Abstract
A series of acids with different strength acidity, heteropolyacid H3PW12O40 (HPW) and oxoacids, H2SO4 and H3BO3 were used as catalysts for the sunflower oil methanolysis with an oil/methanol ratio of 1/29, a reaction temperature of 60 °C and a reaction time of 3 h. The effect of silica support on the catalytic performance of HPW was investigated. (10–50 wt%) HPW supported on silica were characterized by XRD, FT-IR and N2 physisorption (BET specific surface areas, mean pore diameters and pore volumes). The fatty acid methyl esters, reaction products, were analyzed and quantified by gas chromatography. HPW and 30 wt% HPW/SiO2 have similar catalytic behavior with a biodiesel yield of 60–63 %. Side reactions were observed at 100 °C in the presence of 30 wt% HPW/SiO2 resulting in the formation of oxygenated products (alcohol, ketone, acid) in addition to fatty acid methyl esters, as evidenced by GC–MS. A plausible mechanism of supported heteropolyacid catalyzing the sunflower oil methanolysis is proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, the improvement of production systems of biodiesel that can contribute to the development of green chemical processes is a challenging goal. So, several reviews have summarized the recent research [1–3]. Biodiesel fuel, in addition to being non-toxic and biodegradable, has physico–chemical characteristics similar to those of fossil diesel fuel.
The biodiesel, composed of fatty acid methyl (or ethyl) esters is produced from transesterification of vegetable oils as canola, olive, soybean, castor, rapeseed, palm and sunflower oils and cooking oil, renewable feedstocks. The transesterification of oils that can be performed in homogeneous as well as in heterogeneous systems, requires a basic or acidic catalysis and the presence of an alcohol (ethanol or methanol). Methanol is the most used owing to its low cost and its physico–chemical characteristics (polarity and shortest chain alcohol).
The transesterification reaction catalyzed by an acid is much slower than that catalyzed by a base (Na or K alkoxides, hydroxides or carbonates). However, the use of an acid catalyst has certain advantages such as the absence of the saponification reaction that inhibits the transesterification reaction. Moreover, the acid catalysts were able to do transesterification and esterification reactions simultaneously and to convert oil to biodiesel with high amount of free fatty acids, while the presence of free fatty acids in the case of the base catalysis leads to the formation of soaps, as side reaction (reaction between alkali catalyst and free fatty acids) [1–3]. The acid catalysts tested in transesterification of oils are mineral acids (HCl, H2SO4) [4, 5] and Keggin-type heteropolyacids (H3PW12O40, H4SiW12O40) [6, 7]. These latter are known as promising acid catalysts for the clean synthesis [8–10]. Due to their stronger acidity, they usually show higher catalytic activities than the mineral acid catalysts.
The comparison between a heterogeneous system and a homogeneous system showed that the first system may be a best future process to produce biodiesel [1–3]. It reduces the environmental risks lied to corrosion, pollution, etc. Otherwise, in the case of using a solid acid catalyst, the system can operate continuously, the separation of biodiesel and glycerol is much easier and the catalyst can be recovered for other uses. Among the solid acid catalysts, supported H3PW12O40 is the most tested. Thus, HPW supported on silica was used for camphene esterification [11], HPW supported on niobia was tested for both palmitic acid and sunflower oil esterification [12]. Nevertheless, a high alcohol/oil molar ratio (from 12/1 to 50/1) is required in heterogeneous acid catalysis to produce biodiesel unlike base catalysis where an alcohol/oil molar ratio of 6/1 shall be sufficient. It was also reported that high temperatures and high pressures are recommended for obtaining high biodiesel yields from transesterification reaction of oils [1–3]. Among the examined oils, sunflower oil presents some advantages such as specific gravity, viscosity and flash point that decrease linearly with an increase of methyl ester wt% [13].
