Wet milling in a ball mill is used to prepare a suspension based on amorphous and crystallized magnesium aluminum silicate glass. The stabilizing additives used are hydrochloric and oxalic acids, and also an aqueous ammonia solution. Raw material is molded by slip casting in porous molds. Optimum ranges are provided for magnesium aluminum silicate glass suspension casting parameters in order to prepare green workpieces with not more than 30% porosity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Glass ceramic materials are in demand in the space and aviation branches of industry as materials for preparing antenna radomes for rockets of different classes. A number of specifications are laid down for radioparent domes apart from radio engineering properties (low value of dielectric permittivity and dielectric loss angle) providing protection of radio equipment from external action: thermal shock resistance, refractoriness, and low LTEC [1]. The magnesium aluminum silicate system is promising for creating glass ceramic material based upon it satisfying these specifications, whose main crystalline phase is cordierite.
The aim of this work is preparation of highly concentrated ceramic binder suspensions (HCBS) based on magnesium aluminosilicate glass suitable for molding workpieces by slip casting in porous molds in order to prepare dense pore-free glass ceramic material of cordierite composition. Glass ceramic materials were first studied by Stookey in examining processes of photosensitive glass crystallization [2]. Newly developed material pyroceram was prepared from glass by crystallization by a special regime. The material exhibited good thermal shock resistance, improved mechanical, electrical, and other properties [3].
One of the most well-known glass ceramic materials is pyroceram 9606 [4]. This is glass ceramic of the magnesium aluminosilicate system with titanium dioxide as a catalyst. The main phase is cordierite and provides low dielectric losses, good thermal shock resistance, and strength, and also resistance to climatic effects. Porosity is absent from the material, and it is resistant to a high moisture content even without use of coatings.
Various methods are well known for preparing cordierite ceramic: crystallization from glass, growing single crystals from fluxes, sol–gel technology, solid-phase synthesis from oxides or from natural materials [5]. Slip casting is one of the most widespread methods for molding ceramic materials making it possible to prepare objects of any shape with sufficiently uniform distribution of material properties throughout the volume. During subsequent heat treatment there are physicochemical processes of sintering and (or) crystallization, and a material acquires prescribed properties [6].
On the basis of technology developed for preparing magnesium aluminosilicate glass slip general principles have been proposed for the technology of HCBS preparation, including starting material refinement, combining it with a dispersion medium, and stabilization with electrolytes by means of controlling pH and mechanical mixing. Mineral particles within a slip absorb and strongly hold a thin water film at their surface. The thickness of water shells depends on particle fineness and their electrical charge, and is important technologically. The amount of water shell may be regulated by introducing electrolytes whose cations replace cations of adsorbed particles, reducing dissociation and size of a diffusion layer, as a result of which there is an increase in the amount of free water (with retention of its overall content), which thins a suspension [7].
During HCBS preparation the chemical nature of the solid phase, characterized by value of ion potential (IP) of the substance cation, has an exceptional effect on suspension properties. It is well known that IP characterizing cation basicity, may be considered as an approximate measure of an electric field force created by it, and therefore the IP value is closely connected with the acid-basic nature of the solid phase, solubility, and hydration energy. A low solid phase IP of basic materials (IP = 2 – 4 [8]) assumes an increase in content of chemically bonded liquid, an increase in tendency of a system towards thixotropic structure formation, a marked reduction in volume faction of solid phase C V in suspension, and a corresponding increase in casting porosity.
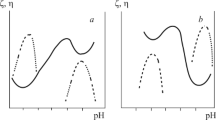
Within the MgO–Al2O3–SiO2 system there is acid oxide SiO2, amphoteric Al2O3, and basic MgO. MgO exhibits a marked water requirement, which strongly limits the process of fine grinding of material of this composition in an aqueous medium and preparation of a sedimentation and aggregatively stable suspension. With stabilization by thinning electrolytes the change in pH of a medium is regulated to values with which a slip develops maximum fluidity [8]. The effect of medium pH is considered as action of a factor changing the value of the ξ-potential and thickness of the diffusion part of a double electric layer. The value of pH with which a slip develops the best fluidity (so-called casting range) is determined by experiment. For suspensions of basic materials existence of two casting ranges is theoretically possible, in both acid and alkali ranges (Fig. 1). Castings are prepared with the greatest density in regions of maximum suspension fluidity.
