Abstract
The kinetics and thermodynamics have been studied for the reactions of the copper(II) complexes with iminodiacetate (ida), 2,2′-bipyridine (bipy) and 1,10-phenanthroline (phen) as ligands. The kinetics of substitution reactions of two aqua ligands for bipy and phen in the [Cu(ida)(H2O)2] coordination compound has been studied in water and three type of aqueous solutions of the following surfactants: anionic sodium dodecyl sulfate (SDS), cationic hexadecyl trimethyl-ammonium bromide (CTAB) and nonionic t-octylphenoxypolyetoxyethanol (Triton X-100). The progress of the substitution reactions in the studied solutions was monitored spectrophotometrically using the stopped-flow method. The studies have allowed the determination of the effect of the type of surfactant solutions on the rate of the substitution reaction. Moreover, the order of studied reactions has been determined. The research performed has also allowed us to propose the reaction mechanism of the [Cu(ida)(H2O)2] binary complex with chelate ligands (bipy or phen). In addition, the thermodynamic stability of complexes under study in aqueous solutions has been examined using the potentiometric titration method. Moreover, the potential scavenging activity of the copper(II) complexes has been investigated towards the superoxide radical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
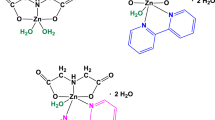
Over the last years, the copper(II) coordination compounds with polycarboxylate anions and chelate ligands containing two or more atoms which have free electron pairs, for example 2,2′-bipyridine (bipy) and 1,10-phenanthroline (phen), have received increasing attention in electronics and technology as new materials of interesting magnetic and catalytic properties [1,2,3,4,5]. Examples of compound structures of this type have been shown in Fig. 1.
The recent results of the studies of our research group have shown that Cu2+ complexes with oda (oxydiacetate) and tda (thiodiacetate) ligands undergo substitution reactions with phen and bipy in aqueous and dimethyl sulfoxide (DMSO) solutions [6]. The observed rate constants of studied reactions increase with the concentration of the binary complex and the temperature in aqueous and DMSO solutions. This dependence is compatible with the activated complex theory [6, 7].
In the literature, several types of surfactants of different types of hydrophilic groups, for example anionic sodium dodecyl sulfate (SDS), cationic hexadecyl trimethyl-ammonium bromide (CTAB) and nonionic t-octylphenoxypolyetoxyethanol (Triton X-100) have been known [8,9,10]. Surfactants are commonly used to mimic many aspects of physical interactions with cell membranes [11]. Consequently, the kinetics of reactions in micellar systems is studied by scientists. The influence of changes in the surfactant concentration on the observed rate constant is commonly studied [12]. Among others, the kinetics of the fading reaction of the malachite green and the bromophenol blue in aqueous solutions of three surfactants: anionic (SDS), cationic (DTAB) and nonionic (TX-100) has been studied. It has been stated that the rate of the fading reaction of malachite green increase in the presence of DTAB, TX-100, and furthermore, it decrease in the presence of SDS [12]. However, in the case of bromophenol blue the rate of the reaction increase in the presence of DTAB, TX-100, and moreover, it does not change in the presence of SDS [12]. Earlier, the stopped-flow method has been used to investigate the kinetics of the [Cu(phen)]+ reaction with phen in the SDS solution [13]. These studies have shown that SDS micelles may inhibit the reactions of Cu(phen) +2 and Cu(phen)+ by forming nonreactive complexes. Another report [14] shows that the substitution of tris(sulfonated triazine)iron(II) complexes by 1,10-phenanthroline, 2,2′-bipyridine and 2,2′,6,2″-terpyridine in the CTAB solution occurs by an associative mechanism, [14] which is confirmed by the low activation energy and high negative entropy.
In addition, the literature reports show that Cu2+ easy changes the oxidation state and, consequently, copper complexes are often used as catalysts in redox reactions [15,16,17]. Moreover, the Cu(II)-polycarboxylate complexes, e.g. [Cu(oda)(4-pic)(H2O)]2H2O (4-pic = 4-picoline) and [Cu(oda)(bipy)(H2O)]4H2O, exhibit an antioxidant activity towards superoxide anion radicals and these complexes are candidates for the synthetic superoxide dismutase mimetics [18, 19].