In this context, the transesterification of sunflower oil in the presence of methanol (methanolysis), using acid catalysts with different strength acidities, heteropolyacid, H3PW12O40, noted HPW (very strong acid) and oxoacids, H2SO4 (strong acid) and H3BO3 (weak acid), was studied under mild experimental conditions similar to those using base catalysts (low reaction temperature: 60 °C) and under more severe operation conditions similar to those required in the case of an acid catalysis (high reaction temperature: 100 °C) at atmospheric pressure and with a molar ratio alcohol/oil of 29/1, to examine the effect of the strength acidity and reaction temperature on the catalytic behavior of material. KOH catalyst was taken for comparison. This is a base catalyst that is widely used in the transesterification process. The effect of silica support on the catalytic performance of HPW was investigated. Silicate gel (SiO2) was selected as support material with 62 µm particle size. HPW supported on silica (10–50 wt%) were prepared and characterized by XRD, FT-IR and their specific surface areas, mean pore diameters and pore volumes were performed by N2 physisorption.
Experimental
Catalyst preparation
The heteropolyacid, HPW, was prepared according to the method described in the literature from its Na2HPW12O40 salt [14]. This latter was obtained by precipitation from aqueous mixture constituted of Na2HPO4, Na2WO4 and HCl in stoichiometric ratios. Na2HPW12O40 salt was then dissolved in an acid aqueous solution (HCl or H2SO4) and was put in a separating funnel. After adding diethyl ether, two layers of liquid were formed. After decantation, the etherate phase, containing the heteropolyacid was recovered. Then, the diethyl ether was removed using a rotary evaporator and HPW was recovered. To obtain a pure product, HPW was crystallized in a minimum amount of water.
The heteropolyacid HPW supported on silica samples with 10–50 wt% (noted S10–S50) were prepared by wet impregnation: a calculated amount of heteropolyacid was dissolved in 30 ml of water under agitation at 60–80 °C, then a calculated amount of silica was added, followed by drying at 110 °C for 5 h under reflux. The obtained mixture was then dried using a rotary evaporator, followed by a drying at 50 °C in a vacuum oven for 6–7 h to remove any traces of water.
Characterization
BET specific surface areas, mean pore diameters and pore volumes were performed by N2 physisorption at liquid nitrogen temperature using a Micromeritics ASAP 2010 equipment after out gassing of sample at 300 °C under vacuum (5.33–6.67 Pa) for about 8 h.
Transmission FT-IR spectra of the prepared catalysts were recorded at room temperature with an Equinox 55 (Bruker) spectrometer, in the 1500–500 cm−1, using KBr disks.
Powder XRD patterns were recorded in the 2θ range between 0.5 and 70° with a Cu Kα radiation (λ = 1.5418 A°) using a PanalyticalX’pert Pro.
Catalytic reaction
The refined sunflower oil methanolysis reaction was carried out at 60 °C, atmospheric pressure under reflux conditions with constant stirring (300 rpm) and a methanol/oil molar ratio of 29/1 to favor the displacement of the equilibrium in the direction of the biodiesel formation [6]. In a catalytic test experiment, 2.61 g of sunflower oil were taken in the glass reactor with 3.50 ml of methanol, after heating to 60 °C, 0.25 g of catalyst was then added to reaction mixture. After 3 h of reaction and adding 10 ml of distilled water through the refrigerant, to recover all of the methanol, the reaction mixture was put in a separating funnel (the catalyst was separated by filtration in the case of supported catalysts). Then 20 ml of chloroform were added for better visibility of two layers of liquid (aqueous and organic phases) and therefore better separation by decantation. After decantation, the chloroform phase, containing the FAMEs (methyl linoleate, methyl oleate, methyl stearate and methyl palmitate), was recovered and the aqueous phase was washed two times with 10 ml of chloroform to extract all esters. Then, the chloroform was removed using a rotary evaporator and the FAMEs recovered were dried over anhydrous Na2SO4 and concentrated under vacuum. The ester content was analyzed and quantified using a gas chromatograph. Peak identification was achieved by comparing the retention time between the samples and a standard and the compositions were calculated as wt% based on the peak areas of each component. The sunflower oil methanolysis in the presence of KOH was carried out in the same conditions.
GC gas chromatograph (Agilent Technologies 7890A) equipped with a flame ionization detector was used under the following conditions: capillary column, carbowax (30 m × 0.25 mm) with nitrogen as a carrier gas (flow rate: 1 ml/min) and injection temperature 260 °C. Product separation was obtained using temperature ramps: from 120 to 180 °C with a rate of 10 °C/min, and from 180 to 260 °C with a rate of 7 °C/min. The temperature was then maintained at 260 °C.
