Q. Decision making deficits in relation to food cues influence obesity: a triadic neural model of problematic eating. Front Psychiatry. 2018;9:264.
Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–27.
Bouret S, Levin BE, Ozanne SE. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev. 2015;95:47–82.
Berthoud H-R, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152:1728–38.
Cameron JD, Chaput J-P, Sjödin AM, Goldfield GS. Brain on fire: Incentive salience, hedonic hot spots, dopamine, obesity, and other hunger games. Annu Rev Nutr. 2017;37:183–205.
Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90.
Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, Avery JA, Hall KD, Martin A. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci. 2013;16:1551–2.
Bickel WK, Snider SE, Quisenberry AJ, Stein JS, Hanlon CA. Competing neurobehavioral decision systems theory of cocaine addiction: From mechanisms to therapeutic opportunities. In: Prog. Brain Res. Elsevier 2016;269–293.
Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90.
Wijngaarden MA, Veer IM, Rombouts S, Van Buchem MA, Van Dijk KW, Pijl H, Van Der Grond J. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res. 2015;287:127–34.
Olivo G, Zhou W, Sundbom M, Zhukovsky C, Hogenkamp P, Nikontovic L, Stark J, Wiemerslage L, Larsson E-M, Benedict C. Resting-state brain connectivity changes in obese women after Roux-en-Y gastric bypass surgery: a longitudinal study. Sci Rep. 2017;7:1–11.
Beyer F, Kharabian Masouleh S, Huntenburg JM, Lampe L, Luck T, Riedel-Heller SG, Loeffler M, Schroeter ML, Stumvoll M, Villringer A. Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Hum Brain Mapp. 2017;38:3502–15.
Kullmann S, Heni M, Linder K, Zipfel S, Häring H-U, Veit R, Fritsche A, Preissl H. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp. 2014;35:6088–96.
Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb Cortex. 2018;28:988–97.
García-García I, Jurado MÁ, Garolera M, Segura B, Sala-Llonch R, Marqués-Iturria I, Pueyo R, Sender-Palacios MJ, Vernet-Vernet M, Narberhaus A. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp. 2013;34:2786–97.
Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-U, Fritsche A, Preissl H. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–61.
Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, Cordes D, Cornier M-A. Altered default network activity in obesity. Obesity. 2011;19:2316–21.
McFadden KL, Cornier M-A, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. NeuroReport. 2013;24:866.
Black WR, Lepping RJ, Bruce AS, Powell JN, Bruce JM, Martin LE, Davis AM, Brooks WM, Savage CR, Simmons WK. Tonic hyper-connectivity of reward neurocircuitry in obese children. Obesity. 2014;22:1590–3.
Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, Wiemerslage L, Larsson E-M, Sundbom M, Benedict C. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int J Obes. 2016;40:1687–92.
Golestani AM, Kwinta JB, Khatamian YB, Chen JJ. The effect of low-frequency physiological correction on the reproducibility and specificity of resting-state fMRI metrics: functional connectivity, ALFF, and ReHo. Front Neurosci. 2017;11:546.
Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, Tian L-X, Jiang T-Z, Wang Y-F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91.
Di X, Kannurpatti SS, Rypma B, Biswal BB. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb Cortex. 2013;23:255–63.
Tomasi D, Volkow ND. Reduced local and increased long-range functional connectivity of the thalamus in autism spectrum disorder. Cereb Cortex. 2019;29:573–85.
McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319:388–96.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Avery JA, Powell JN, Breslin FJ, Lepping RJ, Martin LE, Patrician TM, Donnelly JE, Savage CR, Simmons WK. Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. J Psychopharmacol (Oxf). 2017;31:1475–84.
Wiemerslage L, Zhou W, Olivo G, Stark J, Hogenkamp PS, Larsson E-M, Sundbom M, Schiöth HB. A resting-state fMRI study of obese females between pre-and postprandial states before and after bariatric surgery. Eur J Neurosci. 2017;45:333–41.
