In this study, chamotte refractory samples were analyzed after they were used in the shaft and crucible of a coke gas cupola furnace during the melting of cast iron with spheroidal graphite using carbon waste obtained from aluminum electrolysis as a reducing agent. It was discovered that the structure of the fireclay refractory changed during operation because of corrosion factors specific for each zone of the cupola furnace. Refractory silicates (glass phase and quartz) primarily interact with corrosives. Secondary mullitization, which is associated with the mineralizing effect of fluorine-containing vapors in the furnace atmosphere, was extremely active during refractory operation. In the crucible, the refractory was influenced by mechanical loads exceeding the ultimate compressive strength of the refractory, as well as chemical loads from the metal melts and low-basic slag. Thus, the refractory in the crucible lining was first destroyed mechanically and then chemically, dissolving from the contact surface in the low-basic slag. The glass phase of the products was impregnated with iron oxides, which were reduced in the refractory structure under the influence of the furnace atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In industries, about half of all the refractories are destroyed because of insufficient chemical resistance. Refractories interact with various corrosives, such as slags, metals, and gases. One of the corrosive forces that destroy refractories is dissolution [1], in which a layer of interaction products is formed on the contact surface (refractory corrosion). The particles that do not dissolve on time are subsequently removed with the reaction products (refractory erosion).
Slag aggressiveness depends mostly on ion activity. Free oxygen ions have the highest activity level. Fluorine and alkali ions also make the slag more aggressive, reducing its viscosity and melting point.
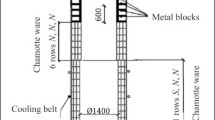
Part 1 of this article [2] demonstrated that chamotte ware had a service life of fewer than 7 days when used in a coke oven gas cupola to smelt cast iron with spheroidal graphite using carbon waste from obtained aluminum production electrolyzers as a carburizer. In this case, the refractories develop a pronounced zonal structure. Table 1 shows the chemical composition of the samples before and after the operation, as well as that of the cupola slag.
Table 1 demonstrates the following:
-
When cast iron was melted with spheroidal graphite in a cupola, slag with a basicity modulus of 0.13 was obtained, implying that it was very acidic and required a high overheating temperature to ensure its fluidity when draining. The alkali oxide concentration was low (0.091 wt.%) despite being high in the carbonaceous part of the charge. This indicates that alkalis and fluorine were evaporated during the production of cast iron;
-
The chemical compositions of the fireclay refractory zones after service in the shaft and crucible were similar, and the working zones contained the same amount of SiO2 as the slag. According to the phase diagram of Al2O3–SiO2–Fe2O3 in the temperature range corresponding to the melting of metals (cast iron), the refractory behavior depends mainly on the Al2O3, SiO2, and Fe2O3 contents.
The fireclay refractory samples were black after use in the crucible and shaft of the cupola because of the saturation of the structure with iron oxides and soot carbon, which was released by the Bell reaction: 2CO ⇄ CO2 + C. The soot carbon release reaction occurs in a volume of fireclay refractories with a porosity of 22% and is accelerated by catalysts (e.g., iron oxides) [3]. Figure 1 and Table 2 present the phase compositions of the initial fireclay refractory and cupola slag.
As shown in Figure 1 and Table 2, the mineral bases of the original fireclay refractory are mullite and cristobalite (quartz), with a moderate concentration of anorthite glass phase. A small amount of corundum indicates that a highalumina additive was used in the refractory charge to increase the mullite content in the product matrix. The cupola smelting slag consisted almost entirely of an iron– aluminate–silica glass phase (fayalite + almandine + anorthite).
Table 3 presents the phase composition of the chamotte ware by zones after service in the shaft and crucible of the cupola furnace. During refractory operation, the concentration of the glass phase increased to 30 – 35%, and its composition changed mainly from anorthite to anorthite–fayalite. In this case, the iron oxide contents in the refractory increased (in the form of calcium ferrites and iron silicates), while the refractory phase contents (mullite and cristobalite) decreased, even when secondary crystallization of mullite was taken into account. The total content of the introduced phases ranged from 9.0% in the crust of the working zone to 2.8% in the impregnated zone.
The phase composition of the refractory changed significantly after use in the cupola crucible. The crust of the refractory working zone consisted almost entirely of a slag skull, namely, a glass phase of ferrite composition (up to 40%) and wustite (32.0%), with remnants of refractory phases, namely, mullite (17.0%) and cristobalite (9.0%). The number of ferrite phases (glass phase and crystalline phases) in the impregnated zones increased, while the number of primary refractory phases, namely, mullite and cristobalite, decreased significantly.
During operation, the physical and chemical properties of the fireclay refractories in the cupola lining change, as well as their structures at both the macro and micro levels. The initial macrostructure of the products consisted of a binder cryptocrystalline mass (matrix) and large grains of chamotte, and a small number of fine corundum and cristobalite grains (aggregates of various fineness). The matrix of the annealed chamotte ware was made up of a cryptocrystalline binder mass of silicate glass and dispersed chamotte grains surrounded by the thinnest colorless rims of metastable cristobalite.
