Abstract
Purpose
This systematic review and meta-analysis aimed to examine the impact of global quality of life (QOL) on mortality risk in patients with cancer, considering cancer type and timepoint of QOL assessment.
Methods
A systematic search was conducted using Cumulated Index to Nursing and Allied Health Literature, PubMed/MEDLINE, and Scopus databases from inception to December 2022. Observational studies that assessed QOL and examined mortality risk in patients with cancer were extracted. Subgroup analyses were performed for cancer types and timepoints of QOL assessment.
Results
Overall, global QOL was significantly associated with mortality risk (hazard ratio: 1.06, 95% confidence interval: 1.05–1.07; p < 0.00001). A subgroup analysis based on cancer type demonstrated that lung, head and neck, breast, esophagus, colon, prostate, hematologic, liver, gynecologic, stomach, brain, bladder, bone and soft tissue, and mixed type cancers were significantly associated with mortality risk; however, melanoma and pancreatic cancer were not significantly associated with mortality risk. Additionally, global QOL was associated with mortality risk at all timepoints (pretreatment, posttreatment, and palliative phase); pretreatment QOL had the largest impact, followed by posttreatment QOL.
Conclusion
These findings provide evidence that QOL is associated with mortality risk in patients with cancer at any timepoint. These results indicate the importance of evaluating the QOL and supportive interventions to improve QOL in any phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2020, an estimated 19.3 million new cancer cases and approximately 10.0 million cancer deaths occurred globally. Cancer remains a crucial public health problem [1,2,3]; however, with the advances in treatment, the death rate has continued to decline [3]. It indicates that patients with cancer may spend their extended lives with physical and mental symptoms associated with cancer and its treatment; therefore, the need to consider the quality of life (QOL) in addition to the quantity of life is extremely important.
The World Health Organization defines QOL as an individual’s perception of their position in life in the context of the culture and value systems where they live in relation to their goals, expectations, standards, and concerns [4]. This implies that there are multiple aspects to QOL and that global QOL is a particularly comprehensive and representative measure that reflects these multiple aspects. It has recently become clear that global QOL and quantity of life, including overall survival, are not independent outcomes but are related. In fact, a previous meta-analysis revealed that disease-specific QOL affects mortality in patients with heart failure [5]. In the field of cancer research, previous systematic reviews have demonstrated that QOL can be associated with mortality risk in patients with various types of cancer [6,7,8]; the challenge is that these studies have not yet been meta-analyzed.
Using a pooled analysis of individual patient data from clinical trials, Quinten et al. [9] showed that physical function QOL was associated with mortality risk, whereas other types of QOL, including global QOL, were not. However, previous studies have found that while global QOL is associated with mortality risk [10, 11], this does not apply to cancers such as melanoma, gynecologic cancers, and thyroid cancer [11]. Specifically, no consensus exists on the relationship between global QOL and mortality risk in patients with cancer. Additionally, these previous studies had the following challenges: only the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) was used for the QOL measurement [9,10,11], only baseline QOL was assessed [9, 10], and categorization according to pre- and posttreatment perspectives was not performed [11]. To the best of our knowledge, there are no meta-analyses that comprehensively integrate all global QOL measures and examine the association with mortality risk in patients with cancer, nor are there any reports that examine cancer type or timing of QOL assessment as a subgroup.
It is hypothesized that once strong evidence of an association between global QOL and mortality risk is established and more relevant cancers and timepoints for QOL assessment are identified, the clinical indications for supportive care to improve QOL will become clearer. Hence, this study aimed to examine the impact of global QOL on mortality risk in patients with cancer, including cancer type and timepoint of QOL assessment.
Methods
This systematic review with meta-analyses was registered in the International Prospective Register of Systematic Reviews (registration number CRD 42023398206), and it followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [12, 13].
Data searches and sources
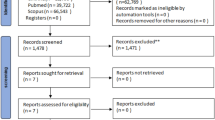
A systematic search was conducted using the Cumulated Index to Nursing and Allied Health Literature (CINAHL), PubMed/MEDLINE, and Scopus databases from inception to December 2022 (Fig. 1). The search strategy performed in each database included QOL, EORTC QLQ-C30 [14], Medical Outcomes Study Short-Form 36-Item Health [15], and Functional Assessment of Cancer Therapy-General (FACT-G) [16], along with keywords, such as cancer, neoplasm, tumor, sarcoma, hematological malignancy, lymphoma, carcinosarcoma, leukemia, mortality, survival, relapse, and recurrence (Appendix 1 [Online Resource 1]).
Study eligibility criteria and study selection
The study eligibility criteria were as follows: (1) observational studies, (2) original human studies published in English, (3) patients with any type of cancer in various settings, and (4) studies examining the association between QOL and mortality. Studies that investigated the relationship between symptoms and mortality were excluded. After removing duplicates, seven reviewers (TF, JN, SM, JI, TO, TT, and KS) independently evaluated study eligibility by reviewing the titles and abstracts of all potential citations according to the eligibility criteria. Full-text articles were retrieved for review when there was an indication that they met the eligibility criteria or when there was insufficient information in the abstract and title to decide. The final inclusion of eligible observational studies was determined in consensus meetings where all authors participated.
Data extraction
Two reviewers (TF and JN) extracted the data. The following data were extracted from each included study: (1) first author’s last name, (2) publication year, (3) nationality, (4) cancer type, (5) number of patients, (6) sex, (7) age, (8) follow-up period, (9) number of deaths, (10) covariates adjusted in the multivariate analysis, and (11) risk estimates for mortality (hazard ratio [HR] and 95% confidence interval [CI]). When several different models of multivariate analyses were indicated, we used the results from the multivariate model with the most complete adjustment for potential confounders.
Quality assessment
The quality assessment of studies, including their risk of bias, was conducted using the Newcastle–Ottawa scale [17]. This tool includes the following eight domains: representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study, comparability of cohorts based on the design or analysis, assessment of outcome, whether the follow-up was sufficiently long for outcomes to occur, and adequacy of cohort follow-up. Two trained reviewers (TF and JN) scored each item according to the criteria [17]. Potential disagreements were resolved during consensus meetings attended by all authors.
Data analysis
Risk estimates of total mortality related to global QOL were analyzed for each type of cancer (lung, head and neck, breast, liver, esophagus, prostate, hematologic, colon, gynecologic, stomach, brain, melanoma, bladder, pancreas, and bone and soft tissue, and mixed type), and all types of cancer were subsequently pooled. In an additional analysis, we investigated the association between global QOL and total mortality during QOL evaluation (pretreatment, posttreatment, and palliative phase). In this study, posttreatment was defined as both during and after treatment. We used adjusted HRs and 95% CIs in multivariate analysis as a measure of the effect size for all studies. The univariate HR was only used if it was reported, rather than the multivariate HR. Inverse variance-weighted averages used the natural logarithmic HR, and the standard error was calculated using a random-effects model. Moreover, we assessed the heterogeneity using the I2 statistic. All statistical analyses were performed using Review Manager version 5.1 (RevMan; The Cochrane Collaboration, London, UK).
Results
The literature search yielded 119,061 articles; this was reduced to 64,247 articles after excluding duplicates. Based on the screening of titles and abstracts, 580 articles were deemed eligible and underwent full-text review. Overall, 104 articles were identified and determined to be suitable for meta-analysis after review (Fig. 1).
Study characteristics
Characteristics of included studies are summarized in Appendix 2 [Online Resource 1]. This meta-analysis included any type of cancer, such as lung [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], head and neck [39–49], breast [50–60], liver [22, 61–66], esophagus [67–73], prostate [23, 74–79], hematologic [80–86], colon [87–95], gynecologic [96–98], stomach [99–101], brain [102–104], melanoma [60, 105, 106], bladder [107, 108], pancreas [109, 110], bone and soft tissue [111], and mixed type cancers [112–121]. QOL was evaluated using the EORTC QLQ-C30 [14, 18, 19, 21, 26,27,28,29,30, 32, 36, 37, 41, 42, 45, 47–53, 55, 58, 61, 63, 65–67, 69–74, 76, 79–81, 84, 85, 87, 88, 90, 91, 94, 95, 97–99, 102, 106–111, 113, 118, 121, 38], FACT-G [16, 20, 22, 23, 57, 62, 77, 82, 83, 92, 93, 96, 103, 104, 114, 117], EuroQOL Five Dimensions [43, 86, 100, 112, 122], Lung Cancer Symptom Scale [24, 25, 31, 34, 35, 123], Rotterdam Symptom Checklist [68, 105, 120, 124], Spitzer Quality of Life Index [33, 44, 60, 64, 125], Quality of Life Index [56, 75, 89, 110], European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative Care [115, 116, 126], QOL for Cancer Patients Treated with Anti-Cancer Drugs [59, 127], self-rated health [78, 119], Auckland Quality of Life Questionnaire [40, 128], General Quality of Life Inventory-74 [54], General Health Questionnaire with Health versus Disease scoring [46], QOL-20 [101], and Neck Radiotherapy Questionnaire [44, 129]. The follow-up period ranged from 1 month to 8.5 years.