Analyzing the literature, there is no information on kinetic studies of reactions of the Cu(II)-iminodiacetate complex in micellar solutions. Thus, the main reason of our studies is to find the correlation between the rate of substitution reactions of aqua ligands for the bipy and phen in the binary complex-[Cu(ida)(H2O)2] and in the presence of surfactant solvents of the different structure. Our studies should answer the question how the surfactant structure impact on the kinetics of copper(II) complexes. Moreover, the thermodynamic stability of the synthesized complex has been investigated as a complement to the study on the kinetic stability of the complexes. We have predicted that the studied copper(II) complexes may exhibit the antioxidant activity against the superoxide anion. Consequently, we have also checked the potential scavenging activity of the synthesized copper(II) complexes.
Experimental
Syntheses
The series of complexes: [Cu(ida)(H2O)2], [Cu(ida)(bipy)]·4H2O, [Cu(ida)(phen)(H2O)]·H2O were synthesized based on the known methods described in the literature [1].
Diaqua(iminodiacetato)copper(II), [Cu(ida)(H2O)2], [Cu(ida)]: portions of Cu(NO3)2·H2O (5.79 g, 24 mmol in water, POCH) were added to the solution of H2IDA (3.19 g, 24 mmol, Fluka analytical) and Na2CO3 anhydrate (2.54 g, 24 mmol, Reachim) in 40 mL of water. The mixture in a round-bottomed flask was then heated to 50 °C with magnetic stirring. The resulting solution was filtered off and the blue solid was left in the air for 7 days.
2,2′-Bipyridine(iminodiacetato)copper(II)-tetrahydrate, [Cu(ida)(bipy)]·4H2O, [Cu(ida)bipy]: portions of Cu(NO3)2·H2O (5.79 g, 24 mmol in water, POCH) were added to the solution of H2IDA (3.19 g, 24 mmol, Fluka analytical) and Na2CO3 anhydrate (2.54 g, 24 mmol, Reachim) in 40 mL of water. Then the mixture was heated in a round-bottomed flask to 50 °C with magnetic stirring. 2,2′-bipyridine (3.74 g, 24 mmol, Fisher Scientific) was added to the solution at the end of the synthesis. The resulting solution was filtered and the blue solid was left in the air for 7 days.
1,10-Phenanthroline(iminodiacetato)(aqua)copper(II) monohydrate, [Cu(ida)(phen)(H2O)]·H2O, [Cu(ida)phen]: Portions of Cu(NO3)2·H2O (5.79 g, 24 mmol in water, POCH) were added to the solution of H2IDA (3.19 g, 24 mmol, Fluka analytical) and Na2CO3 anhydrate (2.54 g, 24 mmol, Reachim) in 40 mL of water. The mixture was then heated to 50 °C in a round-bottom flask with magnetic stirring. 1,10-Phenanthroline (4.76 g, 24 mmol, Stanlab) was dissolved in ethanol and added to the solution at the end of the synthesis. The resulting solution was filtered and the blue solid was left in the air for 7 days.
Elemental analyses of the polycarboxylate complexes were performed with the Vario EL analyzer Cube CHNS. Analysis calculations: for [Cu(ida)(H2O)2]: C, 20.82%, H, 3.90%, N, 6.07%. Found: C, 20.93%, H, 4.03%, N, 6.07%. Anal. Calcd for [Cu(ida)(bipy)]·4H2O: C, 39.76%, H, 4.97%, N, 9.94%. Found: C, 39.57%, H, 5.00%, N, 9.84%. Anal. Calcd for [Cu(ida)(phen)(H2O)]·H2O,: C, 46.96%, H, 4.16%, N, 10.27%. Found: C, 46.32%, H, 4.07%, N, 9.44%.
