Data are provided for preparing fuzed and fuzed stabilized zirconia from baddeleyite and synthetic zirconia in induction, plasma, and electric arc furnaces. Results are discussed and prospects for carbothermal preparation of zirconium dioxide from zircon in an electric arc furnace are demonstrated. A favorable effect of oxidation melting in an electric arc furnace on quality and the process of fuzed product preparation is demonstrated. Data are provided for preparing fuzed zirconia from zircon in plasma furnaces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Zirconium dioxide is a chemical compound of zirconium used most extensively which in natural form (baddeleyite) and synthetic material is used in various branches of industry, mainly for manufacturing refractories, commercial ceramics, and abrasives. In the last decade there has been a significant increase in the use of zirconium dioxide for producing materials for electronics, oxygen sensors, pigments for ceramics, catalysts, etc. A large industry requiring zirconium dioxide is the atomic industry for preparation of nuclear-clean zirconium metal (www.chmz.net).
Alongside natural and synthetic zirconium dioxide there is also considerable demand for its structural modifications, monoclinic and cubic, stabilized with calcium and yttrium oxides, in fuzed form. According to expert evaluation the world production of fuzed zirconium dioxide (FZD) is more than 50 thousand tons; in this case the proportion of stabilized grade does not exceed 20%.
The requirement within Russia for FZD due to a lack of in-house production is accomplished entirely due to imported supplies. In this case the users of FZD are enterprises of strategically important branches of industry (atomic, aviation, space rocket, metallurgical, etc.). Therefore creation of large tonnage production of FZD it not only an important task, but it is also necessary in the case of occurrence of unforeseen external economic and political situations. Research for preparing FZD was quite widespread within the USSR [1, 2] and continues within Russia [3, 4]. Systematic comprehensive study for development of rational methods for preparing FZD is being conducted in NITU MISiS [-57], and also starting from 2010 in OOO Tekhnokeramika (Obninsk, Kaluga Region).
The choice of a melting method is a governing factor for producing FZD. In this case it is necessary to consider that FZD with use of various melting methods may be prepared either in cast form (castings) or in a dispersed form (granules). The form of FZD preparation determines its subsequent use. An example of preparing zirconium dioxide in a cast form is melted and cast refractories containing more than 90% ZrO2 that the final saleable product. Fuzed granulated zirconium dioxide, apart from use as heat insulation material, is a raw material for semifinished product for subsequent processing, etc.
Consideration of heating methods used for preparing FZD makes it possible to take account of rational requirements for its quality and volume of product, and to determine the form of fusion unit. The main fusion methods during FZD preparation by remelting of synthetic zirconium dioxide or baddeleyite concentrate are induction and electric arc melting Induction melting is based on a method of direct HF heating and melting oxide materials in a cold crucible. A periodically operating cold crucible scheme is shown in Fig. 1. The production process includes starting heating of a charge (in this case powder of ZrO2 with additives) to the melting temperature, and an increase in the original melt volume to that prescribed followed by crystallization [8, 9].
A periodic operation induction melting and crystallization unit of the cold crucible type is used for producing ZrO2 single crystals with different additives in order to create heat-barrier protective coating powders in the aerospace industry and also in the jewellery industry (fianite) [10]. The requirements for producing fused oxide materials are most completely satisfied by induction continuous-sequential melting of ingots using a travelling melting and crystallization unit with a moving hearth (Fig. 2).
Features of this process are the continuous melting of an original charge at the molten bath surface and constant crystallization of melt within the bottom part of a bath. The ingot obtained moves upwards from the melting zone by means of a mobile hearth to which it is attached. The specific expenditure for melting in this case is significantly less than with melting in a cold crucible.
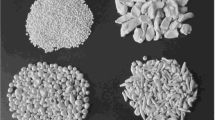
Induction melting technology in a cold crucible for powder FSZD, i.e., melting stabilized zirconium dioxide (with calcium and yttrium oxides) is conducted by the enterprise Tekhnokeramika. The FSZD powder (Fig. 3) contains CaO (4.6%), SiO2 (≤0.3%), Fe2O3 (≤0.1%). Output is organized within the enterprise for powder fractions coarser than 5, 3 – 5, 1 – 3, 0,5 – 1, 0,2 – 0,5 mm, and 40 – 100 μm, and also finely milled powder (finer than 45 μm) with an average particle size of 5 – 10 μm.
Use of an induction heating unit is economically valid with use of raw material with high purity specifications (for example for fianite) making it possible to produce highly profitable product. This melt has low charge material losses, which is very important with use of expensive raw material. However, disadvantages of using these units for preparing a considerable volume of FZD is the low productivity and the difficulty of obtaining fine materials.