Risk of bias assessment
The risk of bias was assessed using the Newcastle–Ottawa scale. Of the included studies, 61, 41, and 2 were considered high (8–9 points), moderate (6–7 points), and low quality, respectively (Appendix 3 [Online Resource 1]).
Impact of global QOL on mortality risk
Overall, 124 datasets from 104 studies were included in a random-effects meta-analysis. The effect of global QOL on mortality risk was estimated using a forest plot of inverse HR and 95% CI. Ultimately, global QOL was significantly associated with mortality risk (HR: 1.06, 95% CI 1.05–1.07, p < 0.00001, I2 = 86%; Fig. 2). A subgroup analysis based on cancer type demonstrated that lung (HR: 1.10, 95% CI 1.07–1.13, p < 0.00001, I2 = 88%), head and neck (HR: 1.09, 95% CI 1.06–1.12, p < 0.00001, I2 = 81%), breast (HR: 1.03, 95% CI 1.01–1.04, p = 0.001, I2 = 79%), esophagus (HR: 1.03, 95% CI 1.01–1.04, p = 0.0002, I2 = 76%), colon (HR: 1.12, 95% CI 1.06–1.17, p < 0.00001, I2 = 94%), prostate (HR: 1.10, 95% CI 1.04–1.17, p = 0.002, I2 = 82%), hematologic (HR: 1.05, 95% CI 1.01–1.10, p = 0.02, I2 = 68%), liver (HR: 1.20, 95% CI 1.08–1.35, p = 0.001, I2 = 89%), gynecologic (HR: 1.15, 95% CI 1.01–1.31, p = 0.03, I2 = 81%), stomach (HR: 1.79, 95% CI 1.34–2.38, p < 0.0001, I2 = 0%), brain (HR: 1.05, 95% CI 1.02–1.08, p = 0.003, I2 = 0%), bladder (HR: 1.98, 95% CI 1.57–2.50, p < 0.00001, I2 = 0%), bone and soft tissue (HR: 8.29, 95% CI 3.13–21.98, p < 0.0001), and mixed type cancers (HR: 1.03, 95% CI 1.01–1.05, p = 0.009, I2 = 94%) were significantly associated with mortality risk; conversely melanoma (HR: 1.27, 95% CI 0.97–1.65, p = 0.08, I2 = 89%) and pancreatic cancer (HR: 1.03, 95% CI 0.99–1.06, p = 0.11, I2 = 18%) were not significantly associated with mortality risk (Appendix 4 [Online Resource 1]).
Impact of global QOL on mortality risk classified based on the time of evaluation
Pretreatment global QOL
A meta-analysis of 80 datasets from 74 studies showed that pretreatment global QOL was significantly associated with mortality risk (HR: 1.06, 95% CI 1.05–1.07, p < 0.0001, I2 = 84%; Fig. 3).
Meta-analysis of the impact of global quality of life on mortality risk classified according to the time of evaluation. aBaseline health-related quality-of-life data as prognostic factors in a phase III multicenter study of women with metastatic breast cancer [52], bhealth-related quality of life parameters as prognostic factors in a nonmetastatic breast cancer population: an international multicenter study [53], cliver, dlung, elung, fprostate, gEuroQol 5 Dimension (index), hEuroQol 5 Dimension (visual analog scale), icolon, jpancreas, kadvanced, lcurable, mHead and Neck Radiotherapy Questionnaire, nSpitzer Quality of Life Index, oAFFIRM study, pPREVAIL study, qduring treatment, r3 months posttreatment, s1 year posttreatment, t2 years posttreatment, unonsurgery, and vsurgery
Posttreatment global QOL
A meta-analysis of 30 datasets from 24 studies showed that posttreatment global QOL was significantly associated with mortality risk (HR: 1.06, 95% CI 1.04–1.08, p < 0.00001, I2 = 85%; Fig. 3).
Palliative phase global QOL
A meta-analysis of five datasets from five studies revealed that palliative phase global QOL was significantly associated with mortality risk (HR: 1.04, 95% CI 1.01–1.06, p = 0.009, I2 = 89%; Fig. 3).
Global QOL was associated with mortality risk at all timepoints (pretreatment, posttreatment, and palliative phase). The largest effect size was for pretreatment QOL (Z = 2.61), followed by posttreatment QOL (Z = 6.28). Although palliative phase QOL was significantly associated with mortality risk, the effect size was relatively small (Z = 2.61; Fig. 3).
Discussion
To the best of our knowledge, this is the first study to reveal the impact of global QOL on mortality risk in patients with cancer, including cancer type and timepoint of QOL assessment. Insufficient evidence exists regarding the relationship between QOL and prognosis in patients with cancer, presenting an issue worth consideration. The main findings of this study were as follows: global QOL is significantly associated with mortality risk in patients with cancer; the relationship between global QOL and mortality risk varies according to cancer type; global QOL is associated with mortality risk at all timepoints (pretreatment, posttreatment, and palliative phase); and pretreatment QOL had the largest impact, followed by posttreatment QOL.
In addition to survival, QOL is one of the most crucial outcomes, particularly in the setting of advanced disease [10]. Therefore, the relationship between QOL and prognosis has attracted increasing attention. A previous pooled analysis study reported the association between QOL and survival in patients with cancer [10]. Although results on the relationship between QOL and prognosis varied among the included studies, and heterogeneity was found after pooling, our meta-analysis revealed that global QOL is significantly associated with mortality risk for any type of cancer, similar to previous research. While the association between QOL and prognosis may be influenced by various factors, including the progression of occult disease and the gradual deterioration of the biological/physiological status of the patients studied, more than 90% of the articles in this meta-analysis controlled for confounding factors, and more than 80% had a sufficient follow-up period. These observations suggest the importance of focusing on QOL when confronting patients with cancer. However, the mechanism of the association between QOL and mortality remains unclear. Previous studies have demonstrated that age [130–132], clinical stage [130–132], symptoms [130], psychosocial problems [130, 132], physical activity [130], and nutritional status [130] were factors associated with QOL in patients with cancer. Global QOL is an indicator that includes these factors and is reported to be associated with prognosis; however, it remains unclear as to which of these concepts related to global QOL may be acting as confounding variables; therefore, identifying these factors acting as confounders warrants further study.
This study aimed to examine the association between global QOL and prognosis based on cancer type; lung, head and neck, breast, esophagus, colon, prostate, hematologic, liver, gynecologic, stomach, brain, bladder, and bone and soft tissue cancers were significantly associated with mortality risk. In addition to cancers that were significant in previous studies (colorectal, rectal, prostate, and hematological malignancies) [11], global QOL in this study was associated with mortality risk for several cancers. Additionally, although not significant in a previous study [11], this association was found to be significant for gynecological cancer in our study, adding to the body of evidence. Nevertheless, this association was not significant for melanoma or pancreatic cancer. Similar to our results, the aforementioned previous study also reported that melanoma was not significant [11]. Determining why only melanoma and pancreatic cancer were not predictors of mortality risk is challenging. This may be because survival rates for these cancers are low. Additionally, fewer original articles exist for this type of cancer; therefore, the relationship between QOL and prognosis may not have been adequately examined.