Kinetic measurements
The substitution reactions of aqua ligands for the bipy and phen in [Cu(ida)(H2O)3] coordination compounds in three type of surfactants aqueous solutions were monitored spectrophotometrically using the stopped-flow method. The concentrations of the binary complex were within the range of 0.25–1.00 mM, whereas the concentration of bipy or phen was kept constant at 0.05 mM in surfactants solvents. The surfactant solvents were the following: anionic sodium dodecyl sulfate SDS (Acros Organics) was 20 mM, cationic hexadecyl trimethyl-ammonium bromide CTAB (Acros Organics) was 2 mM and nonionic t-octylphenoxypolyethoxyethanol Triton X-100 (Sigma Aldrich) was 0.8 mM. Surfactants concentrations have been chosen based on the critical micelle concentration (CMC)-CMCSDS = 8.2 mM, CMCCTAB = 0.92–1.0 mM and CMCTriton X-100 = 0.22–0.24 mM [20]. The kinetic of substitution reactions of [Cu(ida)(H2O)3] complex was monitored in the UV range. [Cu(ida)(H2O)3] and phen or bipy were substrates of the reaction, and [Cu(ida)(phen)(H2O)] or [Cu(ida)(bipy)(H2O)] was the final product of the monitored reaction. The wavelengths necessary for the kinetic studies were chosen on the basis of UV–Vis spectra. The changes of absorbances have been monitored in the case of SDS at 260 nm for Cu(ida)bipy and at 280 nm for Cu(ida)phen; in the case of CTAB, at 242 nm for Cu(ida)bipy and at 245 nm for Cu(ida)phen and in the case of Triton X-100, at 285 nm for Cu(ida)bipy and at 285 nm for Cu(ida)phen.
Instrumentation
UV spectra were recorded using the UV–Vis Lambda 45 Perkin-Elmer spectrophotometer. The progress of the substitution reaction of [Cu(ida)(H2O)2] with bipy or phen ligands in surfactants solutions was monitored using the Applied Photophysics SX 18 MV-R spectrophotometer.
Potentiometric titrations (PT)
Potentiometric titrations were conducted using the Cerko Lab System microtitration unit fitted with a 5-mL Hamilton syringe, a self-made measuring cell equipped with a magnetic stirrer and a pH combined electrode (Schott BlueLine 16 pH type). The 30 mL-cell was thermostated at 298.15 ± 0.10 K (the Lauda E100 circulation thermostat). The electrode was calibrated according to IUPAC recommendations and already tested procedures [21,22,23]. The syringe was calibrated by a weight method. The titrand solutions contained the following species: Cu2+ (1 mM), H2ida (1.5 mM), phen (1 mM) or bipy (1 mM), HClO4 (5 mM). Three solutions: H2ida, Cu2+ + H2ida and Cu2++ H2ida + phen/bipy were potentiometrically titrated with the standardized NaOH solution (50 mM). All titrations were repeated at least three times in order to check the reproducibility of the data. The experimental data have been subjected to calculations to determine the stability constants of the complexes using the Hyperquad2008 program [24, 25].
NBT test
The potential superoxide scavenging activity of the Cu(II) complexes was examined using nitro blue tetrazolium (NBT) according to the method described in the literature [26]. The NBT solutions were prepared by dissolving 10 mg of NBT in 10 mL of DMSO. The solution of superoxide anion radicals contained 6.5 mg of the KO2 powder and 90 mg of 18-crown-6-ether dissolved in DMSO (50 mL) [27]. The tested sample was prepared by mixing 1.5 mL of the superoxide anion radical solution with 0.5 mL of the solution containing the studied complex. The sample was kept for 20 min and then 0.1 mL of the NBT solution was added. The control sample was prepared by mixing 1.5 mL of superoxide anion radical solution with 0.5 mL of the DMSO solution. After mixing all components in the cuvette, the absorbance of the mixture was monitored spectrophotometrically at 560 nm [28].
The percentage of scavenging of the superoxide radical can be calculated using the following equation:
Here is Acontrol is the absorbance of the sample without a potential antioxidant solution, and Asample is the absorbance of the sample with a potential antioxidant solution.