Electric arc melting, whose source of thermal energy is an electric arc, is used extensively in metallurgy and production of fuzed oxide materials [11, 12]. As a result of concentrated energy release within a small volume in an electric arc furnace high temperatures are achieved (2200 – 2700°C) required for melting charge based on refractory oxides (ZrO2, Al2O3, MgO, etc.). An electric arc also provides a fast heating rate, increased unit productivity, and in the majority of cases the cleanliness required for melted material. Electric arc melting is used to prepare casting of fuzed and cast refractories containing ZrO2 up to 94% (refractory ER 1195). In addition, an advantage of electric arc melting is the possibility of preparing fuzed material in a dispersed form.
Three-phase electric arc furnaces with graphite electrodes and an air cooled vessel are used for melting refractory oxides. Furnaces intended for pouring melt into a casting mold and casting preparation, and also melt granulation, have an inclined furnace mechanism (Fig. 4). Electric arc furnaces are also used with melting “en bloc”: a charge is melted within a mold located within the casing of a moving trolley. Casting cooling proceeds within the mold (Fig. 5).
During “en bloc” melting of charges consisting of baddeleyite and chalk in a three-phase electric furnace with power of 400 kV·A (mold 900 mm in diameter and 1200 mm high) for 3 h blocks weighing 680 kg were melted [1]. The electric melting regime: current 3500 – 4000 A, voltage 82 V. The output of melted material is 68% and for unmelted material it is 32%. Melted material has a dark color as a result of partial ZrO2 reduction disrupting its stoichiometry and carbide formation. In order to restore ZrO2 stoichiometry melted material was subjected to oxidation firing at 1500°C and then ground in screw and beam crushers in order to obtain sizes less than 2 mm. The chemical composition of the ground material fraction 0.5 – 2 mm after screening and magnetic separation,%: ZrO2 94.3, CaO 5.5.
FSZD, stabilized with calcium oxide, has also been prepared in a single-phase electric furnace with power of 300 kVA with melting of baddeleyite concentrate with limestone. Melting was conducted “en bloc” with a voltage of 88 V and electrical energy consumption of 2900 kW·h/ton [12, pp. 173, 174]. Data for preparing FSZD have also been provided in articles [14, 15]. In this case it has been demonstrated that the productivity of “pouring” melting is higher by a factor of 2 – 3 than “en bloc” melting.
Melting of zirconium dioxide due to a high melting temperature (2700°C) presents considerable difficulties and requires high energy expenditure. Creation of new electric arc units ÉPD-450 and
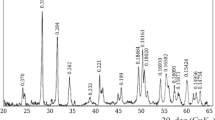
ÉPD-600M has made it possible to prepare FZD in an oxidation melting regime [16, 17]. In an ÉPD-450 electric arc furnace with power of 450 kV·A melting of zirconium dioxide grade TsrO-1 with chemically pure calcium carbonate has been used to prepare casting weighing up to 32 kg (melting duration not more than 60 min). The composition of the crystalline phase of the VTs-2 material obtained according to x-ray microanalysis data,%: ZrO2 91.5 – 92.7, CaO 7.0 – 7.8, impurities 0.4 [18]. The structure of the material was ZrO2 crystals of cubic modification with sizes of 0.15 – 0.35 mm having a shear plane of polygonal isometric shape. A feature of this material is presence of a surrounding crystalline rim 0.002 – 0.003 mm wide around grains of basic crystallization. Probably this points to presence of small amount of ZrO2 of monoclinic modification that is indicated by weak peaks in the x-ray pattern for this substance. However, a high degree of ZrO2 stabilization is confirmed by the dilatometric test curves (Fig. 6) on which there is no hysteresis loop typical for materials of baddeleyite composition. A change should be noted in the color of material prepared from light grey to greenish as a result of an especially selected oxidation melting regime. In contrast to this according to data in [1] with arc reduction melting of a charge based on baddeleyite and chalk due to disturbance of ZrO2 stoichiometry castings acquired a color from dark grey to black. Therefore, for these materials additional firing was necessary in order to restore ZrO2 stoichiometry.
A modernized ÉPD-600M unit with improved energy properties has made it possible to implement an oxidation melting regime and to obtain a heated liquid flowing melt with the possibility of maximum pouring from the furnace. Therefore a systematic study of the FSZD preparation has been performed in an ÉPD-600M furnace with moving graphite electrodes 100 mm in diameter. The raw materials used were baddeleyite powder grade PB-0 containing 98.5% ZrO2 and chalk (GOST 8253). After charge melting the melt was held for some time in the furnace and then poured into a graphite mold with preparation of castings weighing up to 50 kg. A series of castings was also made with preparation of FSZD granules and aggregates with size from 1 to 10 mm (Fig. 7).
