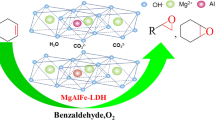
Abstract
Co2Al layered double hydroxide (Co2Al–LDH) could efficiently catalyze the aerobic epoxidation of olefin in the presence of isobutaldehyde (IBA). Various alkenes could transformed to its corresponding epoxide under the catalytic system and the catalyst showed exceptional reusability under the selected reaction conditions. The relationship between catalytic performance and Co2Al–LDH has been studied in the present research. The surface basicity of hydrotalcite was found to be beneficial to the reaction. Based on the controlled results and kinetic study, it was found that basicity not only accelerate the reaction rate, but also benefit the selectivity of epoxide. A possible mechanism of the reaction was proposed based on the controlled results and analysis, which was assumed that the epoxides were generated through two paths involving acylperoxy radical and peroxyacid.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The epoxidation reactions play an important role in the chemical industry, because the epoxides are useful intermediates that can be involved in production of raw materials for pharmaceuticals, surfactants, perfumes and epoxy resins [1,2,3,4]. Traditionally, the epoxidation of olefins is generally carried out with peroxyacids, which is expensive and generates large amounts of wastewater. Developing catalytic protocol for epoxidation using molecular oxygen as an ideal oxidant is highly desirable [5]. Although homogeneous transition-metal complexes are widely used in the aerobic epoxidation of olefins with the co-reactant aldehyde, they suffer from high cost and difficulty in recovery [6].
Various heterogeneous catalysts including GFC-[Mn(L)(OH)] [5], Co-CPF-2 [6], SBA15-[Mn (TPyP)TA] [7], Mn-Porphyrin/GO [8], CoFe2O4@SiO2@CPMS@Mn (III) salen complex [9] and GO*-[Mn (TPyP)OAc]+ Cl− [10] have been reported for the efficient selective epoxidation of alkenes in the presence of IBA (iso-butylaldehyde). The basicity of the catalyst from the introduced 3-aminopropyl was reported to be conductive to increase the initial reaction rate and accelerated the cleavage of O–O bond to give the high oxo intermediates, which can react with the olefins to generate epoxides [11, 12]. Layered double hydroxides (LDHs) are well-known basic materials, which catalyze various reactions. Therefore, we speculated that developing catalyst based on LDHs might give high performance in epoxidation. Some hydrotalcite-based materials including CoPcTs–ZnAl–LDH, CoTPPS/Ni–Al LDH and PW12/LDH [12,13,14], have been reported for epoxidation of olefins, and excellent results have been obtained. However, most of these catalysts are complicated to prepare, which limit their large-scale use. Taking the preparation of CoPcTs-ZnAl–LDH as an example. First, it was necessary to prepare tetra-sulfonic cobalt phthalocyanine tetra-ammonium, and the yield was about 68%. Then, CoPcTs was intercalated into the layers of hydrotalcite by co-precipitation, and the process required to be carried out under N2 atmosphere to ensure that it was not affected by CO2 in the air. The complex preparation method greatly limits the possibility of industrial use, and at the same time, the intercalation samples may also face the problem of active site shedding. From the view of practicality, developing LDHs-based catalyst with simple preparation process for the aerobic epoxidation of olefins is still highly desirable, which can provide a vision for large-scale applications. On this basis, a series of hydrotalcite-like materials with different cations have been prepared simply by coprecipitation in the present study, and systematically investigated in the aerobic epoxidation of olefins.