Cyclic voltammetry (CV)
The scavenging of the superoxide anion generated electrochemically was studied by the cyclic voltammetric technique using the Autolab potentiostat/galvanostat PGSTAT30 (Eco Chemie B.V., the Netherlands) combined with two electronic flow-meters (Bronkhorst El-Flow, equipped with electronic control valves) for oxygen and argon at 298.15 K by using the General Purpose Electrochemical System (GPES 4.9) software [29, 30]. The reference electrode was the Ag/AgCl/0.1 M TBACl in the MeOH solution. The platinum electrode (r = 3 mm) was an auxiliary electrode and it was polished on polishing clothes (Microcloth) with alumina (Buehler) pastes of 0.5 nm decreasing particle size. The glassy carbon electrode was used as a working electrode. In all experiments, 0.1 mol/L tetrabutylammonium perchlorate (TBAP) in DMSO was used as a base electrolyte. All solutions used to measurements were degassed with argon. The oxygen concentration in the measured solution was in the 1.5–2.2 × 10−3 mol/L range. In each voltamperogram a peak of the cathode and the anode has been achieved. The difference between peak heights of the cathode and the anode was calculated by the formula:
This allowed the calculation of the scavenging % of the superoxide anion with the formula:
ΔIp is the difference between the heights of cathodic and anodic peaks, Ipa is the peak height of the anode, Ipc is the peak height of the cathode.
Under the influence of a changing current the values of the potential on the cathode and the anode change since it is a result of the following reactions:
Here L denotes (ida), (ida)(phen) or (ida)(bipy).
The literature [31] confirms that the copper(II) cation has the ability to react with the superoxide anion radical according to the following scheme:
-
1.
Cu2+ + O ·–2 → Cu+ + O2
-
2.
Cu+ + H2O2 → Cu2+ + OH· + OH−
Results and discussion
The kinetic stability of the complexes in surfactants solutions
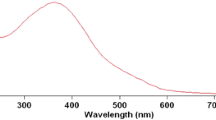
The measurements have been conducted in different solvents: water, SDS(aq), Triton X-100(aq) and CTAB(aq). The surfactant solutions were chosen to check the impact of the micelle effect on the studied substitution rate. In the first step of the investigations, the UV spectra were recorded to select wavelengths for the kinetic measurements. Consequently, the UV spectra of the substrate (phen or bipy), the mixture of phen or bipy with Cu(ida) in the molar ratio 1:1 and the (Cu(ida)phen or Cu(ida)bipy) ternary complex have been registered in four solvents studied. It turned out that the spectra of all compounds and mixtures studies were independent of solvent, i.e. were in all surfactant solutions the same as in water. For this reason, the UV spectra of the substrate (phen or bipy), the mixture of phen or bipy with Cu(ida) and the Cu(ida)phen or Cu(ida)bipy ternary complex in aqueous solutions only are shown in Fig. 2.
a The UV spectra of the phen substrate, the mixture of phen and Cu(ida) in the molar ratio 1:1 and the Cu(ida)phen ternary complex in H2O (T = 298.15 K). b The UV spectra of the bipy substrate, the mixture of bipy and Cu(ida) in the molar ratio 1:1 and the Cu(ida)bipy ternary complex in H2O (T = 298.15 K)
In aqueous or surfactant solutions, the two aqua molecules in the coordination sphere of Cu(II) have been substituted by bipy or phen. A mathematical model for the studied reactions is A → B. Reactions of [Cu(ida)(H2O)3] with bipy and phen may be described using following equations:
Here A is the substrate concentration, B is the product concentration, k is the reaction rate constant, k obs is the observed rate constant, v is the reaction rate, t is the time.
The values of the observed rate constant of the studied substitution have been calculated with the “Glint” program. The linear relationships of the rate of the substitution reaction versus the concentration of the binary complex as well as the temperature have been observed. The order of all studied reactions has been determined by a graphical method. An analysis of the linear fit of absorption changes in a time function and the obtained coefficients of determination R2 allow to conclude that all monitored reactions are of the pseudo-first order (R2 ≅ 1).
