Abstract
In the present work, we demonstrated synthesis of Al-SiO2 and magnetically recoverable Al-SiO2/Fe3O4 systems via grafting of triethylaluminum on SiO2 and SiO2-coated magnetic Fe3O4 nanoparticles, respectively. These materials were characterized by various techniques including elemental and N2-adsorption/desorption analyses, transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FTIR) using CO and pyridine as probe molecules. Amount of grafted Al on the support was found to affect the textural, acid–base properties and catalytic behavior in the isomerization of α-pinene oxide (PO) to campholenic aldehyde (CA). Maximal activity and selectivity towards CA was observed in the presence of sample with 12 wt% of alumina. It was demonstrated that 12%Al-SiO2/Fe3O4 can be used as catalyst for at least four successive cycles without loss of activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terpenes and their epoxides are widely used as precursors for the synthesis of fragrances, flavors and pharmaceuticals [1]. In particular, α-pinene oxide (PO) can be converted by acid-catalyzed transformations in ca. 200 substances [2] which can be applied in the fine chemicals industry. Campholenic aldehyde (CA), trans-carveol (trans-carv) and trans-sobrerol (trans-sobr) are the most important compound among of this diversity (Scheme 1). Thus, CA is utilized as an intermediate for the production of sandalwood fragrances [3] and as an environmental friendly substitute for nitro and polycyclic musks in laundry detergents and softeners [4].
Isomerization of PO to CA mainly proceeds in the presence of systems with Lewis acid sites (LAS), while systems having Brønsted acid sites (BAS) favor to form trans-carveol and trans-sobrerol [5,6,7]. In the presence of Brønsted type catalytic systems selectivity towards CA is 50–60%, while Lewis acids, for example, industrial catalyst ZnCl2, give selectivity up to 80–85% [7]. Note that ZnCl2 application has several disadvantages, such as lack of regeneration, corrosion problems, toxicity, and waste water pollution. Nowadays, the replacement of the homogeneous catalyst by new heterogeneous catalyst is a challenging goal of the fine chemicals industry.
Currently, considerable attention is focused on the development of new solid Lewis catalysts [7,8,9,10,11,12,13] with the main effort being on composite materials with micro-/mesoporous structure. Such type materials improve mass transfer and accessibility of active sites that allow to reduce steric and diffusional limitations, and formation of by-products n catalytic reactions. Thus, a 70% selectivity at a lower conversion of PO (18%) were observed in the presence of microporous 15%Al2O3-SiO2 at 253 K [14]. According to Holderich et al. [7], the dealumination H-US-Y zeolite by HCl allows to produce CA with 70–80% selectivity due to its unique structure (i.e. a three-dimensional large pore system (7.4 Å) with supercages of 12 Å and many mesopores that make USY zeolites). Note that the performance of H-USY zeolite strongly depends on the SiO2/Al2O3 ratio. The selectivity towards CA was about 70% at 398 K after 2 h, when this ratio was 70. Selectivity was improved to about 80% at lower temperatures down to 243 K.
The effect of structure was demonstrated for Al-MSU-SFAU (Si/Al 70) having a mesoporous structure with microporous walls [8]. The high selectivity towards CA was explained by the isomerization of PO within the microporous channels. The short length of the channels favors the rapid movement of the reaction products away from the active site that prevents the further reaction to other isomers occurs. The selectivity towards CA was 86% at 54% conversion of PO. It has been stated in the literature that amount of Al in Al,Si-containing materials also can affect the reaction rate and distribution of products. Thus, Liebens et al. [11] demonstrated that the increasing molar ratio of Si/Al from 10.4 to 60% in HY zeolite framework led to the decreasing conversion of PO from 96 to 49% and increasing selectivity towards CA increased from 49 to 66%. The yield of CA decreased from 72 to 13% after changing of Si/Al molar ratio from 70 to 6 in framework of Al2O3–SiO2 [15].