Casanova R, Hayasaka S, Saldana S, Bryan NR, Demos KE, Desiderio L, Erickson KI, Espeland MA, Nasrallah IM, Wadden T. Relative differences in resting-state brain connectivity associated with long term intensive lifestyle intervention. Psychoneuroendocrinology. 2016;74:231–9.
Chao S-H, Liao Y-T, Chen VC-H, Li C-J, McIntyre RS, Lee Y, Weng J-C. Correlation between brain circuit segregation and obesity. Behav Brain Res. 2018;337:218–27.
Meng Q, Han Y, Ji G, Li G, Hu Y, Liu L, Jin Q, von Deneen KM, Zhao J, Cui G. Disrupted topological organization of the frontal-mesolimbic network in obese patients. Brain Imaging Behav. 2018;12:1544–55.
Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela A-M, Fabian S, Grittner U, Spranger J. Caloric restriction in older adults—differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex. 2017;27:1765–78.
Krafft CE, Pierce JE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, Rodrigue AL, Camchong J, Allison JD, Yanasak NE. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience. 2014;256:445–55.
Ding K, Dragomir A, Bose R, Osborn LE, Seet MS, Bezerianos A, Thakor NV. Towards machine to brain interfaces: Sensory stimulation enhances sensorimotor dynamic functional connectivity in upper limb amputees. J Neural Eng. 2020;17:035002.
Li G, Ji G, Hu Y, Xu M, Jin Q, Liu L, von Deneen KM, Zhao J, Chen A, Cui G. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 2018;39:4755–65.
Li P, Shan H, Liang S, Nie B, Liu H, Duan S, Huang Q, Zhang T, Dong G, Guo Y. Sleeve gastrectomy recovering disordered brain function in subjects with obesity: a longitudinal fMRI study. Obes Surg. 2018;28:2421–8.
Nakamura Y, Ikuta T. Caudate–precuneus functional connectivity is associated with obesity preventive eating tendency. Brain Connect. 2017;7:211–7.
Tang D, Tao S, Ma J, Hu P, Long D, Wang J, Kong D. The effect of short cardio on inhibitory control ability of obese people. Int J Imaging Syst Technol. 2017;27:345–53.
Li G, Hu Y, Zhang W, Ding Y, Wang Y, Wang J, He Y, Lv G, von Deneen KM, Zhao Y. Resting activity of the hippocampus and amygdala in obese individuals predicts their response to food cues. Addict Biol. 2020;e12974.
Frank S, Wilms B, Veit R, Ernst B, Thurnheer M, Kullmann S, Fritsche A, Birbaumer N, Preissl H, Schultes B. Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. Int J Obes. 2014;38:341–8.
García-Casares N, Bernal-López MR, Roé-Vellvé N, Gutiérrez-Bedmar M, Fernández-García JC, García-Arnés JA, Ramos-Rodriguez JR, Alfaro F, Santamaria-Fernández S, Steward T. Brain functional connectivity is modified by a hypocaloric Mediterranean diet and physical activity in obese women. Nutrients. 2017;9:685.
Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C, Stamatakis EA, Verdejo-Garcia A. Disrupted functional connectivity in adolescent obesity. NeuroImage Clin. 2016;12:262–8.
Xia W, Wang S, Sun Z, Bai F, Zhou Y, Yang Y, Wang P, Huang Y, Yuan Y. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology. 2013;38:2493–501.
Yin S, Zhu X, Li R, Niu Y, Wang B, Zheng Z, Huang X, Huo L, Li J. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Brain Stimul Basic Transl Clin Res Neuromodulation. 2015;8:414.
Zhang P, Liu Y, Lv H, Li M, Yu F, Wang Z, Ding H, Wang L, Zhao K, Zhang Z. Integration of neural reward processing and appetite-related signaling in obese females: evidence from resting-state fMRI. J Magn Reson Imaging. 2019;50:541–51.
