New temperature treatment conditions were proposed for the Pervouralsk quartzite PKMI-1V used as a lining material for commercial-frequency induction crucible furnaces. The new conditions allow conducting synthetic pig iron smelting at the temperatures in excess of 1450°C while maintaining high lining resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The commercial-frequency induction crucible furnace (IChT) relates to the principal fixed capital assets of a commercial foundry and directly affects the level of production expenses and productivity. Therefore, an increase in reliability of operation of the melting unit is one of the main objectives providing a necessary degree of reproducibility of the fixed capital assets to ensure the following parameters of the unit: use of lightweight melting stock to charge the furnace [1], alloy smelting flexibility, productivity, high resistance of lining, energy efficiency and maintenance costs [2].
The IChT furnace is designed for alloy smelting at a temperature not exceeding 1450°C while utilizing acid lining. Under proper furnace operating conditions, acid lining can withstand up to 300 – 350 melting cycles. The duration of relining process of the IChT-1 furnace (from the beginning of furnace shut-down to the first complete pouring of the finished hot melt) constitutes from 2.5 to 3 days. For this reason, a decrease in the lining resistance results in increased downtime, labor expenses, as well as material and energy costs.
To ensure maximum lining resistance of the IChT furnace, the early smelting technology provided for leaving a liquid residue (hot heel practice) in the amount of at least 1/3 of the crucible capacity as a mandatory condition. In addition, the allowable quantity of utilized steel scrap did not exceed 30% of the total capacity of the crucible. A limitation of the steel scrap content is caused by its melting temperature. For example, depending on its chemical composition, the melting temperature of the carbon steel scrap constitutes 1400 – 1600°C.
However, the situation on today’s market of the secondary raw materials is as follows: pig-iron scrap is practically non-existent, and the cost of merchant pig iron as well as the transportation cost associated with delivery thereof has increased sharply. Therefore, the only way to facilitate smelting of synthetic cast iron in the IChT furnace is by using the increased quantities of steel scrap, carburizer, and ferroalloys, which would require raising the melting temperature above the allowable level for this furnace. Based on the previous experience, it was shown that the rated capacity of the furnace allows conducting smelting operations at the temperatures above 1450°C. This is explained by the fact that during the IChT furnace design stage, a need for its operation at different melting conditions was considered. Such conditions include partial filling of a crucible with melt, carburization process, crucible drying and sintering. For this purpose, the IChT furnaces are equipped with a dedicated transformer having a constant power at the first 4 – 5 voltage steps due to excess of power (15 – 20%), which enables higher current at a rated power than at a rated voltage [3].
However, there is a problem associated with the acid lining resistance. Based on the prior experience, conducting alloy smelting at 1550°C will reduce the resistance of the lining to 180 melting cycles [4]. Replacement acid lining with the one made of other types of fire-resistant materials would lead to a 4 – 6 time increase in price. In addition, pre-made lining materials are designed for use in the medium-frequency furnaces with embedded inductor, while in the IChT furnace, the inductor is unrestricted and is subject to deformation during cyclic melting conditions, which has a negative effect on the lining resistance. Therefore, preserving high resistance of acid linings when smelting synthetic pig-iron in IChT furnaces at the temperatures exceeding 1450°C is a crucial task [5]. The resistance of the lining is affected by defects resulting from multiple factors associated with the manufacturing process [6], sintering conditions and slag impact [7, 8] (Table 1). In addition, the resistance of the lining is influenced by the quality of utilized quartzite and manufacturing of the lining mass, as indicated below:
-
1.
Quartzite should comply with the product specification (TU) requirements and be of a certain fraction depending on the furnace capacity.
-
2.
Quartzite humidity should not exceed 0.3%.
-
3.
No segregation of the lining mass is allowed when filling the crucible, which may result in the formation of the weakened zones containing coarse-grained quartz particles prone to forming cracks. The segregation causes formation of horizontal cracks of significant thickness and depth in the lining. It is possible even when using quartzite which conforms to the TU requirements, since segregation of grains may occur during transportation.
-
4.
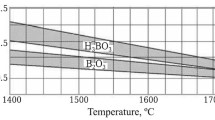
When preparing a lining mass, it is necessary to ensure thorough mixing of quartzite with boron-containing additive. Many commercial foundries use the H3BO3 additive, since its required amount is two times more compared to B2O3 additive, which makes it easier to evenly distribute the additive throughout the bulk of the lining mass. Uneven mixing results in loosening and separation of the lining during the initial operation period.
Temperature-related phase transformations in quartzite were established as early as in 1912 by C. N. Fenner [9]. Further studies of phase transformations in quartzite by using modern techniques did not reveal any substantial changes in their patterns [10, 11]. However, in 2013, I. Vettegren [12] discovered that heating synthetic quartz crystals above 400 K causes formation of tensile stresses in their surface layer, which could reach 300 MPa. The appearance of stresses was explained by an increase in volume of the underlying layer due to β-quartz formation. Therefore, studying phase transformations in quartzite used to line the IChT furnaces is a crucial task.