The magnetic nanoparticles (MNPs) as the carriers for the synthesis of various catalytic systems for many organic transformations are of considerable recent interest [16,17,18,19]. There are several reasons. First of all, the nanoparticles possess a large surface area, which favors the formation of quite an amount of active and accessible centers. Moreover, MNPs allows to simplify separation and filtration and, therefore, to reduce the loss of catalysts in repeated trials. Herein, we wish to demonstrate catalytic properties of the magnetically recyclable Al-SiO2/Fe3O4 samples prepared by a post-synthesis grafting method (Scheme 2), which allows to control amount of Al on the surface of solids and, therefore, textural properties and surface acidity [20]. Al-grafting method was successfully used for the design of catalytic systems based on MCM-41 with Si/Al ratios from 15 to 200 mol/mol for synthesis of bisphenol F from phenol and formaldehyde [21]. Sample with a Si/Al ratio of 70 mol/mol had the highest activity among of Al-MCM-41 samples that was explained by the effect of Al content in MCM-41 on the amount of acid sites. According to the temperature-programmed desorption of ammonia (NH3-TPD), amount of acid sites with weak and medium strength (desorption temperature at 423–673 K) increases with increasing Al content in samples, whereas amount of strong acid sites (desorption temperature at 673–1023 K) is very small and remains constant. We also investigated the effect of Al amount on the textural properties and surface acidity for providing an opportunity to streamline the procedures of Al-SiO2/Fe3O4 preparation. For this aim we used the Al-containing silica (Al-SiO2) with different Al content prepared by a grafting method. In general, the main purpose of our study was the establishment of correlations between amount of Al, aggregation state of Al, nature of acid sites and catalytic behavior of these materials in isomerization of PO to CA. Analysis of main factors affected the reaction rate and isomer selectivity was performed by combination of spectroscopic and catalytic methods.
Experimental
Materials
α-Pinene oxide (95.0%) was purchased from Acros Organics, and SiO2 was purchased from Davison 752 (Fe content was 0.04 wt%). Commercial dichloroethane (0.1 wt% of water), octane, triethylaluminum (TEA) were used without purification. The following reagents of chemical grade purity were used: 23.5% ammonia aqueous solution (Sigma-Aldrich, ≥ 99.99%), FeCl2∙4H2O (Sigma-Aldrich, > 99.0%) and FeCl3·6H2O (Sigma-Aldrich, ≥ 98%).
Synthesis of Al-SiO2 samples
Al-SiO2 samples were synthesised according to Scheme S1 (Supporting Information (SI)). SiO2 was calcined at 973 K for 6 h in air and cooled in an inert atmosphere (N2). Then, a measured amount of 0.329 M TEA in hexane (0.6–20.7 mmol of TEA per 1 g of SiO2) was added to SiO2 and stirred for 2 h at room temperature in a N2 atmosphere. Al-SiO2 samples were air dried and then calcined at 973 K for 4 h. The designation of the samples and the conditions of their synthesis are presented in Table 1.
Synthesis of magnetic Al-SiO2/Fe3O4 samples
Al-SiO2/Fe3O4samples were synthesized according to Scheme 2, which shows the synthesis of magnetic Fe3O4 nanoparticles, SiO2/Fe3O4 nanoparticles and Al-SiO2/Fe3O4 samples. Magnetic Fe3O4 nanoparticles were synthesized was performed according to the procedure reported previously [22]. Magnetic particles were synthesized by co-precipitating Fe2+ and Fe3+ salts ([Fe3+]/[Fe2+] = 2). The total concentration of Fe2+ and Fe3+ ions was 0.15 M. We performed the synthesis under an Ar atmosphere at room temperature (298 K) by mixing the Fe2+ and Fe3+ solution under intense mechanical stirring (500 rpm) with a solution of ammonium hydroxide in deoxygenated water (pH of solution was 11.6). Magnetic Fe3O4 nanoparticles were aged for 3 days in mother liquor at room temperature, then washed with ethanol and used in the next stage of synthesis. TEOS was added to the ethanol solution of magnetic Fe3O4 nanoparticles with size 13.5 nm (0.5 wt%). The amount of TEOS (6.1 mg of TEOS per 1 mg of Fe3O4) was estimated to give a 5 nm SiO2 shell on the magnetic nanoparticles. After dispersion by sonication (44 kHz, 60 W, steel emitter within the mixture) for 1 min, the mixture was hydrolyzed by 23% NH4OH. Then, this mixture was dispersed by sonication for 1 h and stored for 18 h. The resulting SiO2/Fe3O4 was separated from a small amount of solid nonmagnetic parts (~ 1.5 wt% SiO2), washed with ethanol, and dried at room temperature.
The magnetic SiO2/Fe3O4 nanoparticles were calcined at 973 K for 6 h in air and cooled in an inert atmosphere (N2). Then, a measured amount of 0.329 М TEA in hexane was added to SiO2/Fe3O4 and stirred for 2 h at room temperature in an N2 atmosphere. Al-SiO2/Fe3O4 samples were air dried and then calcined at 973 K for 4 h. The synthesis reaction conditions of the Al-SiO2/Fe3O4 samples were similar to that of the Al-SiO2 samples.
Instrumental measurements
The porous structure of the materials was determined from the adsorption isotherm of N2 at 80 K on a Micromeritics ASAP 2400. The specific surface area (SBET) was calculated from the adsorption data over the relative pressure range between 0.05 and 0.20. The total pore volume (Vtotal) was calculated from the amount of nitrogen adsorbed at a relative pressure of 0.99.