The methanolysis products were identified using a GC–MS (GC 6890 plus, MSD 5973, Hewlett Packard-5MS) with HP- INNOWAX column (30 m × 0.25 mm × 0.25 µm). The mass analysis is of Quadripôle type (150 °C). Purified helium was used as the carrier gas with a flow rate of 0.5 ml/min. The samples were diluted with hexane. The initial oven temperature was set at 90 °C for 5 min, then ramped to 280 °C at 4 °C/min and kept at the highest temperature for 5 min. The injector temperature and injector volume were 250 °C and 0.2 µl. The split ratio was 20:1. The ionization source (electronic impact) temperature was kept at 230 °C and that of the interface at 280 °C.
The biodiesel yield was calculated from the content of analyzed methyl esters using the following equation [15]:
Results and discussion
Catalysts characterization
Table 1 shows the BET specific surface areas, mean pore diameters and pore volumes of the silica, heteropolyacid and supported systems (S10–S50). It is known that the specific surface of the Keggin-type heteropolyacid is less than 10 m2 g−1 [16, 17]. After the impregnation of HPW, the BET surface area and the pore volume of the silica decrease gradually, from 159 to 146–93 m2 g−1 and from 0.70 to 0.31 cm3 g−1 with increasing of the HPW loading mass from 10 to 50 wt%. The decrease of these two parameters in the case of supported Keggin-type heteropolyacid has already been reported [7, 18, 19]. It was also emphasized that the decrease of the support surface area can be attributed to the formation of multilayers of HPW active species onto support surface resulting in blocking/stabilization of active sites on the monolayer [18]. This decreasing can also be explained by the reaction between the silanol OH group on the silica surface with one proton of the heteropolyacid to form SiOH2 +, thus, leading to the formation of (=SiOH2 +)(H2PW12O40 −) surface species that are more stable than the free acid form [16, 19, 20]. On the opposite, the pore diameter of the SiO2 support varies little after impregnation of the heteropolyacid (from 113.0 to 114.7–120.2 Å). These results indicate that the HPW molecules with a pore diameter of 84.3 Å can also penetrate inside the pores of the silica support. All these observations suggest that heteropolyanions species are in strong interaction with the silica support. Therefore, the heteropolyacid leaching can be strongly reduced in the reaction medium.
Fig. 1 shows the FT-IR spectra of HPW, silica and HPW supported on silica (S10–S50). Oxygen–phosphorus and oxygen–tungsten characteristic vibration bands of the Keggin anion were observed in the 1500–500 cm−1 region. According to the literature [21], the bands at 1081, 963, 899, 809 and 487 cm−1 correspond to νas(P–Oa), νas (W–Od), νas(W–Ob–W), νas(W–Oc–W) and δas (P–Oa) vibrations. The IR spectrum of the silica exhibits a very strong vibration band between 1200 and 1000 cm−1 assigned to νas(Si–O–Si) and a smaller band at 800 cm−1 that can be assigned to νs(Si–O–Si) in SiO4 groups [16, 22]. FT-IR spectra of supported systems S10, S20, S40 and S50 show that the vibration band corresponding to νas(P–Oa) was completely masked by the broad band of silica. Other vibration bands of metal–oxygen bonds were observed, indicating that the Keggin structure was preserved during the preparation of the material supported on silica.
Fig. 2 shows XRD patterns of HPW, silica and HPW supported on silica. The pattern of the support shows broad peak between 2θ = 15 and 35° indicating the amorphous nature of the silica [22, 23]. The patterns of HPW supported on silica show predominantly peaks related to the support for loading inferior to 40 wt%. In particular, no crystalline phase of heteropolyacid corresponding to its secondary structure (triclinic system) is observed. On the other hand, it was observed that the intensity of the major diffraction peak of support decreased with increasing loadings of acid. Similar observations have been reported in the literature in the case of HPW supported on silica, with low amount of heteropolyacid [11, 16, 17]. With percentages higher than 30, XRD diffraction lines corresponding to the heteropolyanion appear, similar results were obtained with Nb2O5 support [12]. This result suggests that 30 wt% HPW loading correspond to maximum heteropolyacid species that can occupy the total area of the silica.
Catalytic tests
Table 2 presents the fatty acid composition of refined sunflower oil. The major acids are linoleic (C18:2) and oleic (C18:1) followed by stearic (C18:0) and palmitic (C16:0). There is less than 1 % of linolenic acid (C18:3), arachidate (C20:0) and behenic acid (C22:0).