The objective of this work is to study the processes leading to variation in the crystal lattice parameters of PKMI-1V quartzite during heat treatment to ensure the required humidity. PKMI quartzite is produced at the Pervouralsk Silica Plant for manufacturing linings of the induction crucible furnaces installed at the Volga Automobile Plant (VAZ), Kama Automobile Plant (KamAZ), Gorky Automobile Plant (GAZ), Likhachov Automobile Plant (ZIL) and commercial foundries. First, the extracted raw materials are subject to crushing to produce fractions smaller than 5 mm and shipped in bulk by rail in open freight cars. For this reason, majority of foundry designs provided for quartzite drying equipment (SDO type furnaces), runner mills (1A18 type) and quartzite screening into fractions using mesh sieves. Tridymite is known to be the most stable of all phases providing a stable volume at 840 – 1470°C. For this reason, the following temperature conditions were chosen for quartzite treatment to obtain humidity of less than 0.3%: heating to 800 – 900°C with subsequent soaking for 8 – 10 h in stainless steel containers.
The Pervouralsk Silica Plant can supply quartzite with the humidity of less than 0.3%, and (upon customer’s approval) no more than 3.0%. Ensuring humidity of 0.3% is achieved by drying and subsequent annealing (same as for the silica products) at the temperatures above 1000°C [13]. Dry quartzite is supplied in the metal containers, which is a big inconvenience for many enterprises, since metal containers are returnables, but it is difficult to return them by rail within the established time frame due to inability to ensure necessary loading of a rail car. Wet quartzite is supplied in big bags capable of holding 800 – 900 kg of load, which do not have to be returned. Therefore, the use of wet quartzite is more convenient and its price is twice as low. The only disadvantage is that it requires drying.
The variations in the crystal lattice parameters of PKMI-1V quartzite according to TU 1511-022-00190495 –2003 were studied by x-ray diffraction (XRD) analysis by using D8 ADVANCE x-ray diffractometer (Bruker) with Bragg-Brentano focusing geometry. To conduct temperature-related experiments, a high-temperature HTK 16 camera and x-ray tube with copper anode were used. A diffraction spectrum was recorded using a high-speed position-sensitive VÅNTEC-1 detector while recording at 2θ = 10 – 90 degrees with 0.007 degree increments [13]. First, the quartzite batch was recorded at 25°C followed by heating to 800°C and repeated recording after soaking for 2 h. Then, the batch was cooled down to room temperature and recorded again. XRD spectra of quartzite are shown in Fig. 1, and the study results are provided in Table 2.
A previously unknown variation in the quartzite crystal lattice condition consisting in the d-spacing angle shift was discovered. The differences between the quartzite crystal lattice dimensions at 25 and 30°C are insignificant and fall within the measurement error. To prove this finding, it was decided to conduct simultaneous thermal analysis using STA 449C Jupiter instrument (Netzsch). The heating rate was assumed to be 10 K/min, and the sampling rate was 100 points/min. To conduct measurements, two crucibles were used, of which one contained the studied sample, and the other was used as a reference.
The experimental studies were conducted in corundum crucibles [15]. After heating to 800°C and soaking for 2 h, the following thermogram was obtained (see Fig. 2).
At 570°C, a rather intensive phase transformation occurred accompanied by generation of heat. It is characterized by the peak area or the area of the thermal effect of new phase formation (enthalpy) numerically equal to –349.2 mJ (see Fig. 2). The phase transformation process is accompanied by creation of a more rarefied aggregate state (due to increase in volume and decrease in density as a result of crystal lattice expansion). Then it was decided to conduct the same study by performing heat treatment under the following experimentally selected conditions based on quartzite color change (from dark red at 800 – 900°C to natural gray below 300°C): heating to 200°C and soaking for 4 h. The obtained results are shown in Table 3 and on Fig. 3.
It was established that as a result of heating quartzite to 200°C and soaking for 4 h, no variations in crystal lattice are observed. Therefore, it became necessary to conduct the next step of the study by expositing quartzite after removing moisture to different temperature conditions corresponding to the technological operations of melting (Fig. 4). These conditions remain unchanged until complete technological wear of the lining, i.e. until its removal. The study was conducted using XRD analysis, and the results are shown in Table 4.
The applied method of solving the task of changing quartzite heat treatment conditions is one of the commonly used ways of solving different kinds of problems having a complex nature, and relates to a program-oriented and goal-oriented approach intended to achieve certain results [16].
The conducted research proves the efficiency of using heat treatment of the Pervouralsk quartzite (PKMI-1V brand) at 200°C, since the lining based thereon is able to support smelting of synthetic pig-iron at a temperature above 1450°C, overheating required to ensure the maximum carburizer intake, and production of wear-resistant cast iron while maintaining high resistance (250 – 300 melting cycles). In addition, it enables smelting of steel [17] and ferrosilicon [18].
Conclusions
-
1.
In quartzite subjected to heat treatment at 800°C, tridymite formation is observed after sintering, whose content only increases during subsequent melting conditions.
-
2.