High-resolution transmission electron microscopy (HR TEM) images were obtained with a JEOL JEM-2010 microscope with a resolution of 1.4 Å operated at an accelerating voltage of 200 kV. The size distribution of the nanoparticles was calculated based on a representative set of HR TEM images taken at different areas of the sample. The number of measured particles was 546. The Al content in Al-containing samples was carried out by means of inductively coupled plasma-atomic emission spectrometry (ICP-AES) using a PERKIN-ELMER instrument OPTIMA 4300.
The nature of functional groups was studied by IR spectroscopy using probe molecules. Analysis of the Lewis surface acidity by CO adsorption of the Al-SiO2 samples was carried out at 80 K under CO pressure from 13.3 to 1333 Pa. The concentration of Lewis acid sites was estimated from the integral intensity of CO bands in the region above 2180 cm−1 according to [23] (SI, Sect. 2 “IR spectroscopy study of the acid–base properties of the supports”). Brønsted acidity was investigated by pyridine adsorption according to [23]. The IR spectra were recorded on a FTIR-8400S (Shimadzu) spectrometer with DRS-800 diffusion reflection attachment in the region of 700–6000 cm–1 with a resolution of 4 cm–1 using of 100 scans.
Catalytic test
The isomerization of PO was carried out at 303 K in a glass reactor equipped with a magnetic stirrer. Dichloroethane was used as the solvent. Prior to the reaction, all catalysts were activated at 423 K for 4 h in order to remove any adsorbed water. Then, 0.25 mmol PO, 2 mL of C2H4Cl2, and 10 mmol octane (internal standard), 5 mg of the catalyst were added to the reactor. At different time intervals aliquots were taken from reaction mixture and analyzed. A mass-spectrometer (Shimadzu GCMS QP-2010 Ultra with column GsBP1-MS 30 m × 0.32 mm, thickness 0.25 µm) was used for identify the reaction products. A gas chromatograph (Agilent 7820) with a flame ionization detector on capillary column HP-5 was used to analyze reaction products. The experiment reproducibility was 2–3%.
Results and discussion
Optimization of Al-grafting method
Effect of Al content on properties of Al-SiO2 systems
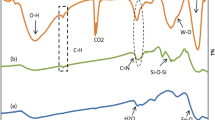
Process of TEA grafting onto SiO2 was monitored by DRIFT spectroscopy. Fig. 1 shows the DRIFT spectra of SiO2 activated at 973 K and Al-SiO2 samples in the Si–OH stretching region. Two bands are observed in all spectra. There are a narrow band at 3745 cm−1 attributed to the stretching vibration of isolated Si–OH groups and a broad and weak band in the range of 3700–3500 cm−1 assigned to the hydrogen-bonded –OH groups formed due to the interaction Al–OH and Si–OH groups [24,25,26]. The intensity of band at 3745 cm−1 decreased with increasing Al content in Al-SiO2 samples (Fig. 1) that can be explained by the interaction between Si–OH groups and TEA. According to Ref. [27], silica dehydroxylated at 973 K contains 2.3 μmol Si–OH per m2 of SiO2. Based on this, we can say that monolayer coverage of silica by TEA is observed in samples with Al content less than 4–6 wt%. This assertion is consistent with investigation of Iengo et al. [20]. They demonstrated that 2.1 mmol of grafted aluminum alkoxide Al(OR)3 on 1 g of SiO2 with a specific surface area of 280 m2/g corresponded to a monolayer coverage, i.e. the Al density was 7.5 μmol Al/m2. In our case, the aluminum monolayer coverage was 1.8 mmol/g (about 5 wt%). The subsequent increases in Al content should lead to the polylayer coverage of the SiO2 surface (i.e. the formation of Al2O3 agglomerates). This suggestion agrees with the change in textural properties of Al-SiO2 samples. One can be seen from Table 1, specific surface area and microporosity of samples raised with increasing Al content up to 12 wt% and then decreased dramatically, that can be related to the impact of Al on the Al2O3 oligomeric state on the surface of Al-SiO2.