Experimental
Materials and methods
All the chemicals were purchased from Energy Chemical (Shanghai) Co., LTD and were used as received. Ni2Al–LDH, Co2Al–LDH, CuMgAl–LDH and Mn2Al–LDH were prepared by coprecipitation method according to the reported previously work [15]. Taking Co2Al–LDH as an example, a solution of NaOH (0.18 mol, 7.2 g) and Na2CO3 (0.015 mol, 1.6 g) in 100 mL deionized water was prepared (solution A). Then, a mixture of Al(NO3)3·6H2O (0.03 mol, 9.6 g) and Co(NO3)2·6H2O (0.06 mol, 17.5 g) were dissolved in 100 mL deionized water to form solution B. Solution A and B were slowly added into 200 mL deionized water in a 500 mL three-neck round bottom flask with mechanical stirring at 60 °C under air atmosphere, and the pH was kept around 9.5. After the titration, the resulting suspension was digested at 65 °C for 24 h. Finally, the resulting precipitate was washed to neutrality with deionized water, and then dried at 80 °C overnight. The Co2Al–LDH sample was obtained in a pink powder. Other catalysts with different compositions were synthesized through similar procedures.
The prepared catalysts were characterized by X-ray diffraction pattern (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectra. The XRD patterns of the prepared catalyst were obtained by a Bruker D8 Advance diffractometer with Cu Kα radiation (λ = 1.5406 Å). Surface morphology was analyzed by scanning electron microscopy (SEM) S-4800. Fourier transform infrared (FT-IR) spectra was recorded on a NicoletIS-10 spectrometer using KBr as the reference.
Catalytic epoxidation of styrene over O 2 /IBA system
In general, the epoxidation of styrene was conducted in a carousel reaction tube under a certain flow rate of oxygen. A suspension of styrene (1 mmol), catalyst (50 mg), acetonitrile (2 mL), IBA (3 mmol), and naphthalene (internal standard, 0.5 mmol), were magnetically stirring at 60 °C. Qualitative and quantitative analysis of the samples were performed on GC–MS (Shimadzu GCMS-2010) and GC-FID (Shimadzu GC-2014C).
Results and discussion
XRD pattern of the prepared hydrotalcites
The XRD pattern of the prepared hydrotalcites were depicted in Fig. 1. Ni2Al–LDH, Co2Al–LDH, Co2MgAl–LDH.
and CuMgAl–LDH show the typical well-crystallized diffraction pattern of a hydrotalcite-like phase. The sharp and asymmetrical diffraction reflections around 2θ = 11.3°, 22.6° and 33.9° are related to (003), (006) and (009), respectively; broad and asymmetrical peak for (018) located at about 40° [16, 17]. The basal spacing distances of LDHs, which are actually determined by the size of the interlayered anions, can be calculated from the reflection (003) via Bragg's Law. Values around 7.89 Å were obtained for these prepared hydrotalcite samples, indicating the anions in the brucite are mainly CO32– and OH–. However, hydrotalcite structure could not be formed for MnAl sample (Fig. 1b), and only MnCO3 phase was obtained under the preparation conditions, which might be related to the Jahn–Teller effect [18]. As shown in Fig. 2 for the infrared spectrum of dfferent hydrotalcites, it can be found that the samples show wide and strong band at about 3456 cm−1 due to the –OH stretching vibration of the interlayer hydroxyl group and water molecules. The absorption peak at 1637 cm−1 is the bending vibration of water molecules inside the hydrotalcite, and the strong stretching vibration at 1384 cm−1 is the carbonate anion between the interlayers. In the 400–1000 cm−1 area, it is due to the vibration of cation-oxygen bond in hydrotalcite. The SEM image of dfferent hydrotalcites obviously show the plate-like agglomerated crystals, also demonstrating the formation of hydrotalcite structure (Fig. 3).
Effect of the reaction conditions
The epoxidation of styrene was selected as the probe reaction, and styrene oxide and benzaldehyde were the main products in the reaction. Various LDHs with different compositions have been studied, and the cobalt-containing layered double hydroxide exhibited better catalytic performance than Ni2Al–LDH, CuMgAl–LDH as well as MnAl hydroxide. However, the difference in selectivity between different samples was not obvious. The catalytic activity of Ni2Al–LDH was the lowest, but a relatively high selectivity 81.3% was obtained. The Jahn–Teller effect of Cu2+ greatly affect the stability of the hydrotalcite laminate [18]. The stability of the copper-containing hydrotalcite will decrease with the increase of amount of Cu2+. Therefore, Mg2+ was added to prepare CuMgAl–LDH in the present study, a stable copper-containing hydrotalcite structure. Nearly 80% of styrene was converted with a 75.4% selectivity of epoxide under the catalysis of CuMgAl–LDH. MnAl sample exhibited moderate catalytic activity, but the selectivity to epoxide was the lowest, suggesting that the unique structure of hydrotalcite was beneficial to the selectivity of epoxide. As shown in Table 1, the order of the catalytic activity is Co > Cu > Mn > Ni in this study, which is consistent with the results reported in the literature [12].