By focusing on the timepoints of QOL assessment, global QOL was found to be associated with mortality risk at all timepoints (pretreatment, posttreatment, and palliative phase). Although the relationship between baseline QOL and prognosis has been investigated, no reports have examined the relationship by timepoint, including the pretreatment, posttreatment, and palliative phases, which we believe to be of interest. Given that global QOL is associated with mortality risk at all timepoints, the importance of evaluating the QOL for any phase of cancer is supported. Furthermore, the effect size by timepoint showed that pretreatment QOL had the highest effect size. Therefore, improving QOL before treatment is important and should be addressed.
Previous studies have demonstrated that supportive care improves QOL in patients with cancer [133–136]. Rehabilitation, which is a form of supportive care, is also considered important before treatment (prehabilitation), and its effectiveness on QOL has been examined [137]. Furthermore, our results demonstrate that posttreatment QOL had the second highest effect size on mortality risk. This suggests that supportive care aimed at improving QOL should be seamless from pretreatment to posttreatment. Although QOL is an important outcome measure, this study is meaningful as it clarifies that global QOL is both an outcome and a prognostic factor in the palliative phase. A previous study showed that palliative care led to significant improvements in QOL and survival [138]. During this phase, patients and their families can benefit from an extended period of high QOL. Considering the above, it is important to periodically conduct a global QOL assessment throughout the pretreatment, posttreatment, and palliative phases, as well as provide supportive care to improve global QOL based on the results of these assessments.
This review has some limitations. First, we used the CINAHL, PubMed/MEDLINE, and Scopus databases for the search. Although this appears to have yielded a sufficient number of articles, the possibility that more articles could have been extracted using additional databases cannot be ruled out. Second, this review was limited to studies published in English; relevant studies published in other languages may offer other findings. Finally, the papers included in this study were varied, and QOL assessment methods were mixed; therefore, heterogeneity was observed.
Conclusions
Global QOL is significantly associated with mortality risk in patients with cancer. Regardless of the phase, a significant relationship was observed between global QOL and mortality risk. These results indicate the importance of periodically evaluating the global QOL at all phases of treatment, as well as supportive care to improve global QOL. Further studies are needed to understand the mechanisms and association between QOL and prognosis in patients with cancer.
References
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Dyba, T., Randi, G., Bray, F., Martos, C., Giusti, F., Nicholson, N., Gavin, A., Flego, M., Neamtiu, L., Dimitrova, N., Negrão Carvalho, R., Ferlay, J., & Bettio, M. (2021). The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. European Journal of Cancer, 157, 308–347. https://doi.org/10.1016/j.ejca.2021.07.039
Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763
WHOQOL—Measuring Quality of Life| The World Health Organization. (n.d.). Retrieved October 30, 2023, from https://www.who.int/tools/whoqol
Xu, J., Sun, Y., Gong, D., & Fan, Y. (2023). Association between disease-specific health-related quality of life and all-cause mortality in patients with heart failure: A meta-analysis. Current Problems in Cardiology, 48(4), 101592. https://doi.org/10.1016/j.cpcardiol.2023.101592
van Nieuwenhuizen, A. J., Buffart, L. M., Brug, J., Leemans, C. R., & Verdonck-de Leeuw, I. M. (2015). The association between health related quality of life and survival in patients with head and neck cancer: A systematic review. Oral Oncology, 51(1), 1–11. https://doi.org/10.1016/j.oraloncology.2014.09.002
Gotay, C. C., Kawamoto, C. T., Bottomley, A., & Efficace, F. (2008). The prognostic significance of patient-reported outcomes in cancer clinical trials. Journal of Clinical Oncology, 26(8), 1355–1363. https://doi.org/10.1200/JCO.2007.13.3439
Montazeri, A. (2009). Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health and Quality of Life Outcomes, 7, 102. https://doi.org/10.1186/1477-7525-7-102
Quinten, C., Coens, C., Mauer, M., Comte, S., Sprangers, M. A. G., Cleeland, C., Osoba, D., Bjordal, K., Bottomley, A., EORTC Clinical Groups. (2009). Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. The Lancet. Oncology, 10(9), 865–871. https://doi.org/10.1016/S1470-2045(09)70200-1
Ediebah, D. E., Quinten, C., Coens, C., Ringash, J., Dancey, J., Zikos, E., Gotay, C., Brundage, M., Tu, D., Flechtner, H. H., Greimel, E., Reeve, B. B., Taphoorn, M., Reijneveld, J., Dirven, L., Bottomley, A., Canadian Cancer Trials Group and the European Organization for Research and Treatment of Cancer. (2018). Quality of life as a prognostic indicator of survival: A pooled analysis of individual patient data from canadian cancer trials group clinical trials. Cancer, 124(16), 3409–3416. https://doi.org/10.1002/cncr.31556
Husson, O., de Rooij, B. H., Kieffer, J., Oerlemans, S., Mols, F., Aaronson, N. K., van der Graaf, W. T. A., & van de Poll-Franse, L. V. (2020). The EORTC QLQ-C30 summary score as prognostic factor for survival of patients with cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. The Oncologist, 25(4), e722–e732. https://doi.org/10.1634/theoncologist.2019-0348
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery, 8(5), 336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., Filiberti, A., Flechtner, H., Fleishman, S. B., & de Haes, J. C. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. JNCI: Journal of the National Cancer Institute, 85(5), 365–376. https://doi.org/10.1093/jnci/85.5.365
Ware, J. E., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30(6), 473–483.
Cella, D. F., Tulsky, D. S., Gray, G., Sarafian, B., Linn, E., Bonomi, A., Silberman, M., Yellen, S. B., Winicour, P., & Brannon, J. (2016). The functional assessment of cancer therapy scale: Development and validation of the general measure. Journal of Clinical Oncology, 11(3), 570–579. https://doi.org/10.1200/JCO.1993.11.3.570
Ottawa Hospital Research Institute. (n.d.). Retrieved 12 Sep 2023, from https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Arraras, J. I., Hernandez, B., Martinez, M., Cambra, K., Rico, M., Illarramendi, J. J., Viudez, A., Ibañez, B., Zarandona, U., Martinez, E., & Vera, R. (2016). Quality of life in Spanish advanced non-small-cell lung cancer patients: Determinants of global QL and survival analyses. Springerplus, 5(1), 836. https://doi.org/10.1186/s40064-016-2559-9
Badaoui, S., Shahnam, A., McKinnon, R. A., Abuhelwa, A. Y., Sorich, M. J., & Hopkins, A. M. (2022). The predictive utility of patient-reported outcomes and performance status for survival in metastatic lung cancer patients treated with chemoimmunotherapy. Translational Lung Cancer Research, 11(3), 432–439. https://doi.org/10.21037/tlcr-21-938
Dharma-Wardene, M., Au, H. J., Hanson, J., Dupere, D., Hewitt, J., & Feeny, D. (2004). Baseline FACT-G score is a predictor of survival for advanced lung cancer. Quality of Life Research, 13(7), 1209–1216. https://doi.org/10.1023/B:QURE.0000037481.36604.eb