The drastic changes of the nature and amount of -OH groups should undoubtedly affect the surface acidity of Al-SiO2 samples. The changes in the surface acidity of Al-SiO2 samples were investigated by FTIR spectroscopy. Pyridine and CO were used as the probe molecules for the analysis of Brønsted and Lewis acidity, respectively. The main results are shown in Table 2 and Fig. S2-S3 (SI). The Table shows that strong BAS form after the TEA grafting to SiO2. Amount of BAS increases until it reaches 12 wt% and then it tends to decrease. Note that strength of BAS also decreases. The existence of BAS is related to the appearance of  groups. Amount of these groups decreases with increasing Al content due to the blocking –Si–OH groups by Al2O3 oligomeric particles. The surface acidity also decreases with the increasing amount of aluminum in Al-SiO2. In general, the total amount of BAS is very small in comparison with that of LAS.
groups. Amount of these groups decreases with increasing Al content due to the blocking –Si–OH groups by Al2O3 oligomeric particles. The surface acidity also decreases with the increasing amount of aluminum in Al-SiO2. In general, the total amount of BAS is very small in comparison with that of LAS.
On the contrary, the total amount of LAS is growing steadily with increasing amount of Al in the sample (Table 2). According to FTIR spectroscopy, three types of LAS, characterized by bands at 2228 (strong LAS), 2210–2215 (medium LAS), and 2180–2200 cm−1 (week LAS), were present in the Al-SiO2 spectra [28]. Most authors [23, 29, 30] suggest that the surface Lewis acid sites of Al2O3 are formed by electron-acceptor sites represented by coordinatively unsaturated aluminum cations, which may be pentacoordinated (i.e., octahedral with one missing ligand and hence one free coordination site), tetracoordinated (normal tetrahedral sites that can expand their coordination or octahedral with two free coordination sites) and trigonal or tricoordinated (octahedral with three free coordination sites or tetrahedral with one free coordination site). The strongest Lewis sites on Al2O3 are formed by tricoordinated Al3+ ions, while medium and weak strength LAS are formed by tetra- and pentacoordinated Al3+ ions, respectively. The distribution of LAS in Al-SiO2 samples is given in Table 2. As can be seen from these data, the Lewis acidity rises with increasing Al content. The rising Al content from 1 to 12 wt% the amount of weak LAS rises from 60 to 350 μmol/g, while the amount of medium and strong LAS changes not so strongly and is in the range of 70–80 μmol/g. Early, effect of Al content on surface acidity was demonstrated for Al-MCM-41 synthesized by grafting method [21]. According to NH3-TPD, Al-MCM-41 materials have moderate acidity. The total amount of acid sites rise with increase in the Al incorporation into the framework of the materials due to the acid sites with weak and medium strength.
Catalytic properties of Al-SiO2 systems
Catalytic properties of Al-SiO2 samples in the isomerization of PO to CA were investigated in dichloroethane at 303 K. The reaction in the presence of Al-SiO2 samples was heterogeneous and that it was investigated by a special test. After 30 min of reaction the 18%Al-SiO2 sample was filtered off via membrane filter. Then, the filtrate was stirred at 303 K for 30 min (Table 3, runs 7–8). Conversion of PO was not observed after removal of the catalyst from the reaction mixture. The main results are shown in Table 3. According to the experimental data, CA was the main product with 48–72% selectivity at 65–80% conversion of 0.25 mmol PO for 30 min. Some by-products were also formed in addition to campholenic aldehyde (CA) during α-pinene oxide isomerization. There are fencholenic aldehyde (FA), trans-carveol, pinocarveol, pinocamphone, trans-sobrerol etc. (Scheme 1). The FA (3–5%) is structural isomer of CA and its mechanism of formation is similar to that of CA. The opening of the epoxide ring of α-pinene oxide over acid sites leads to the formation of trans-carveol, pinocamphone and pinocarveol. The appearance of trans-sobrerol in reaction mixture can be explained by the reaction of trans-carveol with water that is presence of a small amount (0.1 wt%) in dichloroethane.
As our experimental evidence shows (Table 3, runs 1–6 and 8), the Al content affects the catalytic activity of the Al-SiO2 samples. The conversion of PO increases with increasing the Al content up to 4 wt% Al, and then does not change, when Al content is in the range of 4–12 wt%. Conversion of PO dramatically decreases in the next increase in Al content (Table 3, run 7). Conversion of PO was about 43% in the presence of Al2O (Table 3, run 9). Selectivity towards CA also depends on the Al content. The increasing Al from 0.5 to 12 wt% leads to the change in selectivity from 52 to 72%. In the presence of Al2O3 selectivity towards CA was 50% (Table 3, run 9).
Several factors account for these correlations. First of all, isomer selectivity depends on the Al content in Al-SiO2 (Table 1). These data agree with Refs. [14, 31]. Thus, Arate and Tanabe [14] demonstrated that in the presence of Al2O3 and Al2O3-SiO2 (Al2O3—15 wt%) the selectivities towards CA were 37–46% and 70%, respectively. Therefore our results are in line with other studies.