Table 3 summarizes the catalytic performances of HPW, H2SO4 and H3BO3 and HPW supported on silica, evaluated in the sunflower oil methanolysis at 60 °C with a methanol/oil molar ratio of 29/1. The reaction time is 3 h. The obtained results showed that the formed FAME mol number and biodiesel yield are sensitive to the nature of the catalytic material (oxoacid or heteropolyacid) and HPW amount supported on silica. The catalytic results of acids were compared to those obtained with KOH catalyst, tested in the same operation conditions (Table 3).
Catalytic performances of H3BO3, H2SO4 and H3PW12O40
The observed catalytic activities for both H3BO3 and H2SO4 oxoacids and HPW heteropolyacid in the homogeneous methanolysis of sunflower oil showed that there is a parallel between the catalyst acidity strength and biodiesel yield. HPW, the most acid, was also found to be the most active. Thus, the biodiesel yield increases from 6 to 60 % with acid strength of catalyst (H3BO3 < H2SO4 < HPW). It is noteworthy that HPW acid is almost two times more active than H2SO4 (63 against 30 % of biodiesel yield) and this latter is almost five times more active than H3BO3 (30 against 6 % of biodiesel yield). It is known that the three protons of HPW have the same acidity strength that is higher than that of H2SO4. Therefore, more protons for the same acid amount [8–10].
The distribution of different methyl ester products is also sensitive to acidity strength of catalyst. The obtained quantity of methyl palmitate (C16:0) increases from 2.69 × 10−4 to 5.63 × 10−4 mol suggesting that its formation seems to depend of the acidity strength of the catalytic material. The methyl stearate (C18:0) formation requires a strong acidic character (4.02 × 10−4 mol in the presence of H2SO4 and HPW against 1.50 × 10−4 mol in the presence of H3BO3) and HPW, very strong acid, those of methyl oleate (C18:1) (11.41 × 10−4 against 0.77 × 10−4 and 0.30 mol with H2SO4 and H3BO3) and methyl linoleate (C18:2) (35.37 × 10−4 against 17.16 × 10−4 and 0.77 × 10−4 mol with H2SO4 and H3BO3). These results indicate that the high difference in the acid strength between different catalysts clearly has a large impact on homogeneous reactivity and on the nature of formed FAME products. It is noted that C18:0 methanolysis in the presence of H2SO4 and HPW is similar to that in the presence of KOH indicating that the reaction requires either a strong acidic character or a strong basic character.
In the presence of KOH, the biodiesel yield obtained from sunflower methanolysis is of 99 %. This very high yield can be explained by the used high methanol/oil molar ratio (29/1). The alcohol excess has completely to displace the equilibrium in direction of the biodiesel formation. It is known that the base catalysis give more favorable kinetics (reaction rate faster, higher activity, higher yield in short time). It was reported that more than 96 % biodiesel could be obtained from sunflower oil transesterification in 1 h by using 1 % KOH and a methanol/oil molar ratio 6/1 at 65 °C [24].
Effect of silica support for sunflower oil methanolysis
The catalytic activity of HPW supported on silica was studied in the sunflower oil methanolysis under heterogeneous conditions. The influence of the HPW amount on the catalytic performance was examined using loading varying between 10 and 50 wt% of HPW supported on silica (S10–S50). The catalytic results are summarized in Table 3. HPW supported on silica becomes less active than unsupported with 7–60 against 63 % of biodiesel yield. With supported catalysts, biodiesel yield increases from 21 to 60 % with increase of the HPW amount from 10 to 30 wt%, indicating that the catalytic activity increases with the number of active sites. Similar activity trends were observed for sunflower oil methanolysis on HPW/Nb2O5 [17]. Up to 30 wt%, a decrease of biodiesel yield was observed (7 % for S40 and for S50). These lower biodiesel yields can be attributed either to the formation of agglomerates on the silica support surface as it was observed by XRD or the presence of diffusional limitations of reactants. The results indicate that the active site concentration plays an important role in the catalyst activity. Among HPW supported on silica, the higher activity of S30 can be explained by a better dispersion of (–SiOH2 +)(H2PW12O40 −) active surface species, as shown by XRD diffraction, and therefore, a better accessibility of active sites. IR spectroscopy analysis of S30 after sunflower oil methanolysis showed the presence of characteristic vibration bands of Keggin anion indicating that the leaching of the acid has not been total, hypothesis supported by the probable existence of (–SiOH2 +)(H2PW12O40 −) stable surface species. On the other hand, if we assume that there is leaching, S40 and S50 should be the most active, this is not the case. Therefore, this implies that the interaction between the support and the heteropolyacid is very strong.