Erdem, R. (2022). Association between change in quality of life and survival in advanced non-small-cell lung cancer. Turkish Journal of Oncology., 37(3), 246–253. https://doi.org/10.5505/tjo.2022.3375
Fielding, R., & Wong, W. S. (2007). Quality of life as a predictor of cancer survival among Chinese liver and lung cancer patients. European Journal of Cancer (Oxford, England: 1990), 43(11), 1723–1730. https://doi.org/10.1016/j.ejca.2007.05.002
Hui, D., Darke, A. K., Guthrie, K. A., Subbiah, I. M., Unger, J. M., Hershman, D. L., Krouse, R. S., Bakitas, M., & O’Rourke, M. A. (2022). Association between health-related quality of life and progression-free survival in patients with advanced cancer: A secondary analysis of SWOG clinical trials. JCO Oncology Practice, 18(4), e442–e451. https://doi.org/10.1200/OP.21.00407
Jacot, W., Colinet, B., Bertrand, D., Lacombe, S., Bozonnat, M. C., Daurès, J. P., Pujol, J. L., OncoLR health network. (2008). Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 19(8), 1458–1464. https://doi.org/10.1093/annonc/mdn064
Kao, S. C., Vardy, J., Harvie, R., Chatfield, M., van Zandwijk, N., Clarke, S., & Pavlakis, N. (2013). Health-related quality of life and inflammatory markers in malignant pleural mesothelioma. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 21(3), 697–705. https://doi.org/10.1007/s00520-012-1569-6
Langendijk, H., Aaronson, N. K., de Jong, J. M., ten Velde, G. P., Muller, M. J., & Wouters, M. (2000). The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology, 55(1), 19–25. https://doi.org/10.1016/s0167-8140(00)00158-4
Lemonnier, I., Guillemin, F., Arveux, P., Clément-Duchêne, C., Velten, M., Woronoff-Lemsi, M. C., Jolly, D., & Baumann, C. (2014). Quality of life after the initial treatments of non-small cell lung cancer: A persistent predictor for patients’ survival. Health and Quality of Life Outcomes, 12, 73. https://doi.org/10.1186/1477-7525-12-73
Li, T. C., Li, C. I., Tseng, C. H., Lin, K. S., Yang, S. Y., Chen, C. Y., Hsia, T. C., Lee, Y. D., & Lin, C. C. (2012). Quality of life predicts survival in patients with non-small cell lung cancer. BMC Public Health, 12, 790. https://doi.org/10.1186/1471-2458-12-790
Movsas, B., Moughan, J., Sarna, L., Langer, C., Werner-Wasik, M., Nicolaou, N., Komaki, R., Machtay, M., Wasserman, T., & Bruner, D. W. (2009). Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 27(34), 5816–5822. https://doi.org/10.1200/JCO.2009.23.7420
Nieto-Guerrero Gómez, J. M., Silva Vega, G. P., Cacicedo, J., Delgado León, B. D., Herrero Rivera, D., Praena Fernández, J. M., Rivin Del Campo, E., Ortiz Gordillo, M. J., & López Guerra, J. L. (2020). Impact of pre-radiation therapy quality of life in lung cancer survival: A prospective, intention-to-treat, multicenter study. Clinical and Translational Oncology, 22(9), 1635–1644. https://doi.org/10.1007/s12094-020-02310-0
O’Mahony, S., Nathan, S., Mohajer, R., Bonomi, P., Batus, M., Fidler, M. J., Wells, K., Kern, N., Sims, S., & Amin, D. (2016). Survival prediction in ambulatory patients with stage III/IV non-small cell lung cancer using the palliative performance scale, ECOG, and lung cancer symptom scale. The American Journal of Hospice & Palliative Care, 33(4), 374–380. https://doi.org/10.1177/1049909115570707
Pompili, C., Omar, S., Ilyas, M. H., Velikova, G., Dalmia, S., Valuckiene, L., Alexopoulos, P., & Brunelli, A. (2023). Patient-reported physical function is associated with survival after lung resection for non-small cell lung cancer. The Annals of Thoracic Surgery, 116(3), 563–569. https://doi.org/10.1016/j.athoracsur.2022.09.047
Qi, Y., Schild, S. E., Mandrekar, S. J., Tan, A. D., Krook, J. E., Rowland, K. M., Garces, Y. I., Soori, G. S., Adjei, A. A., & Sloan, J. A. (2009). Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, 4(9), 1075–1082. https://doi.org/10.1097/JTO.0b013e3181ae27f5
Sloan, J. A., Cheville, A. L., Liu, H., Novotny, P. J., Wampfler, J. A., Garces, Y. I., Clark, M. M., & Yang, P. (2016). Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health and Quality of Life Outcomes, 14, 66. https://doi.org/10.1186/s12955-016-0461-3
Sloan, J. A., Zhao, X., Novotny, P. J., Wampfler, J., Garces, Y., Clark, M. M., & Yang, P. (2012). Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 30(13), 1498–1504. https://doi.org/10.1200/JCO.2010.33.4631
Trejo, M. J., Bell, M. L., Dhillon, H. M., & Vardy, J. L. (2020). Baseline quality of life is associated with survival among people with advanced lung cancer. Journal of Psychosocial Oncology, 38(5), 635–641. https://doi.org/10.1080/07347332.2020.1765065
Yun, Y. H., Kim, Y. A., Sim, J. A., Shin, A. S., Chang, Y. J., Lee, J., Kim, M. S., Shim, Y. M., & Zo, J. L. (2016). Prognostic value of quality of life score in disease-free survivors of surgically-treated lung cancer. BMC Cancer, 16, 505. https://doi.org/10.1186/s12885-016-2504-x
Montazeri, A., Milroy, R., Hole, D., McEwen, J., & Gillis, C. R. (2001). Quality of life in lung cancer patients: As an important prognostic factor. Lung Cancer (Amsterdam, Netherlands), 31(2–3), 233–240. https://doi.org/10.1016/s0169-5002(00)00179-3
Fang, F. M., Liu, Y. T., Tang, Y., Wang, C. J., & Ko, S. F. (2004). Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer, 100(2), 425–432. https://doi.org/10.1002/cncr.20010
Mehanna, H. M., & Morton, R. P. (2006). Does quality of life predict long-term survival in patients with head and neck cancer? Archives of Otolaryngology-Head & Neck Surgery, 132(1), 27–31. https://doi.org/10.1001/archotol.132.1.27
Oskam, I. M., Verdonck-de Leeuw, I. M., Aaronson, N. K., Kuik, D. J., de Bree, R., Doornaert, P., Langendijk, J. A., & Leemans, C. R. (2010). Quality of life as predictor of survival: A prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology, 97(2), 258–262. https://doi.org/10.1016/j.radonc.2010.02.005
Tarsitano, A., Pizzigallo, A., Ballone, E., & Marchetti, C. (2012). Health-related quality of life as a survival predictor for patients with oral cancer: Is quality of life associated with long-term overall survival? Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 114(6), 756–763. https://doi.org/10.1016/j.oooo.2012.06.022
Lango, M. N., Egleston, B., Fang, C., Burtness, B., Galloway, T., Liu, J., Mehra, R., Ebersole, B., Moran, K., & Ridge, J. A. (2014). Baseline health perceptions, dysphagia, and survival in patients with head and neck cancer. Cancer, 120(6), 840–847. https://doi.org/10.1002/cncr.28482
Xiao, C., Zhang, Q., Nguyen-Tân, P. F., List, M., Weber, R. S., Ang, K. K., Rosenthal, D., Filion, E. J., Kim, H., Silverman, C., Raben, A., Galloway, T., Fortin, A., Gore, E., Winquist, E., Jones, C. U., Robinson, W., Raben, D., Le, Q. T., & Bruner, D. (2017). Quality of life and performance status from a substudy conducted within a prospective phase 3 randomized trial of concurrent standard radiation versus accelerated radiation plus cisplatin for locally advanced head and neck carcinoma: NRG Oncology RTOG 0129. International Journal of Radiation Oncology, Biology, Physics, 97(4), 667–677. https://doi.org/10.1016/j.ijrobp.2016.07.020
Yang, C. J., Roh, J. L., Kim, M. J., Lee, S., Kim, S. B., Choi, S. H., Nam, S. Y., & Kim, S. Y. (2016). Pretreatment quality of life as a prognostic factor for early survival and functional outcomes in patients with head and neck cancer. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 25(1), 165–174. https://doi.org/10.1007/s11136-015-1063-y