Several solvents were then investigated. As shown in Table 1, polar solvents were conducive to the improvement of conversion rate. But there was no significant difference of epoxide selectivity in different solvents. When acetonitrile was used as solvent, styrene was completely converted with a 84.5% selectivity of epoxide. This might be related to the appropriate polarity of the acetonitrile and good solubility for the styrene and IBA. The suitable temperature of the present system was 60 °C. Peroxyisobutyric acid, generated from oxidation of IBA, is unstable at high temperatures, which was not conducive to styrene epoxidation. According to reported literature, peroxyacids can oxidize styrene to the epoxide. The results were still unsatisfactory when prolonging reaction time under 40 °C. Lower temperature might be benefit to the formation of benzaldehyde.
The plots of the amounts of IBA and catalyst to time are depicted in Fig. 4. All the results exhibit the same trend, the conversion of styrene increased with the prolonging of reaction time. It should be noted that the conversion of substrate increased when the amount of IBA increased from 2 to 3 equivalents, however, the epoxidations changed slightly with 4 equivalents of IBA (Fig. 4a). The conversion rate increased with the increase of the amount of catalyst, but when the amount of catalyst was further increased to 100 mg, the conversion of the substrate did not change significantly. 50 mg of catalyst was appropriate for the conversion of 1 mmol of substrate (Fig. 4b).
Subsequently, the reusability of Co2Al–LDH in styrene epoxidation was tested in the O2/IBA system. As shown in Fig. S1A, there was no significant decrease in activity and selectivity of the catalyst after five repeated experiments. The XRD patterns showed that the structure of the catalyst was completely preserved after several reuses (Fig. S1B) indicating that Co2Al–LDH was stable under the selected conditions.
Co2Al–LDH showed outstanding catalytic performance in the epoxidation of styrene by O2/IBA system, which seems to be prior to some reported results [19,20,21,22,23]. Encouraged by the obtained result, the catalytic performance of Co2Al–LDH/O2/IBA system for epoxidation of other olefins was further investigated under the selected reaction conditions (Table 2). α-methylstyrene was oxidized with 99.3% conversion and the corresponding acetophenone was formed as the major by-product (entry 2). 99% p-methylstyrene was converted within 2.5 h (entry 3), while lower reaction rate was observed for p-fluorostyrene (entry 4), and prolonged reaction time was required. These results indicated that the electron-donating substituents at the para position can accelerate epoxidation, while electron-withdrawing substituents are opposite.
When cyclohexene was used as the substrate, it led to lower epoxide yield compared with cyclooctene, which should be due to the less hoop tension of cyclooctene than cyclohexene. Good results could also be obtained under the optimized conditions for the other cycloolefine (entry 7). Apparently, increasing the length of linear olefins triggered lower epoxides [24]. To our delight, 1-octene was epoxidized in good conversion and selectivity with 4 equation of IBA by prolonging the reaction time to 7 h (entry 9). 2,3,5-trimethylcyclohexa-2,5-diene-1,4-dione was epoxidized slowly with 64% conversion, suggesting that electron-withdrawing substituents restrained the epoxidation (entries 10). Under the same condition, the epoxidation of α-pinene has obtained almost a quantitative yield of epoxide, which is a significant commodity in polymers and fine chemicals (entries 11) [25, 26]. In conclusion, the present catalytic system can be applied to the selective epoxidation of various olefins to the corresponding epoxides under optimized conditions.