High temperatures, a reducing atmosphere, and CO penetrating the pores darkened the color of the products and the lining. The crust of the working zone, which is the most altered part of the refractory structure in the shaft and crucible of the cupola, was made up of refractories recrystallized by fluorine mineralizers and impregnated with slag. The working surface of the products was covered with a 2–5 mm thick layer of slag and worn out evenly, and intensive cracking of the structure and penetration of the slag and metal melt into cracks and pores were observed in the crucible zone.
At high magnification (Fig. 2), it can be seen that the sample is composed of individual grains of chamotte and quartz, which are separated by microcracks partially filled with a glass phase with a high reflection coefficient. A portion of the grains had been impregnated with melting products and had transformed into a glassy cryptocrystalline mass.
Since the transition zone in the products underwent fewer structural changes than the working zone, some of the grains degenerated and dissolved in the glass phase; the latter contained inclusions with a high reflection coefficient (metal or lower metal oxides). Fine particles of soot carbon were observed on the surface of the grains and glass phase. The pores were irregular in shape and partially (up to 50%) filled with the glass phase.
The least altered zone of the fireclay refractory after service in the crucible and shaft is the denser, which was stained dark gray because of the penetration of soot carbon. The refractory structure was not impaired, and isolated fine grains of corundum were observed.
The samples presented were selected from the chamotte ware manufactured using the classical multichamotte refractory technology but with an increased amount of Al2O3 in the clay (binder), which was introduced into the joint grinding mixture in the form of alumina. There were no foreign inclusions in the product before the service. During operation, the products were exposed to high temperatures and sudden temperature changes. There were no fluorine-containing phases in the cupola melting slag and the products after operation; however, there was an abnormally small amount of alkali-containing phases, indicating that the fluorine and alkaline components from the carbon scrappage of aluminum production with a furnace atmosphere were removed mainly from the zones above the lined belt.
The refractory wear mechanisms in the shaft and crucible of the cupola differed. In the shaft, the refractory was mainly influenced by the gas phase, namely alkali vapors, perhaps fluorine compounds, and a reducing atmosphere, resulting in the formation of soot carbon throughout the entire volume of the lining. The working surface was also influenced by active metal oxides and the emerging metal phase. All these factors resulted in intense chemical wear of the fireclay refractory, as well as the formation of a large amount of iron-containing glass phase with a low melting point. Refractory silicates (glass phase and quartz) primarily interact with corrosives; secondary mullitization during operation, which is associated with the mineralizing effect of fluorine-containing vapors in the furnace atmosphere, was very active.
In the crucible, the refractory was subjected to mechanical loads that exceeded the ultimate compressive strength of the refractory, as well as chemical loads from the metal melts and low-basic slag. Thus, the refractory in the crucible lining was first destroyed mechanically and then chemically, dissolving from the contact surface in the low-basic slag. The glass phase was impregnated with iron oxides, which were reduced in the structure of the refractory because of the furnace atmosphere.
Conclusion
The formation of the structure of fireclay refractories in the shaft and crucible of the cupola furnace is characterized by the following:
-
the consistency of the mullite content in the different zones of the chamotte ware (Table 3) and the increase in its content in the impregnated zone despite the different service conditions;
-
the absence of alkaline and fluoride phases in the zones of the products due to high partial pressures of gases (vapors) in the pores and structure of the fireclay refractory, as well as the migration of these gaseous phases into the working zone of the cupola with their subsequent removal with furnace gases;
-
additional formation of mullite in some zones because of its synthesis from alumina, which did not react during the fabrication of the fireclay refractories. In this case, fluorine compounds and fluorine act as mineralizers in the aluminosilicate mass of the charge during the operation of the refractories.
Thus, the fireclay refractories were impregnated with gas and liquid phases and restructured after service in the cupola shaft during the smelting of cast iron with spheroidal graphite using fluorine-containing scrappages of electrodes as the carbon component. In the crucible, the products were also saturated with cast iron and slag melts, causing the refractory to wear out not only chemically but also mechanically.
References
I. D. Kashcheev, K. K. Strelov, and P. S. Mamykin, Chemical Technology of Refractories: study guide, Intermet Engineering, Moscow (2007).
A. Z. Isagulov, I. D. Kashcheev, E. A. Sidorina, and K. G. Zemlyanoy, “Behavior of the fireclay lining of a coke gas cupola furnace when melting cast iron using anode scrappage in electrolysis production. Part 1,” Nov. Ogneup., No. 9, 3 – 8 (2021).
K. K. Strelov, I. D. Kashcheev, Theoretical Foundations of Technology of Refractory Materials [in Russian], Metallurgiya, Moscow (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
1Part 1 was published in Novye ogneupory No. 9 (2021).
Translated from Novye Ogneupory, No. 11, pp. 4 – 6, November, 2021.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Isagulov, A.Z., Kashcheev, I.D., Sidorina, E.A. et al. Behavior of the Fireclay Lining of a Coke Gas Cupola Furnace When Melting Cast Iron with Anode Scrappage in Electrolysis Production. Part 21. Refract Ind Ceram 62, 614–617 (2022). https://doi.org/10.1007/s11148-022-00651-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-022-00651-7