Aarstad, H. J., Osthus, A. A., Olofsson, J., & Aarstad, A. K. H. (2014). Level of distress predicts subsequent survival in successfully treated head and neck cancer patients: A prospective cohort study. Acta oto-laryngologica, 134(2), 211–219. https://doi.org/10.3109/00016489.2013.841989
Rogers, S. N., Waylen, A. E., Thomas, S., Penfold, C., Pring, M., Waterboer, T., Pawlita, M., Hurley, K., & Ness, A. R. (2020). Quality of life, cognitive, physical and emotional function at diagnosis predicts head and neck cancer survival: Analysis of cases from the Head and Neck 5000 study. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology—Head and Neck Surgery, 277(5), 1515–1523. https://doi.org/10.1007/s00405-020-05850-x
Ishiyama, H., Kawakami, S., Sekiguchi, A., Kainuma, T., Miyamoto, S., Yamashita, T., & Nakano, M. (2022). Quality of life score as a prognosticator for pharyngeal cancer patients treated with radiotherapy. Scientific reports, 12(1), 2387. https://doi.org/10.1038/s41598-022-06441-y
Liao, K. C., Chuang, H. C., Chien, C. Y., Lin, Y. T., Tsai, M. H., Su, Y. Y., Yang, C. H., Lai, C. C., Huang, T. L., Li, S. H., Lee, T. F., Lin, W. T., Lee, C. H., & Fang, F. M. (2021). Quality of life as a mediator between cancer stage and long-term mortality in nasopharyngeal cancer patients treated with intensity-modulated radiotherapy. Cancers, 13(20), 5063. https://doi.org/10.3390/cancers13205063
Kramer, J. A., Curran, D., Piccart, M., de Haes, J. C., Bruning, P., Klijn, J., Van Hoorebeeck, I., & Paridaens, R. (2000). Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. European Journal of Cancer (Oxford, England: 1990), 36(12), 1498–1506. https://doi.org/10.1016/s0959-8049(00)00144-1
Luoma, M.-L., Hakamies-Blomqvist, L., Sjöström, J., Pluzanska, A., Ottoson, S., Mouridsen, H., Bengtsson, N. O., Bergh, J., Malmström, P., Valvere, V., Tennvall, L., & Blomqvist, C. (2003). Prognostic value of quality of life scores for time to progression (TTP) and overall survival time (OS) in advanced breast cancer. European Journal of Cancer (Oxford, England: 1990), 39(10), 1370–1376. https://doi.org/10.1016/s0959-8049(02)00775-x
Efficace, F., Biganzoli, L., Piccart, M., Coens, C., Van Steen, K., Cufer, T., Coleman, R. E., Calvert, H. A., Gamucci, T., Twelves, C., Fargeot, P., Bottomley, A., & Bottomley, A. (2004). Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. European Journal of Cancer (Oxford, England: 1990), 40(7), 1021–1030. https://doi.org/10.1016/j.ejca.2004.01.014
Efficace, F., Therasse, P., Piccart, M. J., Coens, C., van Steen, K., Welnicka-Jaskiewicz, M., Cufer, T., Dyczka, J., Lichinitser, M., Shepherd, L., de Haes, H., Sprangers, M. A., & Bottomley, A. (2004). Health-related quality of life parameters as prognostic factors in a nonmetastatic breast cancer population: An international multicenter study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 22(16), 3381–3388. https://doi.org/10.1200/JCO.2004.02.060
Epplein, M., Zheng, Y., Zheng, W., Chen, Z., Gu, K., Penson, D., Lu, W., & Shu, X.-O. (2011). Quality of life after breast cancer diagnosis and survival. Journal of Clinical Oncology, 29(4), 406–412. https://doi.org/10.1200/JCO.2010.30.6951
Svensson, H., Hatschek, T., Johansson, H., Einbeigi, Z., & Brandberg, Y. (2012). Health-related quality of life as prognostic factor for response, progression-free survival, and survival in women with metastatic breast cancer. Medical Oncology (Northwood, London, England), 29(2), 432–438. https://doi.org/10.1007/s12032-011-9844-9
Gupta, D., Granick, J., Grutsch, J. F., & Lis, C. G. (2007). The prognostic association of health-related quality of life scores with survival in breast cancer. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 15(4), 387–393. https://doi.org/10.1007/s00520-006-0165-z
DiSipio, T., Hayes, S., Battistutta, D., Newman, B., & Janda, M. (2011). Patterns, correlates, and prognostic significance of quality of life following breast cancer. Psycho-Oncology, 20(10), 1084–1091. https://doi.org/10.1002/pon.1816
De Aguiar, S. S., Bergmann, A., & Mattos, I. E. (2014). Quality of life as a predictor of overall survival after breast cancer treatment. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 23(2), 627–637. https://doi.org/10.1007/s11136-013-0476-8
Takada, K., Kashiwagi, S., Fukui, Y., Goto, W., Asano, Y., Morisaki, T., Takashima, T., Hirakawa, K., & Ohira, M. (2019). Prognostic value of quality-of-life scores in patients with breast cancer undergoing preoperative chemotherapy. BJS Open, 3(1), 38–47. https://doi.org/10.1002/bjs5.50108
Coates, A., & Gebski, V. (1996). On the receiving end. Vl. Which dimensions of quality-of-life scores carry prognostic information? Cancer Treatment Reviews, 22, 63–67. https://doi.org/10.1016/S0305-7372(96)90065-1
Diouf, M., Filleron, T., Barbare, J. C., Fin, L., Picard, C., Bouché, O., Dahan, L., Paoletti, X., & Bonnetain, F. (2013). The added value of quality of life (QoL) for prognosis of overall survival in patients with palliative hepatocellular carcinoma. Journal of Hepatology, 58(3), 509–521. https://doi.org/10.1016/j.jhep.2012.11.019
Steel, J. L., Geller, D. A., Robinson, T. L., Savkova, A. Y., Brower, D. S., Marsh, J. W., & Tsung, A. (2014). Health-related quality of life as a prognostic factor in patients with advanced cancer. Cancer, 120(23), 3717–3721. https://doi.org/10.1002/cncr.28902
Yeo, W., Mo, F. K. F., Koh, J., Chan, A. T. C., Leung, T., Hui, P., Chan, L., Tang, A., Lee, J. J., Mok, T. S., Lai, P. B., Johnson, P. J., & Zee, B. (2006). Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 17(7), 1083–1089. https://doi.org/10.1093/annonc/mdl065
Bonnetain, F., Paoletti, X., Collette, S., Doffoel, M., Bouché, O., Raoul, J. L., Rougier, P., Masskouri, F., Barbare, J. C., & Bedenne, L. (2008). Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: Results from two French clinical trials. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 17(6), 831–843. https://doi.org/10.1007/s11136-008-9365-y
Meier, A., Yopp, A., Mok, H., Kandunoori, P., Tiro, J., & Singal, A. G. (2015). Role functioning is associated with survival in patients with hepatocellular carcinoma. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 24(7), 1669–1675. https://doi.org/10.1007/s11136-014-0895-1
Sternby Eilard, M., Hagström, H., Mortensen, K. E., Wilsgaard, T., Vagnildhaug, O. M., Dajani, O., Stål, P., & Rizell, M. (2018). Quality of life as a prognostic factor for survival in hepatocellular carcinoma. Liver International: Official Journal of the International Association for the Study of the Liver, 38(5), 885–894. https://doi.org/10.1111/liv.13593
Fang, F. M., Tsai, W. L., Chiu, H. C., Kuo, W. R., & Hsiung, C. Y. (2004). Quality of life as a survival predictor for esophageal squamous cell carcinoma treated with radiotherapy. International Journal of Radiation Oncology, Biology, Physics, 58(5), 1394–1404. https://doi.org/10.1016/j.ijrobp.2003.09.100
van Heijl, M., Sprangers, M. A. G., de Boer, A. G. E. M., Lagarde, S. M., Reitsma, H. B., Busch, O. R. C., Tilanus, H. W., van Lanschot, J. J., & van Berge Henegouwen, M. I. (2010). Preoperative and early postoperative quality of life predict survival in potentially curable patients with esophageal cancer. Annals of Surgical Oncology, 17(1), 23–30. https://doi.org/10.1245/s10434-009-0731-y
Bergquist, H., Johnsson, A., Hammerlid, E., Wenger, U., Lundell, L., & Ruth, M. (2008). Factors predicting survival in patients with advanced oesophageal cancer: A prospective multicentre evaluation. Alimentary Pharmacology & Therapeutics, 27(5), 385–395. https://doi.org/10.1111/j.1365-2036.2007.03589.x