Figures showing kobs versus Cu(ida) are available in the Supplementary Information. Results obtained show that in all types of solvents studied the rate constants of the substitution of two aqua ligands with phen or bipy in [Cu(ida)(H2O)3] increase with the Cu(ida) concentration and the temperature. However, in the case of the SDS(aq) solution, the dependence of the rate constants in the temperature function is the strongest. In SDS and Triton X-100 solutions, the reaction of [Cu(ida)(H2O)3] with bipy undergoes 6 times faster than the reaction with phen. In the CTAB solution the similar results have been obtained. The Cu(ida) reaction with bipy undergoes 4.5 times faster than in the case of the reaction with phen. The Cu(ida) reaction with bipy in the SDS solution is about 2 times faster than the same reaction in the CTAB and Triton X-100 solutions. This may be due to the charge of the surfactant molecule. The results of the kinetic measurements show that the presence of micelles in aqueous solutions causes an increase in the rate substitution in Cu(ida) with bipy. A similar dependence occurs in the case of the substitution in Cu(ida) with phen. However, the presence of CTAB and SDS micelles in aqueous solutions causes a decrease in the rate substitution of two aqua ligand with one phen molecule. These differences in the micellar effect may be a result of differences in the basicity of phen and bipy [32]. In the reaction with phen, the impact of Triton X-100 on the rate of the substitution in comparison to the observed rate constant in other surfactants solutions may be explained by the structure of the Triton X-100 molecule. Triton X-100 is a nonionic surfactant. Therefore, its micelles present in solutions have a different impact on the monitored reaction in comparison to ionic surfactants such as SDS and CTAB. The micelles of Triton X-100 may be less likely to interact with studied species (Cu(ida), phen, bipy) than in the case of micelles of ionic surfactants. The effect of the presence of the micelles in the solution on the rate of the chemical reaction can be caused by a change of a reaction mechanism when compared to the observed rate constant in aqueous solutions or elsewhere this effect may be as a result of a change of the activation energy of the reaction.
The thermodynamic stability of the complexes in aqueous solutions
The stability of the title compound in aqueous solutions has been investigated by using the potentiometric titration method. The equilibrium constants defined by equations:
(Here M is Cu2+, L denotes the iminodiacetate ion, B denotes phen or bipy, H is the proton and p, q, r, s are stoichiometric coefficients for the reaction) were refined by least-squares calculations using the Hyperquad computer program. All potentiometric titrations have been performed involving the ternary system which contained Cu2+, ida as the primary ligand as well as bipy or phen as the secondary ligand. The calculated logarithms of the stability constants values (log β pqrs) of the Cu2+ complexes are collected in Table 1. In our studies, based on the best fit of the calculated data to the experimental ones, the equilibrium model has been determined using the Hyperquad2008 program and the formation of CuB or CuB2 has been neglected. The results show that the copper(II)-iminodiacetic complex with phen has higher thermodynamic stability in aqueous solution that the Cu2+ complex with bipy as a ligand. This result may be explained by the fact that phen is more basic ligand than bipy. The thermodynamic stability of the complexes increases with an increasing basicity of ligands. The analysis of calculated log β pqrs values allow us to conclude that in aqueous solutions, the Cu2+-iminodiacetic complex is less stable by four orders of magnitude compared to Cu(ida)phen and by three orders of magnitude compared to the Cu(ida)bipy complex. According to the general rule, the greater is the basicity of the ligand, the more stable complexes are formed [32]. In our study, phen is the more basic ligand than bipy and, consequently, forms more stable complexes. All studied complexes, Cu(ida), Cu(ida)(phen) and Cu(ida)(bipy) are more stable in comparison with Cu-phen and Cu-bipy complexes in aqueous solutions. For Cu(phen)2+ and Cu(bipy)2+ log β 1010 equals to 9.25 and 8.00, respectively [33]. The analysis of log β pqrs values shows that in aqueous solutions, the Cu(ida) complex is more stable by one order of the magnitude whencompared to Cu(phen)2+ and by two orders when compared to the Cu(bipy)2+ complex. Moreover, Cu(ida)(phen) and Cu(ida)(bipy) complexes are more stable by five orders of the magnitude when compared to Cu(phen)2+ and Cu(bipy)2+, respectively.
In aqueous solutions the Cu(ida) complex dominates in the solution at pH ≅2, Cu(ida)bipy and Cu(ida)phen dominate at pH ≅ 4 (Fig. 3). In the case when bipy occurs in the investigated mixture, the Cu(ida) complex is present at pH = 2 at a higher percentage concentration than in the case of the mixture containing phen. The pH value higher than 4 favors the formation of the ternary complexes Cu(ida)phen and Cu(ida)bipy.