The nature of FAMEs obtained from sunflower oil methanolysis is also very sensitive to the amount of HPW supported on silica. In the presence of S10, methyl oleate is the only observed product with a quantity of 19.01 × 10−4 mol. This latter decreases to 0.96 × 10−4 mol with increase of the HPW amount from 10 to 50 wt%. Unsupported HPW and S30 have a similar behavior toward of the formation of different methyl esters with equivalent FAME mol number.
These results seem to suggest that the distribution of FAME products in heterogeneous catalysis is function of the accessibility of Brønsted acid active sites localized on support to different radicals (R1, R2 and R3) of the triglyceride and to methanol and probably to preferential reaction pathway that take place on the surface of the solid catalyst and to the desorption rate of FAME products.
Effect of temperature for sunflower oil methanolysis
From obtained results, S30 (heterogeneous catalysis) and HPW acid (homogeneous catalysis) were found be the most efficient for sunflower oil methanolysis under mild experimental conditions (60 °C, atmospheric pressure and 3 h of reaction time) with 63 and 60 % of biodiesel yield. However, these yields are smaller than that obtained with the KOH (99 %) in the same operation conditions. Several works have reported that high biodiesel yields were obtained with more severe operation conditions (high reaction temperature and high pressure). Shin et al. have obtained a content of FAME of 92 % from the used vegetable oil transesterification, carried out at 260 °C, 20 MPa and molar ratio of oil/methanol: 1/40 with Cs-doped heteropolyacid as catalyst [25]. In the work published by Jiménez-morales et al., a maximum of biodiesel yield of 89 % was obtained, using mesoporous tantalum phosphate as catalyst for sunflower oil methanolysis performed at 200 °C with a Parr high pressure reactor and a molar ratio oil/methanol of 1/12 [26]. Nambo et al. have carried out the methanolysis of olive oil over ZnO nanorods, with a molar ratio methanol/oil of 50/1 and a reaction temperature of 150 °C [27]. High biodiesel yield was obtained in this study (95 %).
With the aim to increase the biodiesel yield, S30 was tested at 100 °C, atmospheric pressure and 3 h of reaction time (same procedure as described in the experimental part). The GC analysis showed, in addition, to peaks corresponding to different FAMEs, other products have appeared (Fig. 3), confirmed qualitatively by GC–MS analysis (Table 4).
GC chromatogram of reaction products after sunflower oil methanolysis at 100 °C on 30 wt% HPW/SiO2. Operation conditions: mcatalyst = 250 mg, nmethanol/noil = 29/1 (moil = 2.61 g, Vmethanol = 3.5 mL), T = 100 °C at atmospheric pressure, reaction time: 3 h, agitation rate: 300 rpm. Products: C16:0-methyl palmitate, C18:0-methyl stearate, C18:1-methyl oleate, C18:2-methyl linoleate, cyclohexanol, cyclohexanone, 2,4-decadienal, methylbutanoicanhydride, diethyl isobutylmalonate, methyl palmitoleate, methyl palmetate and methyl tetradecanoate
Table 4 shows that FAMEs corresponding to C18 and C16 acids are the major products and the other observed products resulting side-reactions are cyclohexanol, cyclohexanone, 2,4-decadienal, methylbutanoicanhydride, diethyl isobutylmalonate, methyl palmitoleate, methyl palmitate and methyl tetradecanoate. Among them, it may be noted the formation of methyl esters (methyl palmitoleate, methyl tetradecanoate, methyl eicosanoate) resulting of the methanolysis of corresponding acids. The presence of these methyl esters shows that the biodiesel yield can be superior to 60 %.