Moreover, nature of active site also can affect the reaction rate and selectivity of reaction. The LAS promote the formation of CA, whereas BAS mainly favour the generation of trans-carveol [7]. Indeed, a larger amount of LAS in comparison with that of BAS (Table 2) is one of the reasons for the high isomer selectivity towards CA (Table 3). The strength of LAS also affects the distribution products. We can assume that activation of oxygen atom of epoxy-group of PO preferably takes place on the centers with medium and strong strength. LAS with weak strength also can take part in the reaction process, but their contribution is likely negligible. Correlations between the amount of medium and strong LAS and selectivity towards CA confirm this assertion (Fig. S3, SI). Note that the strength of LAS is one of the main parameters which can affect the reaction rate in the presence of Al-SiO2. As shown in Fig. S3 (SI), the increase in the strength of LAS from 1190 kJ/mol (1%Al-SiO2) to 1130 kJ/mol (4–12%Al-SiO2) leads to increasing PO from 50 to 75–80%. The low conversion of PO (65%) in the presence of 18%Al-SiO2 can account for the low strength of LAS (1210 kJ/mol).
The dramatically decreasing selectivity of CA in the presence of 18%Al-SiO2 leads us to think that one of the important parameters for activity and selectivity of the reaction also is the oligomeric state of Al2O3 species on the surface of Al-SiO2. The changing the oligomeric state with variation of Al content was qualitatively estimated from the analysis of changing of the yield of CA based on SBET (YCA/SBET, μmol/(m2 g−1)):
The decreasing this value is related to the increasing amount of oligomeric Al2O3 species. The effect of the oligomeric state of the metal oxide particles on the efficiency of the catalyst is not a unique in catalysis and was demonstrated for isomerization of PO to CA in the presence of iron modified zeolites (beta and ZSM-5), MCM-41, SiO2 and Al2O3 [32], Fe-VSB-5 and Fe-SBA-3 [33].
The changes in textural properties with increasing Al content in Al-SiO2 also should be taken into account. Conversion of PO and selectivity towards CA correlate with specific surface area (SBET) and porosity of samples (Vμ/VΣ) (Table 1). The reaction rate and selectivity rise with increasing SBET and Vμ/VΣ (Table 1, 1%Al-SiO2–12%Al-SiO2 samples). Catalytic properties of 18%Al-SiO2, however, are being fall dramatically by the decreasing SBET and Vμ/VΣ. Thus, we can say that catalytic properties of Al-SiO2 materials are tunable by the variation of micro-/mesoporous structure. Note that this phenomenon was demonstrated by Ravindra et al. [8] for Al-MSU-SFAU (Si/Al 70), which was synthesized from nanoclustered zeolite Y seeds as framework precursors, and possessed a mesoporous structure with the walls having microporosity. In the presence of Al-MSU-SFAU the selectivity towards CA was 86% at 54% conversion of PO. The high selectivity was explained by the short length of channels that did not allow further reactions to other isomers occur.
Synthesis and investigation of magnetically Al-SiO2/Fe3O4 systems
Fe3O4 magnetic nanoparticles were used as a support for the synthesis of the Al-SiO2/Fe3O4 nanocomposite catalysts with controllable reactivity and magnetic recyclability. Synthesis of Al-SiO2/Fe3O4 samples was based on the Al-SiO2 synthesis strategy and had three stages (Scheme 2): (i) synthesis of magnetite Fe3O4 nanoparticles, (ii) coating magnetic Fe3O4 particles with silica, and (iii) grafting of Al-organic compounds to silica and the following calcination at 973 K. In general, synthesis of Al-SiO2/Fe3O4 materials was based on our knowledge of the synthesis of Al-SiO2 materials under which Al content on the surface of Al-SiO2 affects its catalytic properties. At this point, we synthesized the corresponding Al-SiO2/Fe3O4 samples with 4, 12 and 18 wt% Al content.
The morphology and size distribution of the particles were examined by high-resolution transmission electron microscopy. Fig. 2 shows the HR-TEM image of 12%Al-SiO2/Fe3O4 sample. It can be seen that the size of magnetite Fe3O4 nanoparticles has uniform distribution with an average dimension of 8–15 nm. The surface of the Fe3O4 nanoparticles is coated with silica layer. The thickness of the layer is about 5 nm thick. The following coating of Al2O3 by post-synthesis grafting method leads to form Al species with an island structure (Fig. 2c (C)).