According to spectrographic analysis data FSZD has a completely crystalline structure of quite coarse ZrO2 crystals. Castings have coarser crystals (0.40 – 0.60 mm) compared with granules (0.20 – 0.40 mm). The crystalline phase of granular FSZD according to x-ray microanalysis data contains 91.5 – 92.4% ZrO2 and 7.6 – 8.5% CaO. Results of x-ray phase analysis have confirmed that the sole crystalline compound of all melted castings and granules is a cubic modification of ZrO2.
The examples provided of using induction and electric- arc melting point to the possibility of preparing FZD and FSZD by remelting quite expensive powder raw material, i.e., baddeleyite or synthetic ZrO2. Synthetic ZrO2, prepared by a prolonged and complex hydrometallurgical method is a highly pure an fine grained powder that is used most rationally in the preparation of structural and electronic ceramics. In order to produce the majority of types of refractories and commercial ceramics this form of raw material is quite expensive, which retards effective development. Therefore in order to reduce raw material expenditure methods have been developed for preparing FZD of monoclinic and cubic modifications from zircon material whose cost is an order to magnitude lower than synthetic ZrO2. The main method for preparing ZrO2 from zircon, implemented practically, concerns electric arc and plasma induction melting.
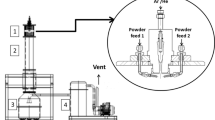
Basic plasma and plasma-induction melting are dissociation of zircon introduced into a high temperature zone (7000 – 9000°C) of a plasma reactor within which it dissociates to ZrO2 and SiO2 followed by SiO2 removal by a hydrometallurgical method. The fundamental equipment layout of a pilot plant unit for producing FZD from zircon sand in a plasma furnace is shown in Fig. 8 [19. 21].
Zircon sand, ground to a size of 200 μm, is fed uniformly into a plasma discharge created by an arc plasmatron. During fractions of a second of dwell time in a plasma there is melting and almost complete (99%) breakdown of zircon into oxides:
After cooling and solidification a product contains spherical particles of monoclinic ZrO2 coated with a layer of amorphous silica; the latter as a result of increased reaction capacity is selectively leached with 1% caustic soda solution at 120°C. After two-stage countercurrent leaching ZrO2 is obtained with an SiO2 content less than 1% and also sodium silicate solution being a useful subsidiary product. Zirconium dioxide consists of particles represented by complete spheres 0.1 – 0.2 μm in diameter. Depending on user specifications for the product the process may be shortened by using just one leaching. According to data in [19] it is possible to obtain three forms of product containing 70% ZrO2 and 30% SiO2, 96% ZrO2 and 4% SiO2 or 99% ZrO2 and 0,5% SiO2. Other examples of FZD preparation from zircon by plasma decomposition have been provided in publications [22,23, – 24]. Serious disadvantages of arc plasmatrons are the impossibility of prolonged continuous operation with a power of more than 300 kW, significant electrode erosion, and use of argon of a plasma-forming gas.
Use of high-frequency induction (HFI) plasma units for preparing FZD is more effective compared with the arc plasma technique [4]. In a test HFI-plasma unit with power of 1 MV·A the Tekhnokeramika enterprise conducted experiments for thermal decomposition of zircon in an air plasma (Fig. 9) [25]. A zircon particle is shown in Fig. 10 after plasma treatment represented by sphere consisting of a matrix of zirconium dioxide whose space is filled with amorphous silicon dioxide exhibiting high reaction capacity that accelerates the process of pure zirconium dioxide extraction, In addition, work has been conducted in treating zirconium concentrate decomposition product obtained in an air plasma followed by ammonium fluoride extraction and preparation of materials containing 99.9% ZrO2. Pilot plant batches of ZrO2 product have been produced by technology for preparing ZrO2 with programmed treatment of plasma-dissociated zircon developed in the Tekhnokeramika enterprise together with the Tomsk Polytechnic University.