Dependence of viscosity (1) and ξ-potential (2) on basic material slip medium pH [6].
Fineness is a no less important HCBS property, specified by content of fine (<5 μm) and coarse (>50 μm—T 50) fractions. From the point of view of an increase in sedimentation stability of a suspension it is expedient to increase the fineness of particles comprising them, and in order to reduce shrinkage of material based on HCBS it is desirable to achieve particle polydispersion. Polydispersion of a system determines the possibility not only of achieving a high solid phase concentration in a suspension, but also preparation of high density semifinished product, prepared by slip casting [6]. This is achieved due to the fact that during semifinished product structure formation within the main mass relatively coarse particles with a size of 5 – 100 μm contain fine particles (1 – 5 μm), and between them there are ultrafine particles with a size of 0.1 – 1.0 μm, and nanoparticles within gaps.
EXPERIMENTAL SECTION
Crystallizing glass OTM-554 (TU 1-596-488–2012) in the form of glass granules of the following composition was used in the work: MgO (11.8 ± 1.3) %, Al2O3 (29.8 ± 1.0) %, SiO2 (45.9 ± 2.1) %, TiO2 (12.0 ± 1.0) %, As2O3 (1.85 ± 1.0) %. Glass was used both in original form, and crystallized. The crystallization regime was two-stage heat treatment with a temperature of the first stage of 850°C and soaking for 3 h, then the temperature for the second stage was 1230°C with soaking for 4 h. The main crystal phase of crystallized glass was cordierite.
Material refinement was accomplished in a ball mill with a corundum lining charged with amorphous or crystallized glass with a lump size of about 15 × 15 × 5 mm (Fig. 2) and Al2O3 milling bodies from 15 to 40 mm in diameter in a ratio 1:(3 – 4). The dispersion medium used was distilled water; water acidified to pH 2.5 by addition of hydrochloric or oxalic acids; water made alkaline to pH 10.8 with addition of aqueous ammonia solution. After wet milling the suspension was stabilized by mechanical mixing.
The hydrogen ion concentration and electrokinetic potential were determined in an I-500 instrument (ionometric converter). Solid phase particle size composition of a suspension was determined by screen analysis and a sedimentation method. Detailed information about particle fineness in this work was obtained from data for integral and differential distribution in an LA-950 laser scanning analyzer. On the curves obtained (Figs. 3–5) the right-hand scale concerns the integral distribution (smooth curve), and the left-hand scale concerns differential particle distribution (histogram).
Calculation of the slid phase volume content C V was performed by an equation [8]
where ρs.p and ρi are suspension solid and liquid phase density; W is suspension moisture content.
Specimens were molded in gypsum molds, and castings were prepared in the form of rectangular plates air dried for two days, after which apparent density and porosity were determined from results of hydrostatic weighing of paraffin treated specimens.
RESULTS AND DISCUSSION
A typical feature of wet milling technology for starting material is a combination of crushing lump material and fine milling. An important factor governing the efficiency and results of milling is the amount of dispersion medium, for example water. A newly-formed surface during milling absorbs and retains water well, creating a dense hydrated shell around new particles, part of which is chemically-bonded water. Test milling showed that the minimum amount of water in order to obtain a suspension of magnesium aluminum silicate glass is about 25% of the ground material weight. In order to obtain a highly dense suspension this amount of water is too much, and a smaller amount of water in view material basicity is inadequate, since ground mix is compacted and held at the mill walls, excluding further milling. Some examples of parameters for the suspensions obtained and casting properties are provided in Table 1.
Milling amorphous glass by a wet method with use of HCl as a stabilizer did not provide the results required. The slip mix did not achieve the fluidity required even with a high suspension moisture content of 23 – 24%; this also facilitated a greater amount of particles of small (<5 μm) sizes (see Fig. 3), increasing the overall solid particle specific surface. Thus, it has been confirmed by experiment that due to the tendency of MgO towards hydration in order to prepare satisfactory suspension fluidity a lot of water is necessary, as a result of which castings are highly porous.