Djärv, T., & Lagergren, P. (2011). Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. European Journal of Cancer (Oxford, England: 1990), 47(4), 530–535. https://doi.org/10.1016/j.ejca.2010.10.014
Chang, Y. L., Tsai, Y. F., Chao, Y. K., & Wu, M. Y. (2016). Quality-of-life measures as predictors of post-esophagectomy survival of patients with esophageal cancer. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 25(2), 465–475. https://doi.org/10.1007/s11136-015-1094-4
McKernan, M., McMillan, D. C., Anderson, J. R., Angerson, W. J., & Stuart, R. C. (2008). The relationship between quality of life (EORTC QLQ-C30) and survival in patients with gastro-oesophageal cancer. British Journal of Cancer, 98(5), 888–893. https://doi.org/10.1038/sj.bjc.6604248
van Kleef, J. J., Dijksterhuis, W. P. M., van den Boorn, H. G., Prins, M., Verhoeven, R. H. A., Gisbertz, S. S., Slingerland, M., Mohammad, N. H., Creemers, G. J., Neelis, K. J., Heisterkamp, J., Rosman, C., Ruurda, J. P., Kouwenhoven, E. A., van de Poll-Franse, L. V., van Oijen, M. G. H., Sprangers, M. A. G., van Laarhoven, H. W. M., Dutch Upper GI Cancer Group (DUCG). (2021). Prognostic value of patient-reported quality of life for survival in oesophagogastric cancer: analysis from the population-based POCOP study. Gastric Cancer: Official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24(6), 1203–1212. https://doi.org/10.1007/s10120-021-01209-1
Collette, L., van Andel, G., Bottomley, A., Oosterhof, G. O. N., Albrecht, W., de Reijke, T. M., & Fossà, S. D. (2004). Is baseline quality of life useful for predicting survival with hormone-refractory prostate cancer? A pooled analysis of three studies of the European Organisation for Research and Treatment of Cancer Genitourinary Group. Journal of Clinical Oncology: Official journal of the American Society of Clinical Oncology, 22(19), 3877–3885. https://doi.org/10.1200/JCO.2004.07.089
Lis, C. G., Gupta, D., & Grutsch, J. F. (2008). Patient satisfaction with health-related quality of life: Implications for prognosis in prostate cancer. Clinical Genitourinary Cancer, 6(2), 91–96. https://doi.org/10.3816/CGC.2008.n.014
Gupta, D., Braun, D. P., & Staren, E. D. (2013). Prognostic value of changes in quality of life scores in prostate cancer. BMC urology, 13, 32. https://doi.org/10.1186/1471-2490-13-32
Beer, T. M., Miller, K., Tombal, B., Cella, D., Phung, D., Holmstrom, S., Ivanescu, C., Skaltsa, K., & Naidoo, S. (2017). The association between health-related quality-of-life scores and clinical outcomes in metastatic castration-resistant prostate cancer patients: Exploratory analyses of AFFIRM and PREVAIL studies. European Journal of Cancer (Oxford, England: 1990), 87, 21–29. https://doi.org/10.1016/j.ejca.2017.09.035
Gupta, D., Patel, K., & Lis, C. G. (2015). Self-rated health supersedes patient satisfaction with service quality as a predictor of survival in prostate cancer. Health and Quality of Life Outcomes, 13, 137. https://doi.org/10.1186/s12955-015-0334-1
Roy, S., Morgan, S. C., Spratt, D. E., MacRae, R. M., Grimes, S., Malone, J., Mukherjee, D., & Malone, S. (2022). Association of baseline patient-reported health-related quality of life metrics with outcome in localised prostate cancer. Clinical Oncology (Royal College of Radiologists (Great Britain)), 34(1), e61–e68. https://doi.org/10.1016/j.clon.2021.10.007
Jerkeman, M., Kaasa, S., Hjermstad, M., Kvaløy, S., & Cavallin-Stahl, E. (2001). Health-related quality of life and its potential prognostic implications in patients with aggressive lymphoma: A Nordic Lymphoma Group Trial. Medical Oncology (Northwood, London, England), 18(1), 85–94. https://doi.org/10.1385/MO:18:1:85
Jung, H. A., Park, S., Cho, J. H., Kim, S., Ko, Y. H., Kim, S. J., & Kim, W. S. (2012). Prognostic relevance of pretreatment quality of life in diffuse large B-cell lymphoma patients treated with rituximab-CHOP: Results from a prospective cohort study. Annals of Hematology, 91(11), 1747–1756. https://doi.org/10.1007/s00277-012-1516-0
Hamilton, B. K., Law, A. D., Rybicki, L., Abounader, D., Dabney, J., Dean, R., Duong, H. K., Gerds, A. T., Hanna, R., Hill, B. T., Jagadeesh, D., Kalaycio, M. E., Lawrence, C., McLellan, L., Pohlman, B., Sobecks, R. M., Bolwell, B. J., & Majhail, N. S. (2015). Prognostic significance of pre-transplant quality of life in allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplantation, 50(9), 1235–1240. https://doi.org/10.1038/bmt.2015.122
Shaw, B. E., Brazauskas, R., Millard, H. R., Fonstad, R., Flynn, K. E., Abernethy, A., Vogel, J., Petroske, C., Mattila, D., Drexler, R., Lee, S. J., Horowitz, M. M., & Rizzo, J. D. (2017). Centralized patient-reported outcome data collection in transplantation is feasible and clinically meaningful. Cancer, 123(23), 4687–4700. https://doi.org/10.1002/cncr.30936
Huang, H., Datye, A., Fan, M., Knapp, A., Nielsen, T., Bottos, A., Paulson, J. N., Trask, P. C., & Efficace, F. (2022). Patient-reported outcomes provide prognostic information for survival in patients with diffuse large B-cell lymphoma: Analysis of 1239 patients from the GOYA study. Cancer Medicine, 11(17), 3312–3322. https://doi.org/10.1002/cam4.4692
Lindberg, Å., Eskelund, C. W., Albertsson-Lindblad, A., Kolstad, A., Laurell, A., Räty, R., Grønbaek, K., Geisler, C. H., & Jerkeman, M. (2022). Pre-treatment health-related quality of life parameters have prognostic impact in patients >65 years with newly diagnosed mantle cell lymphoma: The Nordic Lymphoma Group MCL4 (LENA-BERIT) experience. Hematological Oncology, 40(1), 22–30. https://doi.org/10.1002/hon.2940
Kurosawa, S., Yamaguchi, T., Mori, A., Tsukagoshi, M., Okuda, I., Ikeda, M., Fuji, S., Yamashita, T., Ogawa, C., Ito, A., Tanaka, T., Inamoto, Y., Kim, S. W., & Fukuda, T. (2021). Prognostic impact of pretransplantation quality of life and its post-transplantation longitudinal change after allogeneic hematopoietic cell transplantation: A prospective study that administered the Short-Form Health Survey (SF-12) and EuroQol 5. Transplantation and Cellular Therapy, 27(11), 935.e1-935.e9. https://doi.org/10.1016/j.jtct.2021.07.026
Maisey, N. R., Norman, A., Watson, M., Allen, M. J., Hill, M. E., & Cunningham, D. (2002). Baseline quality of life predicts survival in patients with advanced colorectal cancer. European Journal of Cancer (Oxford, England: 1990), 38(10), 1351–1357. https://doi.org/10.1016/s0959-8049(02)00098-9
Efficace, F., Bottomley, A., Coens, C., Van Steen, K., Conroy, T., Schöffski, P., Schmoll, H., Van Cutsem, E., & Köhne, C. H. (2006). Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? European Journal of Cancer (Oxford, England: 1990), 42(1), 42–49. https://doi.org/10.1016/j.ejca.2005.07.025
Lis, C. G., Gupta, D., Granick, J., & Grutsch, J. F. (2006). Can patient satisfaction with quality of life predict survival in advanced colorectal cancer? Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 14(11), 1104–1110. https://doi.org/10.1007/s00520-006-0100-3
Efficace, F., Innominato, P. F., Bjarnason, G., Coens, C., Humblet, Y., Tumolo, S., Genet, D., Tampellini, M., Bottomley, A., Garufi, C., Focan, C., Giacchetti, S., Lévi, F., & Lévi, F. (2008). Validation of patient’s self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: Results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. Journal of Clinical Oncology, 26(12), 2020–2026. https://doi.org/10.1200/JCO.2007.12.3117
Braun, D. P., Gupta, D., Grutsch, J. F., & Staren, E. D. (2011). Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health and Quality of Life Outcomes, 9, 62. https://doi.org/10.1186/1477-7525-9-62