Electric-arc melting for FZD preparation by zircon carbothermal decomposition is based on zircon decomposition at high temperature to oxide by reaction (1) and reduction of the SiO2 separated to volatile SiO monoxide [26]:
Silicon monoxide volatilizes from the furnace working zone and is subsequently oxide to SiO2 whose particles and captured in dust catchers. In order to implement carbothermal decomposition electric arc furnaces have been used with “en bloc” melting (see Fig. 3) and “pouring” (see Fig. 4). In order to prepare FSZD from zircon (65 – 67% ZrO2) reduction melting “en bloc” is used during which the SiO2 content is reduced from 33 to 0.5 – 0.9% [12, pp. 172, 173]. A charge included zircon, iron shavings and metallurgical coke. The melted product was ground, demagnetized and calcined at 1400 – 1450°C for carbide decomposition. FSZD composition, %: ZrO2 94 – 95, CaO 4 – 5.
Implementation of zircon carbothermal decomposition in an ÉDP-450 electric arc unit has made it possible in the first stage to reduce the amount of SiO2 in zircon by almost a factor of three and also to prepare raw semifinished products containing more than 80% ZrO2. With use of an oxidation melting regime and casting coke as carbon reducing agent fuzed product has been prepared in the form agglomerates with sizes of 10 – 15 mm [27]. The high content of residual silica in fuzed product is explained by significant wear and combustion of coke in the furnace charging period, which led to a reduction on its reducing function. Achievement of a high degree of silicon removal requires appropriate adjustment of the technology (change in melting regime and conditions, increase in reducing agent efficiency, charge compaction, etc.).
It should be noted that although adequate research has been conducted for preparation of FZD and FSZD pilot plant verification in our country has not been completed due to liquidation of the pilot plant experimental bases as a result of the economic situation in the 1990s. In selecting a melting unit for industrial production of FZD and FSZD it is necessary to consider many years of practice of using electric arc furnaces for preparing fuzed oxide materials [11, 12]. On the basis of this equipment it is possible to adopt electric arc furnaces types DS-05, DSPM-1.5, and OKB 2126A [16] modernized in accordance with specifications of fused product and on a production scale.
Conclusion
1. Results of analyzing melting units indicate that in order to create pilot plant production of FZD and FSZD from baddeleyite concentrate or synthetic ZrO2 it is more rational to use an electric arc furnace with the possibility of performing an oxidation melting regime. Creation of a pilot plant electric arc unit based on the DS-0.5 equipment has made it possible to produce within a year by the melting method up to 1000 tons of fuzed granular ZrO2 of monoclinic modification and ZrO2 stabilized with calcium or yttrium oxides. This product is intended primarily for the main users, i.e., enterprises for manufacturing refractories, abrasives, ceramics, and pigments. In order to prepare structural and electronic ceramic oxide sensors with increased specifications with respect to raw material cleanliness it is possible to use induction and plasma units taking account of the economic expediency.
2. In order to prepare FZD and FSZD from zirconia raw material two versions are possible: electric arc carbothermal and plasma with use of hydrometallurgy. Use of an electric arc furnace for carbothermal treatment of zircon will make it possible to organize quite large production (several thousand tons a year) of FZD and FSZD. However, this requires special melting equipment, which besides the high temperature of the process should provide removal of considerable amount of silicon monoxide; in this case melt granulation during casting is required.
3. Use of plasma units will make it possible to organize medium tonnage production with output of individual batches of FZD and FSZD. The ZrO2 produced in this case exhibits good cleanliness and has a particle size suitable for use in various industries without additional crushing and grinding.
References
A. G. Karaulov, A. D. Malyuk, V. G. Druzhinin, and A. V. Oistrakh Karaulov, “Fusing baddeleyite in an electric arc furnace,” Refractories, 24(2), 208 – 210 (1983).
A. G. Karaulov, T. E. Sudarkina, Ya. G. Gaponov, et al., “Experience of melting baddeleyite in an OKB 955N electric arc furnaces,” Ogneupory, No. 6, 21 – 24 (1983).
V. A. Sokolov, “Preparation of fused stabilized zirconium dioxide,” Refract. Ind. Ceram., 56(2), 119 – 121 (2015).
A. B. Lisafin, “Development and study of high-frequency induction plasma unit with a capacity of 1 MV·A for new technology for preparing zirconium dioxide from zircon concentrate,” Diss. Cand. Techn. Sci., Moscow (2017).
V. A. Sokolov, S. V. Markov, and E. V. Bogatyreva, “Development of rational methods for preparing zirconium dioxide,” Proc. Internat. Conf. of Refractory Workers and Metallurgists(7 – 8 April, 2016, Moscow), Novye Ogneupory, No. 3, 26 – 27 (2016).
V. A. Sokolov, E. V. Bogatyreva, and S. V. Markov, “development of ecological security for preparing zirconium dioxide from zirconia concentrate,” Proc. Sci.-Pract. Conf. “Intensification of hydrometallurgical processes for treatment natural technogenic raw material. Technology and equipment,” St. Petersburg, 28 May- 1 June 2018.