These results evidenced that the transesterification reaction carried out at 100 °C is followed by side-reactions. These observations have been emphasized by other authors, but at higher reaction temperatures (>200 °C) [28–31]. The observed side reactions at 100 °C and no at higher temperatures can probably result from the fact that HPW supported on silica is a more acidic material compared to the Cs-doped heteropolyacid [25], mesoporous tantalum phosphate [26], sulfated zirconia [28, 29] and supported metal catalysts [30]. The side-reactions take place simultaneously with the oil transesterification, thus leading to the formation of products that can come from either catalytic decarboxy-cracking or the conversion of triglyceride and that of oxygenated products from catalytic cracking of unsaturated fatty acids [28–30]. It is known that the activation of the C–H bond requires a strong acidic character of the catalyst [31, 32].
Catalytic stability of HPW supported on silica
The leaching of HPW contained within HPW supported on silica (S30) was verified. In a catalytic test experiment, methanol and catalyst (S30) were mixed during 30 min, then the solid is removed by filtration and the sunflower oil was added (methanol/oil molar ratio of 29/1) and the reaction takes places during 3 h at 60 °C (same procedure as described in the experimental part). The GC analysis showed only peaks corresponding to tri-, di- and monoglycerides characteristic of sunflower oil, indicating accordingly, meaning that the transesterification reaction does not take place. It can be concluded that either there was no leaching or the amount of leached acid was not sufficient to catalyze the reaction of transesterification.
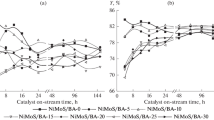
The catalytic performance of used S30 catalyst was also evaluated in order to test its activity as well as its stability. The results are represented in Table 5. Each cycle lasts 3 h. After the first cycle, S30 catalyst was separated easily by simple filtration and the biodiesel is recovered. The methanolysis of sunflower oil was carried out with the used catalyst, under the optimized conditions (reaction temperature: 60 °C, methanol/oil: 29/1). A decrease of catalytic activity of S30 took place and the achieved FAME yield was of 35 % after the second run against 60 % after the first cycle. When the test was repeated a third time with the same used catalyst, 15 % of FAME yield were obtained. These observations seem to suggest that either the leaching of the heteropolyacid would take gradually and slowly or deactivation of the catalyst.
Probable reaction mechanism
Fig. 4 shows a plausible mechanism of supported heteropolyacid catalyzing the sunflower oil methanolysis. The initial step is the protonation of methanol on a Brønsted site at the support surface and at the same time, protonation of triglyceride on another Brønsted site to give a carbocation. The methanol is then deprotonated to give a nucleophilic that will attack the carbocation that generates a tetrahedral intermediate. This latter eliminates a diglyceride and leads to the ester. The mechanism, thus, continues until obtaining of both glycerol and various FAMEs. This reaction mechanism is similar to that proposed in the case of the soybean oil methanolysis using Amberlyst A26-OH as a basic resin catalyst [33]. The authors suggested that methanol adsorption is the key step in reactions where methanol is present in excess.
The obtained results in this study with acid catalysts (oxoacids, and unsupported and supported heteropolyacid) for sunflower oil transesterification show that at low reaction temperature (60 °C), only FAMES are formed and at higher reaction temperature (100 °C) in the presence of HPW supported on silica (S30), oxygenated compounds as alcohol, ketone, aldehydes and acids are formed. Therefore, to increase the biodiesel yield with strong acid catalyst, it should be examined, other parameters such as reaction time and methanol to oil ratio.
Conclusion
In this study, the examination of the catalytic properties of a series of acids with different strength acidities: H3PW12O40, H2SO4 and H3BO3 for the sunflower oil methanolysis to produce biodiesel showed at low reaction temperature (60 °C), only FAMES are formed. With HPW supported on silica (30 wt%) tested at higher reaction temperature (100 °C), it was observed in addition to C16 and C18 methyl esters, other methyl esters and oxygenated compounds (alcohol, ketone, aldehydes) are also formed. 30 wt% HPW/SiO2 has a similar catalytic behavior to that of HPW with a biodiesel yield of 60–63 %. The use of an acid heterogeneous catalysis could to be the future development to produce biodiesel thus avoiding problems inherent in homogeneous processes as corrosion and polluting. Other investigations are necessary to increase the biodiesel yield with acid catalysts without to provoke side-reactions as decarboxy-cracking and catalytic cracking studying operation conditions as concentration, morphology and particle size of catalyst and temperature and time reaction, and alcohol/oil ratio.