In quartzite subjected to heat treatment at 200°C, cristobalite formation is observed after sintering (skipping the tridymite phase), whose content only increases during subsequent melting conditions. This phase can withstand higher temperatures (1450 – 1600°C), thus, enabling the use of up to 80 – 90% of steel scrap when charging the furnace.
-
3.
By changing the temperature of heat treatment of raw quartzite in order to remove moisture from 800 to 200°C, it becomes possible to use low-temperature heating furnaces, and therefore, reduce production expenses. This is particularly important in terms of energy consumption and replacement of stainless steel with carbon steel used to make containers for drying quartzite. For example, the cost of a stainless steel sheet is 3 – 5 times higher compared to carbon steel, and the consumption of electric power per 1 ton of charge (furnace model SDO 15.15.10/12, capable of heating quartzite to 900°C) is 3 times higher compared to furnace model SDOS 10.12,5.10/2,5IZ for heating the same charge to 200°C.
References
V. S. Shumikhin, P. P. Luzan, and M. V. Zhelnis, Synthetic cast iron [in Russian], Naukova dumka, Kiev, 160 (1971).
V. V. Kukartsev and O. A. Antamoshkin, “Combined decision-making method in recreation of fixed capital assets,” Problemy Mashinostroeniya i Avtomatizatsii, Issue 2, 56 – 60 (2011).
A. A. Prostyakov, Induction furnaces and mixers for cast iron smelting [in Russian], Énergiya, Moscow, 216 (1977).
V. S. Sassa, Lining of induction melting furnaces and mixers [in Russian], Énergoatomizdat, Moscow, 120 (1983).
I. Riposan, M. Chisamera, and S. Stan, “Enhanced quality in electric melt grey cast irons,” ISIJ Int. 53(10), 1683 – 1695 (2017).
A. A. Kassie and S. B. Assfaw, “Minimization of casting defects IOSR,” J. Eng. (IOSRJEN), 3(5), 31 – 38 (2013).
J. Zuno-Silva, A. Bedolla-Jacuinde, J. M. Martínez-Vázquez, et al., “Laboratory scale study of uncommon degradation of SiO2 refractories used in induction furnaces,” Revista Electronica Nova Scientia, 6(11), 113 – 134 (2013).
V. A. Kukartsev, “Causes of quartzite lining failure during operation of induction crucible furnace and ways of its prevention,” Zagotovitelnoe Proizvodstvo, 9, 7 – 9 (2013).
C. N. Fenner, “The various forms of silica and their mutual relations,” J. Wasn. Acad. Sci., 2, 471 – 480 (1912).
F. M. Wahl, R. E. Grim, and R. B. Graf, “Phase transformations in silica as examined by continuous x-ray diffraction,” The American Mineralogist, 46, January – February (1961). Access at: http://minsocam.org/ammin/AM46/AM46_196.pdf.
A. B. Thompson and M. Wennemer, “Heat capacities and inversions in tridymite, cristobalite, and tridymite-cristobalite mixed phases,” The American Mineralogist, 64, 1013 – 1026 (1979). [Electronic resource]. Access at: http://minsocam.org/ammin/AM64/AM64_1018.pdf.
V. I. Vettegren, R. I. Mamaliyev, and G. A. Sobolev, “Diffuse phase transition in a superficial layer of quartz during temperature variations,” Fizika Tverdogo Tela, 55(10), 1987 – 1992 (2013).
I. D. Kashcheev, K. K. Strelov, and P. S. Mamykin, Chemical technology of refractory materials, Intermet Engineering, Moscow, 752 (2007).
V. A. Kukartsev and A. K. Abkaryan, “Study by x-ray methods of the effect of temperature on crystal lattice interplanar distances of Pervouralsk quartzite used for induction furnace lining,” Refractories and Industrial Ceramics, 54(5), 413 – 415 (2013).
V. A. Kukartsev, A. I. Trunova, and A. V. Kukartsev, “Thermal analysis of quartzite used to line a crucible-equipped industrial-frequency induction furnace,” Refractories and Industrial Ceramics, 55(3), 220 – 222 (2014).
K. I. Gorlevskii and A. V. Kukartsev, “Principles of innovative business process management at an aerospace industry enterprise,” Ekonomika i Management System Upravleniya, No. 1(11), 44 – 52, (2014).
V. A. Kukartsev, “Steel smelting using commercial-frequency induction crucible furnaces (IChT),” Liteishchik Rossii, 12, 35 – 36 (2012).
M. Chaabet and E. Dötsch, “Steelmaking based on inductive melting heat processing,” Heat Processing, 10(1), 49 – 58 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 5, pp. 28 – 33, May, 2018.
Rights and permissions
About this article
Cite this article
Kukartsev, V.A., Kukartsev, V.V. & Kukartsev, A.V. Effect of the Temperature Treatment of Quartzite on the Lining Resistance of Commercial-Frequency Induction Crucible Furnaces. Refract Ind Ceram 59, 252–256 (2018). https://doi.org/10.1007/s11148-018-0216-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-018-0216-2