Concentration distribution curves of the complexes as a function of pH calculated based on the values of log β pqrs collected in Table 1
The results of kinetic and thermodynamic studies confirm that Cu(ida)bipy and Cu(ida)phen complexes are more stable in aqueous solutions than the Cu(ida) complex. In SDS and Triton X-100, both Cu(ida)bipy and Cu(ida)phen are more stable than in aqueous solutions. In the case of the Cu(ida)bipy complex, the presence of nonionic micelles of Triton X-100 increase about 2 times the substitution reaction when compared to SDS and CTAB solutions. In the case of the Cu(ida) reaction with phen, such a dependence of the micelles structure on rate constant values does not occur.
The antioxidant activity of Cu(ida), Cu(ida)bipy and Cu(ida)phen against the superoxide radical
The potential scavenging activity of the copper(II) complexes with iminodiacetate anion and bipy or phen towards the superoxide anion radical anion has been studied by NBT test (Fig. 4a). The results show that the Cu2+ complex with phen as a ligand exhibits the highest antioxidant activity. It is interesting that the presence of the phen and bipy ligand in the coordination sphere of Cu2+ causes a decrease of the scavenging activity of the complexes. For the Cu(ida) complex, the value of the ascorbic acid equivalent (EA) has been calculated and it equals 2.412. However, for the Cu(ida)bipy and Cu(ida)phen complexes, the values of EA could not be calculated due to lack of data: the studied concentrations of Cu(ida)bipy and Cu(ida)phen do not cause the 50% inhibition of the superoxide anion radical due to too low solubility in aqueous solutions. Among the synthesized complexes, only Cu(ida) exhibits a sufficient solubility in an aqueous solutions to determine the concentration of complex, which causes the 50% scavenging activity against the superoxide anion. The presence of bipy and phen in the complexes reduces the ability of the Cu2+ cation to change the state of an oxidation.
Cyclic voltammetry allows to study the antioxidant activity of the synthesized complexes towards the superoxide anion radical which was generated electrochemically (Fig. 4b). The CV results show that the copper(II) complex with phen and ida most effectively remove the superoxide anion radical. However, the presence of bipy ligand in the copper(II) complex reduce the compound reactivity with the superoxide anion.
Taking into account the results of the NBT and CV tests it can be concluded that Cu(ida) may play a role as a potential antioxidant or redox catalyst. Although this complex exhibits about 2.5 times lower scavenging activity than ascorbic acid it can be proposed as an antioxidant compound since Cu(ida) mimics the superoxide dismutase interactions with the superoxide anion radical. The complexes containing bipy and phen have too low solubility in aqueous solutions to determine the concentration causing the 50% inhibition of the superoxide anion radical using the NBT test.
Conclusions
The kinetics of the aqua ligands substitution in the [Cu(ida)(H2O)2] complex with phen and bipy has been studied. Furthermore, the micellar effect on the rate constants has been investigated in aqueous solutions and in three type of surfactants: cationic CTAB, anionic SDS and nonionic Triton X-100. It has turned out that all monitored reactions are of the first-order rate and undergo reactions according to the associative mechanism. An impact of the type of micelles depends on the basicity of the ligand (phen or bipy) which is substituted in the coordination sphere of Cu2+ and, depends on the type of the solved surfactant. A lower basicity of an associated ligand to a complex compound causes the acceleration of the substitution in surfactant solvents in comparison to the reaction with ligands of higher basicity. The potentiometric titrations have shown that in aqueous solutions the iminodiacetate Cu2+ complexes with phen or bipy have the higher thermodynamic stability than the Cu(ida) complex. Moreover, the results obtained by the NBT test and the CV method have proven that the three Cu2+ complexes exhibit the scavenging activity towards the superoxide anion radical. The results of the NBT and CV methods allow to conclude that Cu(ida) is a potential antioxidant and may be used for studies on the application of Cu(ida) as a redox catalyst.