References
Chouhan APS, Sarma AK (2011) Renew Sust Energy Rev 15:4378–4399
Shahid EM, Jamal Y (2011) Renew Sust Energy Rev 15:4732–4745
Borges ME, Díaz L (2012) Renew Sust Energy Rev 16:2839–2849
Samios D, Pedrotti F, Nicolau S, Reiznautt QB, Martini DD, Dalcin FM (2009) Fuel Process Technol 90:599–605
Salvini A, Giomi D, Cipriani G, Bartolozzi G, Alfini R, Brandi A (2012) RSC Adv 2:4864–4868
Zieba A, Matachowski L, Gurgul J, Bielan´ska E, Drelinkiewicz A (2010) J Mol Catal A Chem 316:30–44
Pesaresi L, Brown DR, Lee AF, Montero JM, Williams H, Wilson K (2009) Appl Catal A Gen 360:50–58
Misono M (1987) Catal Rev Sci Eng 29:269–321
Okuhara T, Mizuno N, Misono M (1996) Adv Catal 41:113–252
Kozhevnikov IV (1998) Chem Rev 98:171–198
Meireles ALP, Silva Rocha KA, Kozhevnikov IV, Gusevskaya EV (2011) Appl Catal A Gen 409–410:82–86
Srilatha K, Lingaiah N, Devi BLAP, Prasad RBN, Venkateswar S, Prasad PSS (2009) Appl Catal A Gen 365:28–33
Ghanei R, Moradi GR, TaherpourKalantari R, Arjmandzadeh E (2011) Fuel Process Technol 92:1593–1598
Tsigdinos GA (1974) Ind Eng Chem Prod Res Dev 13:267–274
Cannilla C, Bonura G, Rombic E, Arena F, Frusteri F (2010) Appl Catal A Gen 382:158–166
Benadji S, Eloy P, Leonard A, Su BL, Bachari K, Rabia C, Gaigneaux EM (2010) Microporous Mesoporous Mater 130:103–114
Zieba A, Matachowski L, Lalik E, Drelinkiewicz A (2009) Catal Lett 127:183–194
Shringarpure PA, Patel A (2011) Chem Eng J 173:612–619
Lefebvre F (1992) J Chem Soc Chem Comm 10:756–757
Tatibouet JM, Montalescot C, Bruckman K, Haber J, Che M (1997) J Catal 169:22–32
Rocchiccioli-Deltcheff C, Fournier M, Frank R, Thouvenot R (1983) Inorg Chem 22:207–216
Benadji S, Eloy P, Leonard A, Su BL, Rabia C, Gaigneaux EM (2012) Comptes Rendus Chimie 15:658–668
Pei S, Yue B, Qian L, Yan S, Cheng J, Zhou Y, Xie S, He H (2007) Appl Catal A Gen 329:148–155
Refaat AA, Attia NK, Sibak HA, El Sheltawy ST, Eldiwani GI (2008) Int J Environ Sci Technol 5:75–82
Shin HY, An SH, Sheikh R, Park YH, Bae SY (2012) Fuel 96:572–578
Jiménez-morales I, Santamaría-Gonzàlez J, Maireles-Torres P, Jiménez-López A (2012) Appl Catal A Environ 123–124:316–323
Nambo AA, Miralda CM, Jasinski JB, Carreon MA (2015) React Kinet Mech Catal 114:583–595
Rattanaphra D, Harvey AP, Thanapimmetha A, Srinophakun P (2012) Fuel 97:467–475
Rattanaphra D, Harvey A, Srinophakun P (2010) Top Catal 53(11–12):773–782
Morgan T, Grubb D, Santillan-Jimenez E, Crocker M (2010) Top Catal 53(11–12):820–829
Dimitratos N, Védrine JC (2003) Catal Today 81:561–570
Sultan M, Paul S, Fournier M, Vanhove D (2012) Appl Catal A Gen 259:141–149
Jamal Y, Rabie A, Boulanger BO (2015) React Kinet Mech Catal 114:63–74
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Idrissou, Y., Mazari, T., Benadji, S. et al. Homogeneous and heterogeneous sunflower oil methanolysis over 12-tungstophosphoric, sulfuric and boric acids. Reac Kinet Mech Cat 119, 291–304 (2016). https://doi.org/10.1007/s11144-016-1042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1042-5