The relationship between catalytic performance and Co2Al–LDH was further studied, and a series of controlled experiments have been conducted. The results showed that the substrate could transfer to some extent in the absence of catalysts (Table 3, entry 1). However, the conversion of styrene and the selectivity of the epoxide were reduced without the catalyst. The results suggested that Co2Al–LDH could accelerate the conversion of olefins as well as increase the selectivity of the epoxide. No transformation could be observed without IBA (entry 2), indicating the crucial role of IBA. Co(NO3)2 gave 84% conversion of styrene while the selectivity of the epoxide was only 33.3% (entry 3), indicating that Co(NO3)2 was more conducive to the generation of benzaldehyde. Besides, Mg2Al–LDH could not accelerate the reaction while gave high selectivity of the epoxide (entry 4), which might be related to the surface basicity. These results implied that cobalt was the active center. In addition, Co2Al–LDH exhibited higher catalytic activity than Co(NO3)2, as well as the higher epoxide selectivity (entry 5). The surface basicity of the hydrotalcite might have advantage in the epoxidation of styrene. It is known that the addition of Mg2+ could increase the basicity of hydrotalcite. To verify the speculation, Co2MgAl–LDH was prepared and introduced into the reaction system under the selected conditions. To our delight, styrene was completely converted in 2 h with a 94% selectivity of the epoxide (entry 6). Co2MgAl–LDH exhibited better catalytic performance than Co2Al–LDH. The Hammett indicator method was used to analyze the basic strength of the catalysts instead of CO2–TPD analysis (temperature-programmed desorption of carbon dioxide), because LDH samples required high-temperature pretreatment in CO2-TPD analysis, which is bound to destroy the structure of the LDH samples. The data in Table 4 showed that the basicity of Co2MgAl–LDH was higher than Co2Al–LDH. To test the effect of basicity on the catalytic performance in the epoxidation of styrene, 10 mg Na2CO3 was introduced to the oxidation when Co(NO3)2 was used as the catalyst (entry 7). Both the conversion of styrene and the selectivity of epoxide increased to some extent. Co2Al–LDH with Na2CO3 obtained 92% selectivity of the epoxide which further confirmed the above speculation (entry 8). Co2Al–LDH with Mg2Al–LDH was further introduced into the system (entry 9), the selectivity of the epoxide also increased. These results further indicated that the basicity of the hydrotalcite benefited the selectivity of the epoxide.
On the other hand, the basicity of hydrotalcite may also benefit the reaction rate in the current system (entry 6). Kinetic study was further investigated based on Co2Al–LDH and Co2MgAl–LDH. Least squares fitting was used to obtain the relevant parameters. The term “least squares” means that the parameter values are determined for which the square of the difference between measured points and the theoretical function is minimal. The reliability of the values was also assessed through estimating the standard deviation of the parameters. The detailed methods of the least-squares fitting and the calculation of standard deviation were according to literature [27]. After excluding the effects of internal and external diffusion (see the Supplementary Information for the detailed information), the experiments were investigated under different temperatures to study the kinetic aspects of the epoxidation of styrene. The substrate concentration was exponential with time. The fitting results of both Co2Al–LDH and Co2MgAl–LDH indicated that the oxidation is a first order reaction. The rate constant k of different temperatures were obtained through least-squares fitting to the exponential curve (Eq. 1).
“k” represents the rate constant (min−1). “t” represents reaction time (min). “C0” is the intial concentration of the substrate. “C” is the measured quantity, the concentration of the substrate.
The parameters of the Arrhenius equation (Eq. 2) can be deduced from the least-squares fitting of rate constant (k) to temperature (T).
“k” represents the rate constant (min−1). “A” is the pre-exponential factor (min−1). “Ea” represents activation energy (kJ/mol). “R” represents molar gas constant with a value of 8.314 J/(mol·K). “T” is the temperature (K).