The catalytic properties of the Al-SiO2/Fe3O4 samples were investigated in the isomerization of PO to CA. Experimental conditions were similar to that in the presence of Al-SiO2. The main results are presented in Table 3. In this case the main products also were CA, trans-carv and trans-sobr. Reaction did not proceed without catalyst, because after removal of the catalyst conversion of PO and distribution of products did not change (Table 3, runs 14–15). As expected, reaction rate and selectivity depended on the Al content in Al-SiO2/Fe3O4. The optimal Al content was 12 wt%. However, in contrast to 12%Al-SiO2, 12%Al-SiO2/Fe3O4 gave lower conversion of PO and selectivity towards CA, readily accounted for by differences in textural properties and Al aggregation state (Table 3, runs 6 and 13).
Several reasons might be adduced to explain the difference in catalytic properties of 12%Al-SiO2 and 12%Al-SiO2/Fe3O4. First of all, it may be related to the differing textural properties (Table 1). 12%Al-SiO2 possesses larger specific surface area, total pore volume and micropore volume in compared with 12%Al-SiO2/Fe3O4. At the same time, pore diameter (Dpore) and Vμ/VΣ value are larger for 12%Al-SiO2/Fe3O4. The largest Vμ/VΣ can provoke diffusion problems and, therefore, to decrease reaction rate, whereas the largest diameter of pore can affect the spatial arrangement and configuration of intermediates and, thereby determine the isomer selectivity. Other reason can be related to the impact of Fe on catalytic properties of Al-SiO2/Fe3O4 samples as confirmed by the catalytic test in the presence of SiO2/Fe3O4 (Table 3, run 10).
The catalytic potential of Al-SiO2 and Al-SiO2/Fe3O4 materials
First of all, it is interesting to compare the catalytic behavior of Al-SiO2 with that of Fe-VSB-5 and Fe-containing mesoporous silica materials (Fe-MMM-2) [33]. Within the Fig. S4 (SI) is contained correlations between surface acidity and reaction rate and selectivity towards CA for these materials. As can be seen from these correlations, selectivity towards CA rises with increasing amount of LAS in Al-SiO2 that is similar to Fe-VSB-5. At the same time, the opposite trend is observed with respect to Fe-MMM-2 that is related to a large oligomeric iron oxide species in framework of solid. Therefore, all these results indicate that the high selectivity is a result of well-dispersed Lewis acid sites in a matrix.
Furthermore, we compared efficiency of these samples with that of Al- and Fe-containing materials reported in literature. Because experimental conditions were different, productivity of catalysts was calculated using Eq. 2:
As is clear from Table S1 (SI) (runs 1–3), in the presence of 12%Al-SiO2 selectivity towards CA is comparable with that in the presence of Al2O3-SiO2 commercial and Al2O3 sulfated systems. In general, productivity of 12%Al-SiO2 is higher in comparison with other samples. However, in spite of low productivity of Al-MSU (Si/Al = 70) selectivity towards CA is higher (86%) in comparison with 12%Al-SiO2 (72%) (Table S1 (SI), runs 1 and 7). The comparison of catalytic properties of 12%Al-SiO2/Fe3O4 with that of Fe-SiO2 and Fe-Al2O3 prepared by the impregnation method [32] points the advantage of post-synthesis grafting method (Table S1 (SI), runs 9–14).
Against this background, the central question also was to investigate the reusability and the recycling use of the 12%Al-SiO2/Fe3O4 catalyst. Taking into account the magnetic response of Al-SiO2/Fe3O4 materials, after each catalytic experiment, the used 12%Al-SiO2/Fe3O4 catalyst was separated from the reaction mixture by the external permanent magnet (Fig. 3). Then, the catalyst was washed by dichloroethane and used in the next cycle. According to experimental data, 12%Al-SiO2/Fe3O4 can be used repeatedly without significant loss of catalytic activity during at least four catalytic cycles (Fig. 3).
Conclusions
Herein, we demonstrated synthesis of the Al-SiO2 and magnetically recyclable Al-SiO2/Fe3O4 samples with 0.5–18 wt% Al content. Synthesis of samples was based on the (i) grafting of triethylaluminum to silica or coating of magnetic Fe3O4 particles by silica under anhydrous conditions and (ii) the following calcination at 973 K. Samples were characterized by various techniques including elemental and N2-adsorption/desorption analyses, transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FTIR) using CO and pyridine as probe molecules. It was found that LAS and BAS formed after modification of SiO2 by triethylaluminum. The strength and amount of these sites depended on the Al content. The aluminum monolayer coverage by triethylaluminum was found to be about 5–6 wt%. The next increase in Al content led to the polylayer cover of the SiO2 surface (i.e. the formation of Al2O3 agglomerates). The 12%Al-SiO2 sample had optimal acidity.