Zhang Y, Wang J, Zhang G, Zhu Q, Cai W, Tian J, Zhang YE, Miller JL, Wen X, Ding M. The neurobiological drive for overeating implicated in Prader-Willi syndrome. Brain Res. 2015;1620:72–80.
Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo A, White SM. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32.
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66.
de Araujo IE, Geha P, Small DM. Orosensory and homeostatic functions of the insular taste cortex. Chemosens Percept. 2012;5:64–79.
Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980;190:259–82.
O’doherty JP, . Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76.
Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7.
Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23:100–7.
Geha P, Cecchi G, Todd Constable R, Abdallah C, Small DM. Reorganization of brain connectivity in obesity. Hum Brain Mapp. 2017;38:1403–20.
Hare TA, O’doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–30.
Rudebeck PH, Murray EA. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014;84:1143–56.
De Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178:230–5.
Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes. 2010;34:407–19.
Duehlmeyer L, Hester R. Impaired learning from punishment of errors in smokers: Differences in dorsolateral prefrontal cortex and sensorimotor cortex blood-oxygen-level dependent responses. NeuroImage Clin. 2019;23:101819.
Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE. Emotion regulation model in binge eating disorder and obesity–a systematic review. Neurosci Biobehav Rev. 2015;49:125–34.
Steward T, Picó-Pérez M, Mata F, Martínez-Zalacaín I, Cano M, Contreras-Rodríguez O, Fernández-Aranda F, Yucel M, Soriano-Mas C, Verdejo-García A. Emotion regulation and excess weight: Impaired affective processing characterized by dysfunctional insula activation and connectivity. PloS One. 2016;11:e0152150.
Steward T, Picó-Pérez M, Mestre-Bach G, et al. A multimodal MRI study of the neural mechanisms of emotion regulation impairment in women with obesity. Transl Psychiatry. 2019;9:194.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–60.
Voigt K, Razi A, Harding IH, Andrews ZB, Verdejo-Garcia A. Neural network modelling reveals changes in directional connectivity between cortical and hypothalamic regions in obesity. bioRxiv. 2020.
Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage. 2008;40:409–14.
Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26:609–18.
Parsons N, Bowden SC, Vogrin S, D’Souza WJ. Single-subject manual independent component analysis and resting state fMRI connectivity outcomes in patients with juvenile absence epilepsy. Magn Reson Imaging. 2020;66:42–9.
Reynaud O, Jorge J, Gruetter R, Marques JP, van der Zwaag W. Influence of physiological noise on accelerated 2D and 3D resting state functional MRI data at 7 T. Magn Reson Med. 2017;78:888–96.
Gordon EM, Scheibel RS, Zambrano-Vazquez L, Jia-Richards M, May GJ, Meyer EC, Nelson SM. High-fidelity measures of whole-brain functional connectivity and white matter integrity mediate relationships between traumatic brain injury and post-traumatic stress disorder symptoms. J Neurotrauma. 2018;35:767–79.
Harding IH, Andrews ZB, Mata F, Orlandea S, Martinez-Zalacain I, Soriano-Mas C, Stice E, Verdejo-Garcia A. Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. Int J Obes. 2018;42:448–54.
Frank GKW, Favaro A, Marsh R, Ehrlich S, Lawson EA. Toward valid and reliable brain imaging results in eating disorders. Int J Eat Disord. 2018;51:250–61.
Preibisch C, Bührer M, Riedl V. Evaluation of multiband EPI acquisitions for resting state fMRI. PloS One. 2015;10:e0136961
Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–8.
Seo HS, Jang KE, Wang D, Kim IS, Chang Y. Accelerated resting-state functional magnetic resonance imaging using multiband echo-planar imaging with controlled aliasing. Investig Magn Reson Imaging. 2017;21:223–32.
Feinberg DA, Setsompop K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J Magn Reson. 2013;229:90–100.