Park, S., Eo, W., & Lee, S. (2018). The relationship between health-related quality of life and survival in metastatic colorectal cancer patients treated with Korean medicine. Integrative Cancer Therapies, 17(1), 65–72. https://doi.org/10.1177/1534735416684015
Dodson, R. M., McQuellon, R. P., Mogal, H. D., Duckworth, K. E., Russell, G. B., Votanopoulos, K. I., Shen, P., & Levine, E. A. (2016). Quality-of-life evaluation after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Annals of Surgical Oncology, 23(Suppl 5), 772–783. https://doi.org/10.1245/s10434-016-5547-y
Ratjen, I., Schafmayer, C., Enderle, J., di Giuseppe, R., Waniek, S., Koch, M., Burmeister, G., Nöthlings, U., Hampe, J., Schlesinger, S., & Lieb, W. (2018). Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: A German cohort study. BMC Cancer, 18(1), 1156. https://doi.org/10.1186/s12885-018-5075-1
Hamers, P. A. H., Vink, G. R., Elferink, M. A. G., Stellato, R. K., Dijksterhuis, W. P. M., Punt, C. J. A., Koopman, M., May, A. M., QUALITAS study group. (2022). Quality of life and survival of metastatic colorectal cancer patients treated with trifluridine-tipiracil (QUALITAS). Clinical Colorectal Cancer, 21(2), 154–166. https://doi.org/10.1016/j.clcc.2022.03.002
Ashing-Giwa, K. T., Lim, J.-W., & Tang, J. (2010). Surviving cervical cancer: Does health-related quality of life influence survival? Gynecologic Oncology, 118(1), 35–42. https://doi.org/10.1016/j.ygyno.2010.02.027
Carey, M. S., Bacon, M., Tu, D., Butler, L., Bezjak, A., & Stuart, G. C. (2008). The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecologic Oncology, 108(1), 100–105. https://doi.org/10.1016/j.ygyno.2007.08.088
Kim, M. K., Sim, J. A., Yun, Y. H., Bae, D. S., Nam, J. H., Park, C. T., Cho, C. H., Lee, J. M., & Park, S. Y. (2016). Health-related quality of life and sociodemographic characteristics as prognostic indicators of long-term survival in disease-free cervical cancer survivors. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society, 26(4), 743–749. https://doi.org/10.1097/IGC.0000000000000665
Park, S. H., Cho, M. S., Kim, Y. S., Hong, J., Nam, E., Park, J., Cho, E. K., Shin, D. B., Lee, J. H., & Lee, W. K. (2008). Self-reported health-related quality of life predicts survival for patients with advanced gastric cancer treated with first-line chemotherapy. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 17(2), 207–214. https://doi.org/10.1007/s11136-008-9307-8
Terashima, M., Fujitani, K., Ando, M., Sakamaki, K., Kawabata, R., Ito, Y., Yoshikawa, T., Kondo, M., Kodera, Y., Kaji, M., Oka, Y., Imamura, H., Kawada, J., Takagane, A., Shimada, H., Tanizawa, Y., Yamanaka, T., Morita, S., Ninomiya, M., & Yoshida, K. (2021). Survival analysis of a prospective multicenter observational study on surgical palliation among patients receiving treatment for malignant gastric outlet obstruction caused by incurable advanced gastric cancer. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24(1), 224–231. https://doi.org/10.1007/s10120-020-01114-z
Ogoshi, K., Hayashi, F., Takenoshita, S., & Isono, K. (2022). Baseline QOL, QOL-relevant HLA-restricted HERV gene-derived peptides, and survival outcomes in gastric cancer. Annals of Cancer Research and Therapy, 30(2), 55–66. https://doi.org/10.4993/acrt.30.55
Mauer, M. E. L., Taphoorn, M. J. B., Bottomley, A., Coens, C., Efficace, F., Sanson, M., Brandes, A. A., van der Rijt, C. C., Bernsen, H. J., Frénay, M., Tijssen, C. C., Lacombe, D., van den Bent, M. J., EORTC Brain Cancer Group. (2007). Prognostic value of health-related quality-of-life data in predicting survival in patients with anaplastic oligodendrogliomas, from a phase III EORTC brain cancer group study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 25(36), 5731–5737. https://doi.org/10.1200/JCO.2007.11.1476
Bragstad, S., Flatebø, M., Natvig, G. K., Eide, G. E., Skeie, G. O., Behbahani, M., Pedersen, P. H., Enger, P. Ø., & Skeie, B. S. (2018). Predictors of quality of life and survival following Gamma Knife surgery for lung cancer brain metastases: A prospective study. Journal of Neurosurgery, 129(1), 71–83. https://doi.org/10.3171/2017.2.JNS161659
Sehlen, S., Lenk, M., Hollenhorst, H., Schymura, B., Aydemir, U., Herschbach, P., & Dühmke, E. (2003). Quality of life (QoL) as predictive mediator variable for survival in patients with intracerebral neoplasma during radiotherapy. Onkologie, 26(1), 38–43. https://doi.org/10.1159/000069862
Chiarion-Sileni, V., Del Bianco, P., De Salvo, G. L., Lo Re, G., Romanini, A., Labianca, R., Nortilli, R., Corgna, E., Dalla Palma, M., Lo Presti, G., Ridolfi, R., Italian Melanoma Intergroup (IMI). (2003). Quality of life evaluation in a randomised trial of chemotherapy versus bio-chemotherapy in advanced melanoma patients. European Journal of Cancer (Oxford, England: 1990), 39(11), 1577–1585. https://doi.org/10.1016/s0959-8049(03)00372-1
Brandberg, Y., Johansson, H., Aamdal, S., Bastholt, L., Hernberg, M., Stierner, U., von der Maase, H., Hansson, J., Nordic Melanoma Cooperative Group. (2013). Role functioning before start of adjuvant treatment was an independent prognostic factor for survival and time to failure. A report from the Nordic adjuvant interferon trial for patients with high-risk melanoma. Acta Oncologica (Stockholm, Sweden), 52(6), 1086–1093. https://doi.org/10.3109/0284186X.2013.789140
Roychowdhury, D. F., Hayden, A., & Liepa, A. M. (2003). Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. Journal of Clinical Oncology, 21(4), 673–678. https://doi.org/10.1200/JCO.2003.04.166
Westhofen, T., Eismann, L., Buchner, A., Schlenker, B., Giessen-Jung, C., Becker, A., Stief, C. G., & Kretschmer, A. (2022). Baseline health-related quality of life predicts bladder cancer-specific survival following radical cystectomy. European Urology Focus, 8(6), 1659–1665. https://doi.org/10.1016/j.euf.2022.02.001
Gupta, D., Lis, C. G., & Grutsch, J. F. (2006). The European organization for research and treatment of cancer quality of life questionnaire: Implications for prognosis in pancreatic cancer. International Journal of Gastrointestinal Cancer, 37(2–3), 65–73. https://doi.org/10.1007/s12029-007-0001-9
Lis, C. G., Gupta, D., & Grutsch, J. F. (2006). Patient satisfaction with quality of life as a predictor of survival in pancreatic cancer. International Journal of Gastrointestinal Cancer, 37(1), 35–44. https://doi.org/10.1385/IJGC:37:1:35
Eichler, M., Singer, S., Hentschel, L., Richter, S., Hohenberger, P., Kasper, B., Andreou, D., Pink, D., Jakob, J., Grützmann, R., Fung, S., Wardelmann, E., Arndt, K., Heidt, V., Bonilla, S. A. Z., Gaidzik, V. I., Jambor, H. K., Weitz, J., Schaser, K. D., … Schuler, M. K. (2022). The association of Health-Related Quality of Life and 1-year-survival in sarcoma patients-results of a Nationwide Observational Study (PROSa). British Journal of Cancer, 126(9), 1346–1354. https://doi.org/10.1038/s41416-022-01702-z
Park, S. M., Park, M. H., Won, J. H., Lee, K. O., Choe, W. S., Heo, D. S., Kim, S. Y., Lee, K. S., & Yun, Y. H. (2006). EuroQol and survival prediction in terminal cancer patients: A multicenter prospective study in hospice-palliative care units. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 14(4), 329–333. https://doi.org/10.1007/s00520-005-0889-1
Coates, A., Porzsolt, F., & Osoba, D. (1997). Quality of life in oncology practice: Prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. European Journal of Cancer (Oxford, England: 1990), 33(7), 1025–1030. https://doi.org/10.1016/s0959-8049(97)00049-x