The value of the apparent activation energy (Ea) and the pre-exponential factor (A0) of Co2Al–LDH were 39.8 kJ/mol and 9.4 × 103 min−1, and their relative standard deviation were 0.003 and 0.031, respectively. Kinetic study of Co2MgAl–LDH was also investigated by the same method, and the value of Ea and A0 were 31.4 kJ/mol and 4.7 × 102 min−1, and their relative standard deviation were 0.004 and 0.038, respectively. The apparent activation energy in the case of Co2MgAl–LDH was lower than that of Co2Al–LDH, indicating the higher catalytic activity of Co2MgAl–LDH, which should be related to the higher bacisity of Co2MgAl–LDH.
Discussion of mechanism
The reaction mechanism has also been preliminarily investigated. To verify the free radical pathway, 2,6-di-tert-butyl-4-methylphenol (BHT) was introduced into the reaction as a free radical scavenger. The results in Table 3 (Table 3, Entry 10) show that no products were generated, suggesting that the free radical species formed is a significant intermediate. Two main mechanistic paths were proposed by researchers for metal-based catalysis [28]. The oxidation of IBA was firstly initiated by the metal to produce isobutyryl radical, which turned into an isobutyric acrylperoxy radical with an oxygen molecular [29,30,31]. In addition to peroxyl radicals, peracid intermediate were also suggested as the active species [29, 30].
3-chloroperoxybenzoic acid (m-CPBA) was used in place of IBA/O2 to verity peroxyacid paths in the absence of catalyst (Entry 11). Both epoxides and benzaldehyde were observed, the results indicated that peroxyacid might be one of the intermediates. Further, one equivalent of BHT was added, only the epoxides compound was obtained accompany with decrease of styrene conversion (Entry 12). The above results demonstrate that the two paths of free radicals and peroxyacid existed in the current system at the same time, and benzaldehyde might be generated through the radical path.
According to the reported literature, the formation of benzaldehyde might undergo two possible paths [32]. As shown in Scheme 1a, in order to determine the formation path of benzaldehyde, the influence of molecular oxygen flow rate on the selectivities of styrene oxide and benzaldehyde was studied. It is obvious that the selectivity of benzaldehyde increases with decreasing oxygen flow rate (Entries 13–15), which is inconsistent with path I. This result indicates that the formation of benzaldehyde may have passed Path II [33].
The plausible mechanism is proposed based on the obtained results and some literatures (Scheme 1b) [29,30,31,32]. Co2Al–LDH reacted with IBA to generate isobu tyryl radical (a) at first, and then combined with one molecular of oxygen to give an acylperoxy radical (b). The radical b could react with olefins to produce a new radical intermediate (d), which was supposed to play two roles. On one hand, it transferred to epoxides accompany with isobutyric acid. On the other hand, the radical intermediate d transferred to benzaldehyde through path II in scheme 1a. The acylperoxy radical could also seize a hydrogen from IBA to give peroxyacid (c). The epoxides were formed by the reaction of the peroxyacid with styrene through intermediate e.
Conclusions
In summary, bifunctional catalyst Co2Al–LDH exhibited remarkable catalytic activities in the epoxidation of olefins. Various alkenes transformed to its corresponding epoxide under the O2/IBA system and the catalyst showed exceptional reusability under the selected reaction conditions. Co2MgAl–LDH exhibited higher catalytic activity than Co2Al–LDH based on the kenetic experiments, which may be related to the higher bacisity of Co2MgAl–LDH. It was found that basicity not only accelerate the reaction rate, but also benefit the selectivity of epoxide based on the controlled results and kinetic study. A possible mechanism has been proposed based on the controlled experiments and analysis, which demonstrated that the epoxide was formed by two possible pathways involving acylperoxy radical and peroxyacid.