References
Roman-Alpiste MJ, Martin-Ramos JD, Castineiras-Campos A, Bugella-Altamirano E, Sicilia-Zafra AG, Gonzalez-Perez JM, Niclos-Gutierrez J (1999) Polyhedron 18:3341–3351
Ren YP, Long LS, Mao BW, Yuan YZ, Huang RB, Zheng LS (2003) Angew Chem Int Ed 115:550–553
Hong-Bin X, Li-Kai Y, Zhong-Min S, Shu-Mei Y, Heng-Już Z, Kui-Zhan S, Ya-Hui Z (2004) Transit Met Chem 29:471–476
Selvakumar B, Rajendiran V, Maheswari PU, Stoeckli-Evans H, Palaniavar M (2006) J Inorg Biochem 100:316–330
Pavlishchuk AV, Kolotilov SV, Zeller M, Thompson LK, Addison AW (2014) Inorg Chem 53:1320–1330
Pranczk J, Jacewicz D, Wyrzykowski D, Tesmar A, Chmurzyński L (2015) J Chem Sci 127:1845–1852
Holyer RH, Hubbard CD, Kettle SDA, Wilkins RG (1956) Inorg Chem 4:929–935
Bunton CA (2006) Adv Colloid Interface Sci 123–136:333–343
Dwars T, Paetzold E, Oehme G (2005) Angew Chem Int Ed 44:7174–7199
Ruiz CC (1995) Colloid Polym Sci 273:1033–1040
Singh A, Van Hamme JD, Ward OP (2007) Biotechnol Adv 25:99–121
Samiey B, Toosi AR (2009) Bull Korean Chem Soc 30:2051–2056
Hodges HL, De Araujo MA (1982) Inorg Chem 21:3236–3239
Bellam R, Anipindi NR (2014) Transit Met Chem 39:311–326
Wang JS, Matyjaszewski K (1995) Macromolecules 28:7901–7910
Fridovich I (1995) Annu Rev Biochem 64:97–112
Harrison PG, Ball IK, Azelee W, Daniell W, Goldfarb D (2000) Chem Mater 12:3715–3725
Siddiqi ZA, Sharma PK, Shahid M, Khalid M, Siddique A, Kumar S (2012) Eur J Med Chem 57:102–111
Siddiqi ZA, Sharma PK, Shahid M, Khalid M, Kumar S (2011) J Mol Struct 994:295–301
Singh V, Tyagi R (2015) J Taibah Univ Sci 9:477–489
Brariz I, Barriada J, Vilarino T, de Vicente MS (2004) Monatsh Chem 135:1475–1488
Chmurzyński L (1996) Anal Chim Acta 326:267–274
Chmurzyński L, Nesterowicz M, Wawrzyniak G, Kaczmarczyk E, Warnke Z (1996) Aust J Chem 49:931–942
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Alderighi L, Gans P, Ienco A, Peters D, Sabatini A, Vacca A (1999) Coord Chem Rev 184:311–318
Pranczk J, Jacewicz D, Wyrzykowski D, Chmurzynski L (2014) Curr Pharm Anal 10:293–304
Audri RL, Allen AO, Bielski BH (1981) FEBS Lett 135:265–267
Pranczk J, Jacewicz D, Wyrzykowski D, Wojtczak A, Tesmar A, Chmurzyński L (2015) Eur J Inorg Chem 20:3343–3349
Wyrzykowski D, Inkielewicz-Stępniak I, Czupryniak J, Jacewicz D, Ossowski T, Woźniak M, Chmurzyński L (2013) Z Anorg Allg Chem 639:1795–1799
Pranczk J, Wyrzykowski D, Jacewicz D, Sikorski A, Tesmar A, Chmurzyński L (2015) Polyhedron 100:74–81
Rosi M, Sgamellotti A, Tarantelli F, Bertini I, Luchinat C (1986) Inorg Chem 25:1005–1008
Wyrzykowski D, Pranczk J, Jacewicz D, Tesmar A, Pilarski B, Chmurzyński L (2014) Cent Eur J Chem 12:107–114
Sillen LG, Martel AE (1966) Stability constants of metal-ion complexes. The Chemical Society, London
Acknowledgements
This work was supported by National Science Centre, Poland under Grant Number 2015/19/N/ST5/00276.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Drzeżdżon, J., Piotrowska, A., Wyrzykowski, D. et al. Kinetics and thermodynamics of the reaction of iminodiacetate copper(II) complexes with 1,10-phenanthroline and 2,2′-bipyridine in aqueous, anionic, cationic and nonionic surfactants solutions. Reac Kinet Mech Cat 122, 729–740 (2017). https://doi.org/10.1007/s11144-017-1269-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1269-9