V. A. Sokolov, “Preparation of fuzed stabilized zirconium dioxide,” Proc. Internat. Conf. of Refractory Workers and Metallurgists, 3 – 4 April 2014, Moscow, Novye Ogneupory, No. 3, 24 (2014).
V. I. Aleksandrov, V. V. Osiko, V. M. Tataritsev, et al., “Melting refractory dielectrics by direct HF heating method in and a “cold” container,” Neorgan. Materialy, 9(2), 236 – 238 (1973).
Yu. B. Petrov, A. V. Shkul’kov, and I. V. Shurygina, “Induction melting of refractory oxide materials in cold crucibles,” Refractories, 21, 536 – 541 (1980).
D. I. Kravchuk and V. I. Kravchuk, “Features of production by a fianite skull method,” Molodoi Uchenyi, No. 10(90), 236 – 239 (2015).
O. N. Popov, P. T. Rubalkin, V. A. Sokolov, and S. D. Ivanov, Production and Application of Melting and Casting Refractories [in Russian], Metallurgiya, Moscow 91985).
A. N. Sokolov, Yu. B. Ashimov, A. V. Bolotov, et al., Fuzed Refractory Oxides [in Russian], Metallurgiya, Moscow 91988).
SÅÐR Group Catalogue. Refractory Products for Glass Furnaces. Fused cast refractories. Fused cast products.
G. I. Kuznetsov and E. S. Borisovskii, “Fused refractories: New developments and prospects,” Refractories, 35, 389 – 391 (1994).
V. A. Perepelitsyn, A. M. Gorokhovskii, A. V. Fedortseva, et al., “Production of fuzed zirconium dioxide in AO Pervoural Dinas Plant,” Novye Ogneupory, No. 7, 25 – 29 (2016).
V. A. Sokolov, M. D. Gasparyan, and P. P. Mamochkin, “Arc melting plant synthesizing and producing fusion-cast,” Refract. Ind. Ceram., No. 3, 185 – 188 (2009).
M. D. Gasparyan and V. A. Sokolov, “Pilot plant unit ÉPD- 600M for melting refractory oxide materials,” Coll. VI Internat. Sci.-Pract. Conf. “Energy saving technology in industry. Furnace units. Ecology,” ITEP NITU MISiS, Moscow (2012).
V. A. Sokolov, “Fusion-cast refractories in high-zirconia region of the ZrO2–SiO2–CaO system,” Refract. Ind. Ceram., 46(3), 197 – 200 (2005).
M. L. Thorpe and P. H.Wilks, “Electric-arc furnace turns zircon sand to zirconia,” Chem. Eng. (New York), 78(26), 117 – 119 (1971).
A. N. Zelikman, Refractory Rare Metal Metallurgy [in Russian], Metallurgiya, Moscow 9186).
Yu. N. Tumanov, Plasma, High-Frequency, Microwave, and Laser Technology in Chemical-Metallurgical Processes [in Russian], Fizmatlit, Moscow (2010).
P. H. Wilks, “Arc-plasma dissociation of zircon,” Chem. Eng. (New York), 82(24), 56 – 57 (1975).
G. Barnett, M. Houchin, and D. Jenkins, “Zircon to zirconia from sand to high performance ceramics,” Mineralogy-Petrology Symposium, Sidney, 6 – 8 February 1989. Parkville (1989).
F. M. Evans and J. P. Williamson, “The influence of quench rates on the microstructure and properties of plasma-dissociated zircon,” J. Mater. Sci., 14(3), 680 – 686 (1979).
G. A. Farnasov and A. B. Lisafin, “Dissociation of zirconia after treatment in air by a high-frequency induction plasma,” Fiz. Khim. Obrab. Materialov, No. 2, 29 – 34 (2015).
V. A. Sokolov, “Decomposition of zirconia by melting in an electric arc furnace,” Svet. Met., No. 7, 59 – 63 (2006).
V. A. Sokolov, “Melting zircon in electric-arc furnace — a method for preparing refractory materials and green semifinished products,” Refract. Ind. Ceram., 55(3), 191 – 193 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 12, pp. 3 – 9, December, 2020.
Rights and permissions
About this article
Cite this article
Sokolov, V.A., Gorbanenko, M.A., Lisafin, A.B. et al. Selection of Melting Unit for Fuzed Zirconium Dioxide Production. Refract Ind Ceram 61, 619–625 (2021). https://doi.org/10.1007/s11148-021-00531-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-021-00531-6