References
Mizuno N, Yamaguchi K, Kamata K (2005) Coord Chem Rev 249:1944–1956
Blaser HU (2003) Chem Commun 3:293–296
Ghiami S, Nasseri M, Allahresani A, Kazemnejadi M (2019) React Kinet Mech Cat 126:383–398
Majid MF, Alavijeh MK, Hosseini MS (2020) React Kinet Mech Cat 130:303–315
Abbasi V, Hosseini-Monfared H, Hosseini SM (2017) New J Chem 41:9866–9874
Zhao M, Wu CD (2017) Catal Commun 99:146–149
Berijani K, Hosseini-Monfared H (2018) Inorg Chim Acta 471:113–120
Berijani K, Farokhi A, Hosseini-Monfared H, Janiak C (2018) Tetrahedron 74:2202–2210
Hemmat K, Nasseri MA, Allahresani A (2019) Appl Organomet Chem 33:1–12
Ahadi E, Hosseini-Monfared H, Schlüsener C, Janiak C, Farokhi A (2019) Catal Lett 150:861–873
Fan G, Li F, Evans DG, Duan X (2014) Chem Soc Rev 43:7040–7066
Zhou W, Zhou J, Chen Y, Cui A, Sun FA, He M, Xu Z, Chen Q (2017) Appl Catal A 542:191–200
Shen C, Ma J, Zhang T, Zhang S, Zhang C, Cheng H, Ge Y, Liu L, Tong Z, Zhang B (2020) Appl Clay Sci 187:105478
Ma J, Yang M, Chen Q, Zhang S, Cheng H, Wang S, Liu L, Zhang C, Tong Z, Chen Z (2017) Appl Clay Sci 150:210–216
Shan RR, Yan LG, Yang K, Yu SJ, Hao YF, Yu HQ, Du B (2014) Chem Eng J 252:38–46
Tong DS, Liu M, Li L, Lin CX, Yu WH, Xu ZP, Zhou CH (2012) Appl Clay Sci 70:1–7
Adachi-Pagano M, Forano C, Besse JP, Mater J (2003) Chem 13:1988–1993
Wu LY, Peng B, Li QZ, Wang QW, Yan X (2020) New J Chem 44:5293–5302
Malko D, Guo Y, Jones P, Britovsek G, Kucernak A (2019) J Catal 370:357–363
Li Z, Wu S, Ding H, Lu H, Liu J, Huo Q, Guan J, Kan Q (2013) New J Chem 37:4220–4229
Yang Y, Zhang Y, Hao S, Kan Q (2011) Chem Eng J 171:1356–1366
Yang Y, Zhang Y, Hao S, Guan J, Ding H, Shang F, Qiu P, Kan Q (2010) Appl Catal A 381:274–281
Chen L, Yang Y, Guo Z, Jiang D (2011) Adv Mater 23:3149–3154
Betz D, Raith A, Cokoja M, Kuhn FE (2010) Chemsuschem 3:559–562
Gallezot P (2007) Catal Today 121:76–91
Maksimchuk N, Melgunov M, Chesalov Y, Mrowiecbialon J, Jarzebski A, Kholdeeva O (2007) J Catal 246:241–248
Lente G (2015) Deterministic kinetics in chemistry and systems biology. Springer, Debrecen
Serra AC (2011) Rocha Gonsalves AMdA. Tetrahedron Lett 52:3489–3491
Bastienne PLA, Wentzel B (2004) J Org Chem 69:3453–3464
Wonwoo Nam HJK, Raymond YN (1996) Inorg Chem 35:1045–1049
Vanoye L, Wang J, Pablos M, Favre-Réguillon A (2016) Catal Sci Technol 6:4724–4732
Haber J, Kłosowski M, Połtowicz J (2003) J Mol Catal A Chem 201:167–178
Silva M, Freire CB, Castro J, Figueiredo L (2006) J Mol Catal A Chem 258:327–333
Acknowledgements
The authors are grateful to Analysis and Testing Center of Nanjing University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, X., Huang, J., Tang, S. et al. Efficient aerobic epoxidation of olefins accelerated by a bifunctional Co2Al layered double hydroxide. Reac Kinet Mech Cat 131, 283–296 (2020). https://doi.org/10.1007/s11144-020-01867-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01867-9