Feinberg DA, Yacoub E. The rapid development of high speed, resolution and precision in fMRI. Neuroimage. 2012;62:720–5.
Krause F, Benjamins C, Eck J, Lührs M, Hoof R, Goebel R. Active head motion reduction in magnetic resonance imaging using tactile feedback. Hum Brain Mapp. 2019;40:4026–37.
Friston KJ. Functional and Effective Connectivity: A Review. Brain Connect. 2011;1:13–36.
Tomasi D, Volkow ND. Association between brain activation and functional connectivity. Cereb Cortex. 2019;29:1984–96.
Augustijn MJCM, Di Biase MA, Zalesky A, Van Acker L, De Guchtenaere A, D’Hondt E, Lenoir M, Deconinck FJA, Caeyenberghs K. Structural connectivity and weight loss in children with obesity: a study of the “connectobese.” Int J Obes. 2019;43:2309–21.
Mumford JA. A power calculation guide for fMRI studies. Soc Cogn Affect Neurosci. 2012;7:738–42.
García-García I, Jurado MÁ, Garolera M, Marqués-Iturria I, Horstmann A, Segura B, Pueyo R, Sender-Palacios MJ, Vernet-Vernet M, Villringer A, Junqué C. Functional network centrality in obesity: A resting-state and task fMRI study. Psychiatry Res Neuroimaging. 2015;233(3), pp.331-338.
Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, Wiemerslage L, Larsson EM, Sundbom M, Benedict C, Schiöth HB. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int J Obes (Lond). 2016 Nov;40(11):1687-1692. https://doi.org/10.1038/ijo.2016.105. Epub 2016 Jun 7. PMID: 27349694; PMCID: PMC5116051.
Funding
NP is supported by a Deakin University Postgraduate Research Scholarship (DUPRS). HA is supported by the Special Research Fund of Ghent University (grant No. BOF16/DOC/282; https://www.ugent.be/), and a grant for long stay abroad from the Fund for Scientific Research-Flanders (FWO–V; awarded to HA; http://www.fwo.be). TS is supported by a NHMRC/MRFF Investigator Grant (MRF1193736), a BBRF Young Investigator Grant, and a University of Melbourne McKenzie Fellowship. The authors Rebecca Clohesy and Leonie Duehlmeyer received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This literature review did not require Ethics approval.
Consent to participate
This literature review did not require participants’ consent.
Consent for publication
The authors Nicholas Parsons, Trevor Steward, Rebecca Clohesy, Hannes Almgren, and Leonie Duehlmeyer consent for this article to be published.
Conflicts of interest
The authors Nicholas Parsons, Trevor Steward, Rebecca Clohesy, Hannes Almgren, and Leonie Duehlmeyer have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11154_2021_9665_MOESM1_ESM.tiff
Supplementary file1 Online Resource Fig. 1. PRISMA Flow Diagram Screening process applied to identified studies including identification, screening, eligibility assessments. A total of 1045 studies were identified, of which 24 met inclusion criteria (TIFF 964 KB)
11154_2021_9665_MOESM2_ESM.tiff
Supplementary file2 Online Resource Fig. 2. Aggregated MRI acquisition parameters. This figure shows aggregated MRI scanning parameters: Panel A shows repetition time in milliseconds; Panel B shows scan time in minutes; Panel C shows the number of volumes. In most studies, scans were acquired with 2000 ms repetition times using between 150 and 200 EPI volumes. Over 50% of studies used a scan duration of between 6 and 7 min (TIFF 2580 KB)
Rights and permissions
About this article
Cite this article
Parsons, N., Steward, T., Clohesy, R. et al. A systematic review of resting-state functional connectivity in obesity: Refining current neurobiological frameworks and methodological considerations moving forward. Rev Endocr Metab Disord 23, 861–879 (2022). https://doi.org/10.1007/s11154-021-09665-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-021-09665-x