It was possible to reduce the effect of the MgO solubility factor somewhat into a bonded form by crystallizing the original glass by means of two-stage heat treatment, as a result of which material was obtained with the following main crystal phases: indialite (cordierite system), and rutile; cristobalite and enstatite were contained in an insignificant amount. During milling of crystallized glass using HCl it was possible to obtain a suspension with density 1.99 – 2.04 g/cm3 with pH 3.0 – 4.0 and fineness (residue on a screen with cell size 0.063 mm) T 63 = 3 – 4%. The open porosity of green workpieces did not exceed 30%. Use of aqueous ammonia solution NH4OH as a stabilizer for slip preparation with good casting properties in an alkali medium did not have a favorable effect (the slip mass was lumpy, and stuck to mill walls).
Apart from electrostatic stabilization in order to prepare slips with good casting properties, thinning additives were used, whose operating principle in contrast to electrolytes, is based on electrosteric stabilization. One of the simplest substances capable of having a similar effect on a dispersed medium is oxalic acid C2H2O4, which is related to a class of higher fatty acids with an open chain of carbon atoms and is the simplest dibasic carbonic acid. Acid was used both in the form of saturated aqueous solution, and in the form of powder added during milling. A good result was obtained by the method of limited saturation, when for a suspension maximum possible amount of glass powder is added up to an instant with which viscosity started to increase. Suspension density before molding was 1.99 g/cm3 and moisture content 20.16%, C V 0.60 (see Table 1). Particle distribution of the solid phase within a slip (see Fig. 5) was close to the optimum. In spite of this selection of green workpieces (mix structure formation) was carried out too rapidly, casting porosity was 33 – 35%, and they were distinguished by considerable brittleness and friability.
This work has shown that the most effective method for HCBS preparation based on magnesium aluminum silicate glass is milling of crystallized glass using HCl as a stabilizer. Casting porosity in this case is not more than 30%. Comminution of crystallized glass should be carried out to preparation of slip with density 1.99 – 2.04 g/cm3 with pH 3.0 – 4.0 and fineness with residue on a screen with a cell size of 0.63 mm of 0 – 10%. The output of slip parameters these limits leads to an increase in porosity to more than 35%, and this makes it almost impossible to sinter it during heat treatment to zero porosity.
CONCLUSION
Suspensions have been prepared by wet milling in a ball mill based on amorphous and crystallized magnesium aluminum silicate glass using hydrochloric and oxalic acids as stabilizing additives. The best results have been obtained using crystallized glass as starting material. Milling of crystallized glass should be carried out up to preparation of a slip with density of 1.99 – 2.04 g/cm3 with pH 3.0 – 4.0 and fineness with residue on a screen with a cell size of 0.63 mm of 0 – 10%. Castings prepared from these suspensions have porosity not more than 30% and are suitable for preparing dense pore-free glass ceramic material of cordierite composition.
References
V. G. Lisachuk, R. V. Krivobok, A. V. Zakarov, et al., “Prospective radioparent materials for rocket and space engineering,” Visnik NTU KhGTs, No. 28(1071), 72 – 79 (2014).
S. D. Stookey, “Catalyzed crystallization of glass in theory and practice,” J. Indust. Eng. Chem., 51(7), 805 – 808 (1959).
N. M. Pavlushkin, Bases of Sitall Technology [in Russian], Stroizdat, Moscow (1970).
N. E. Uvarova, Yu. E. Anan’eva, E. G. Bolokina, et al., “Radioparent glass-ceramic materials,” Uspekhi Khim. Khim. Tekhnol., Vol. XXI, No. 7(75) (2007).
V. N. Antsiferov, S. E. Porozova, and S. N. Peshcherenko, “Effect of raw materials on cordierite ceramic properties,” Ogneupor. Tekhn. Keram., No. 10, 20 – 23 (1997).
A. G. Dobrovol’skii, Slip Casting [in Russian], Metallurgiya, Moscow (1974).
S. S. Voyutskii, Colloidal Chemistry Course [in Russian], Khimiya, Moscow (1975).
Yu. E. Pivinskii, Ceramic Binders and Ceramic Concretes [in Russian], Metallurgiya, Moscow (1990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 11, pp. 29 – 33, November 2015.
Rights and permissions
About this article
Cite this article
Suzdal’tsev, E.I., Zaichuk, T.V., Ustinova, Y.S. et al. Preparation of Highly Concentrated Binder Suspensions Based on Magnesium Aluminum Silicate Glass. Refract Ind Ceram 56, 601–604 (2016). https://doi.org/10.1007/s11148-016-9896-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-016-9896-7