The catalytic properties of the Al-SiO2 and Al-SiO2/Fe3O4 samples were investigated in the isomerization of PO to CA. The reaction rate and isomer selectivity towards CA were found to dramatically decrease when Al content was more than 12 wt%. This phenomenon was related to the changing of textural and acid–base properties. Investigation of 12%Al-SiO2/Fe3O4 stability pointed that sample can be used repeatedly without significant loss of catalytic activity during at least four catalytic cycles.
References
Erman WE (1985) Chemistry of the monoterpenes, An encyclopedic handbook. Marcel Dekker, New York
Volcho KP, Salakhutdinov NF (2008) Transformations of terpenoids on acidic clays. Mini-Rev Org Chem 5:345–354. https://doi.org/10.2174/157019308786242151
Ohloff G, Winter B, Fehr C, Muller PM, Lamparsky D (eds) (1991) Perfumes, art, science and technology. Elsevier, New York, pp 287–330
da Silva Rocha KA, Hoehne JL, Gusevskaya EV (2008) Phosphotungstic acid as a versatile catalyst for the synthesis of fragrance compounds by α-pinene oxide isomerization: solvent induced chemoselectivity. Chemistry 14:6166–6172. https://doi.org/10.1002/chem.200800184
Lewis JB, Hedrick GW (1965) Reaction of α-pinene oxide with zinc bromide and rearrangement of 2,2,3-trimethyl-3-cyclopentenep derived there from. J Org Chem 30:4271–4275. https://doi.org/10.1021/jo01023a064
Kaminska J, Schwegler MA, Hoenfnagel AJ, van Bekkum H (1992) The isomerisation of α-pinene oxide with Brønsted and Lewis acids. Recl Trav Chim Pays-Bas 111:432–437. https://doi.org/10.1002/recl.19921111004
Holderich WF, Roseler J, Heitmann G, Liebens AT (1997) The use of zeolites in the synthesis of fine and intermediate chemicals. Catal Today 37:353–366. https://doi.org/10.1016/S0920-5861(97)81094-2
Ravindra DB, Nie YT, Jaenicke S, Chuah GK (2004) Isomerisation of α-pinene oxide over B2O3/SiO2 and Al-MSU catalysts. Catal Today 96:147–153. https://doi.org/10.1016/j.cattod.2004.06.117
Kunkeler PJ, van der Waal JC, Bremmer J, Zuurdeeg BJ, Downing RS, van Bekkum H (1998) Application of zeolite titanium Beta in the rearrangement of α-pinene oxide to campholenic aldehyde. Catal Lett 53:135–138. https://doi.org/10.1023/A:1019049704709
Jarry B, Launay F, Nogier JP, Bonardet JL (2007) Comparative study of the catalytic activity of Al-SBA-15 and Ga-SBA-15 materials in α-pinene isomerisation and oxidative cleavage of epoxides. Stud. Surf. Sci. Catal. 165:791–794
Liebens AT, Mahaim C, Holderich WF (1997) Selective isomerization of α-pinene oxide with heterogeneous catalysts. Stud Surf Sci Catal 108:587–594. https://doi.org/10.1016/S0167-2991(97)80954-8
Panadero MP, Velty A (2019) Readily available Ti-beta as an efficient catalyst for greener and sustainable production of campholenic aldehyde. Catal Sci Technol 9:1293–4303. https://doi.org/10.1039/c9cy00957d
Štekrova M, Kubů M, Shamzhy M, Musilova Z, Cejka J (2018) α-Pinene oxide isomerization: role of zeolite structure and acidity in the selective synthesis of campholenic aldehyde. Catal Sci Technol 8:2488–2501. https://doi.org/10.1039/c8cy00371h
Arata K, Tanabe K (1979) Isomerization of α-pinene oxide over solid acids and bases. Chem Lett. https://doi.org/10.1246/cl.1979.1017
Ravasio N, Zaccheria F, Guidotti M, Psaro R (2004) Mono-and bifunctional heterogeneous catalytic transformation of terpenes and terpenoids. Top Catal 27:157–168. https://doi.org/10.1023/B:TOCA.0000013550.28170.6a
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset J-M (2011) Magnetically recoverable nanocatalysts. Chem Rev 111:3036–3075. https://doi.org/10.1021/cr100230z
Liu J, Qiao SZ, Hu QH, Lu GQ (2011) Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small 7:425–443. https://doi.org/10.1002/smll.201001402
Shylesh S, Schenemann V, Thiel WR (2010) Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 49:3428–3459. https://doi.org/10.1002/anie.200905684
Baig RBN, Varma RS (2013) Magnetically retrievable catalysts for organic synthesis. Chem Commun 49:752–770. https://doi.org/10.1039/C2CC35663E
Iengo P, Di Serio M, Sorrentino A, Solinas V, Santacesaria E (1998) Preparation and properties of new acid catalysts obtained by grafting alkoxides and derivatives on the most common supports note I—grafting aluminium and zirconium alkoxides and related sulphates on silica. Appl Catal A 167:85–101. https://doi.org/10.1016/S0926-860X(97)00303-7
Jana SK, Kugita T, Namba S (2004) Aluminum-grafted MCM-41 molecular sieve: an active catalyst for bisphenol F synthesis process. Appl Catal A 266:245–250. https://doi.org/10.1016/j.apcata.2004.02.013
Kirillov VL, Balaev DA, Semenov SV, Shaikhutdinov KA, Martyanov ON (2014) Size control in the formation of magnetite nanoparticles in the presence of citrate ions. Mater Chem Phys 145:75–81. https://doi.org/10.1016/j.matchemphys.2014.01.036
Panchenko VN, Danilova IG, Zakharov VA, Semikolenova NV, Paukshtis EA (2017) Effect of the acid-base properties of the support on the catalytic activity of ethylene polymerization using supported catalysts composed of Cp2ZrX2 (X = Cl, Me) and Al2O3(F). Reac Kinet Mech Cat 122:275–287. https://doi.org/10.1007/s11144-017-1215-x
Caillot M, Chaumonnot A, Digne M, van Bokhoven JA (2014) Creation of Brønsted acidity by grafting aluminum isopropoxide on silica under controlled conditions: determination of the number of Bronsted sites and their turnover frequency for m-xylene isomerization. Chem Catal Chem 6:2–841. https://doi.org/10.1002/cctc.201300824
Leydier F, Chizallet C, Chaumonnot A, Digne M, Soyer E, Quoineaud AA, Costa D, Raybaud P (2011) Brønsted acidity of amorphous silica-alumina: the molecular rules of proton transfer. J Catal 284:215–229. https://doi.org/10.1016/j.jcat.2011.08.015
Zaki MI, Knøzinger H (1987) Carbon monoxide: a low temperature infrared probe for the characterization of hydroxyl group properties on metal oxide surfaces. Mater Chem Phys 17:201–221. https://doi.org/10.1016/0254-0584(87)90056-3
Zhuravlev LT (2000) The surface chemistry of amorphous silica. Zhuravlev Model Colloids Surf A 173:1–38. https://doi.org/10.1016/S0927-7757(00)00556-2
Glazneva TS, Kotsarenko NS, Paukshtis EA (2008) Surface acidity and basicity of oxide catalysts: From aqueous suspensions to in situ measurements. Kinet Catal 49:859–867. https://doi.org/10.1134/S0023158408060104
Busca G (2014) Structural, surface, and catalytic properties of aluminas. Adv Catal 57:319–404. https://doi.org/10.1016/B978-0-12-800127-1.00003-5
Belskaya OB, Danilova IG, Kazakov MO, Mironenko RM, Lavrenov AV, Likholobov VA (2012). FTIR spectroscopy of adsorbed probe molecules for analyzing the surface properties of supported Pt (Pd) catalysts, infrared spectroscopy—materials science, engineering and technology, Prof. Theophanides Theophile (ed.), pp 149–178. ISBN: 978-953-51-0537-4
Nigam IC, Levi L (1968) Essential oils and their constituents. XLII. Isomerization of epoxides on active alumina. Can J Chem 46:1944–1947. https://doi.org/10.1139/v68-321
Stekrova M, Kumar N, Aho A, Sinev I, Grunert W, Dahl J, Roine J, Arzhumanov AS, Maki-Arvela P, Murzin DYu (2014) Isomerization of alfa-pinene oxide using Fe-supported catalysts: Selective synthesis of campholenic aldehyde. Appl Catal A 470:162–176. https://doi.org/10.1016/j.apcata.2013.10.044
Timofeeva MN, Panchenko VN, Hasan Z, Khan NA, Mel'gunov MS, Abel AA, Matrosova MM, Volcho KP, Jhung SH (2014) Effect of iron content on selectivity in isomerization of α-pinene oxide to campholenic aldehyde over Fe-MMM-2 and Fe-VSB-5. Appl Catal A 469:427–433. https://doi.org/10.1016/j.apcata.2013.10.016
Acknowledgements
This work was conducted within the framework of the budget Project AAAA-A17-117041710082–8 for Boreskov Institute of Catalysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panchenko, V.N., Kirillov, V.L., Gerasimov, E.Y. et al. Isomerization of α-pinene oxide to campholenic aldehyde in the presence of Al-SiO2 and magnetic Al-SiO2/Fe3O4 catalysts. Reac Kinet Mech Cat 130, 919–934 (2020). https://doi.org/10.1007/s11144-020-01811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01811-x