Sehlen, S., Marten-Mittag, B., Herschbach, P., Schweden, M., Book, K., Henrich, G., Dühmke, E., & Dinkel, A. (2012). Health-related quality of life supersedes other psychosocial predictors of long-term survival in cancer patients undergoing radiotherapy. Acta Oncologica (Stockholm, Sweden), 51(8), 1020–1028. https://doi.org/10.3109/0284186X.2012.683879
Lee, Y. J., Suh, S. Y., Choi, Y. S., Shim, J. Y., Seo, A. R., Choi, S. E., Ahn, H. Y., & Yim, E. (2014). EORTC QLQ-C15-PAL quality of life score as a prognostic indicator of survival in patients with far advanced cancer. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 22(7), 1941–1948. https://doi.org/10.1007/s00520-014-2173-8
Koyama, N., Matsumura, C., Shitashimizu, Y., Sako, M., Kurosawa, H., Nomura, T., Eguchi, Y., Ohba, K., & Yano, Y. (2021). The role of EORTC QLQ-C15-PAL scores and inflammatory biomarkers in predicting survival in terminally ill patients with cancer. BMC Cancer, 21(1), 304. https://doi.org/10.1186/s12885-021-08049-3
Hwang, S. S., Scott, C. B., Chang, V. T., Cogswell, J., Srinivas, S., & Kasimis, B. (2004). Prediction of survival for advanced cancer patients by recursive partitioning analysis: Role of Karnofsky performance status, quality of life, and symptom distress. Cancer Investigation, 22(5), 678–687. https://doi.org/10.1081/cnv-200032911
Vigano, A., Donaldson, N., Higginson, I. J., Bruera, E., Mahmud, S., & Suarez-Almazor, M. (2004). Quality of life and survival prediction in terminal cancer patients: A multicenter study. Cancer, 101(5), 1090–1098. https://doi.org/10.1002/cncr.20472
Shadbolt, B., Barresi, J., & Craft, P. (2002). Self-rated health as a predictor of survival among patients with advanced cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 20(10), 2514–2519. https://doi.org/10.1200/JCO.2002.08.060
Bozzetti, F., Cozzaglio, L., Biganzoli, E., Chiavenna, G., De Cicco, M., Donati, D., Gilli, G., Percolla, S., & Pironi, L. (2002). Quality of life and length of survival in advanced cancer patients on home parenteral nutrition. Clinical Nutrition (Edinburgh, Scotland), 21(4), 281–288. https://doi.org/10.1054/clnu.2002.0560
Grande, G. E., Farquhar, M. C., Barclay, S. I. G., & Todd, C. J. (2009). Quality of life measures (EORTC QLQ-C30 and SF-36) as predictors of survival in palliative colorectal and lung cancer patients. Palliative & Supportive Care, 7(3), 289–297. https://doi.org/10.1017/S1478951509990216
Group, T. E. (1990). EuroQol-a new facility for the measurement of health-related quality of life. Health Policy, 16(3), 199–208. https://doi.org/10.1016/0168-8510(90)90421-9
Hollen, P. J., Gralla, R. J., Kris, M. G., & Potanovich, L. M. (1993). Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). European Journal of Cancer (Oxford, England: 1990), 29A(Suppl 1), S51–S58. https://doi.org/10.1016/s0959-8049(05)80262-x
de Haes, J. C., van Knippenberg, F. C., & Neijt, J. P. (1990). Measuring psychological and physical distress in cancer patients: Structure and application of the Rotterdam Symptom Checklist. British Journal of Cancer, 62(6), 1034–1038. https://doi.org/10.1038/bjc.1990.434
Spitzer, W. O., Dobson, A. J., Hall, J., Chesterman, E., Levi, J., Shepherd, R., Battista, R. N., & Catchlove, B. R. (1981). Measuring the quality of life of cancer patients: A concise QL-index for use by physicians. Journal of Chronic Diseases, 34(12), 585–597. https://doi.org/10.1016/0021-9681(81)90058-8
Groenvold, M., Petersen, M. A., Aaronson, N. K., Arraras, J. I., Blazeby, J. M., Bottomley, A., Fayers, P. M., de Graeff, A., Hammerlid, E., Kaasa, S., Sprangers, M. A., Bjorner, J. B., EORTC Quality of Life Group. (2006). The development of the EORTC QLQ-C15-PAL A shortened questionnaire for cancer patients in palliative care. European Journal of Cancer (Oxford, England: 1990), 42(1), 55–64. https://doi.org/10.1016/j.ejca.2005.06.022
Kurihara, M., Shimizu, H., Tsuboi, K., Kobayashi, K., Murakami, M., Eguchi, K., & Shimozuma, K. (1999). Development of quality of life questionnaire in Japan: Quality of life assessment of cancer patients receiving chemotherapy. Psycho-Oncology, 8(4), 355–363. https://doi.org/10.1002/(SICI)1099-1611(199907/08)8:4%3c355::AID-PON401%3e3.0.CO;2-I
Morton, R. P., & Witterick, I. J. (1995). Rationale and development of a quality-of-life instrument for head-and-neck cancer patients. American Journal of Otolaryngology, 16(5), 284–293. https://doi.org/10.1016/0196-0709(95)90055-1
Browman, G. P., Levine, M. N., Hodson, D. I., Sathya, J., Russell, R., Skingley, P., Cripps, C., Eapen, L., & Girard, A. (1993). The Head and Neck Radiotherapy Questionnaire: A morbidity/quality-of-life instrument for clinical trials of radiation therapy in locally advanced head and neck cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 11(5), 863–872. https://doi.org/10.1200/JCO.1993.11.5.863
El Haidari, R., Abbas, L. A., Nerich, V., & Anota, A. (2020). Factors associated with health-related quality of life in women with breast cancer in the Middle East: A systematic review. Cancers, 12(3), 696. https://doi.org/10.3390/cancers12030696
Ngo, N. T. N., Nguyen, H. T., Nguyen, P. T. L., Vo, T. T. T., Phung, T. L., Pham, A. G., Vo, T. V., Dang, M. T. N., Nguyen Le Bao, T., & Duong, K. N. C. (2023). Health-related quality of life in breast cancer patients in low-and-middle-income countries in Asia: A systematic review. Frontiers in Global Women’s Health, 4, 1180383. https://doi.org/10.3389/fgwh.2023.1180383
Odeo, S., & Degu, A. (2020). Factors affecting health-related quality of life among prostate cancer patients: A systematic review. Journal of Oncology Pharmacy Practice: Official Publication of the International Society of Oncology Pharmacy Practitioners, 26(8), 1997–2010. https://doi.org/10.1177/1078155220959414
Rueda, J., Solà, I., Pascual, A., & Subirana Casacuberta, M. (2011). Non-invasive interventions for improving well—being and quality of life in patients with lung cancer. The Cochrane Database of Systematic Reviews, 2011(9), CD004282. https://doi.org/10.1002/14651858.CD004282.pub3
de van der Schueren, M. A. E., Laviano, A., Blanchard, H., Jourdan, M., Arends, J., & Baracos, V. E. (2018). Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Annals of Oncology, 29(5), 1141–1153. https://doi.org/10.1093/annonc/mdy114
Mishra, S. I., Scherer, R. W., Snyder, C., Geigle, P. M., Berlanstein, D. R., & Topaloglu, O. (2012). Exercise interventions on health-related quality of life for people with cancer during active treatment. The Cochrane Database of Systematic Reviews, 2012(8), CD008465. https://doi.org/10.1002/14651858.CD008465.pub2
Fukushima, T., Nakano, J., Hashizume, K., Ueno, K., Matsuura, E., Ikio, Y., Ishii, S., Morishita, S., Tanaka, K., & Kusuba, Y. (2021). Effects of aerobic, resistance, and mixed exercises on quality of life in patients with cancer: A systematic review and meta-analysis. Complementary Therapies in Clinical Practice, 42, 101290. https://doi.org/10.1016/j.ctcp.2020.101290
Molenaar, C. J., van Rooijen, S. J., Fokkenrood, H. J., Roumen, R. M., Janssen, L., & Slooter, G. D. (2022). Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. The Cochrane Database of Systematic Reviews, 5(5), CD013259. https://doi.org/10.1002/14651858.CD013259.pub2
Temel, J. S., Greer, J. A., Muzikansky, A., Gallagher, E. R., Admane, S., Jackson, V. A., Dahlin, C. M., Blinderman, C. D., Jacobsen, J., Pirl, W. F., Billings, J. A., & Lynch, T. J. (2010). Early palliative care for patients with metastatic non-small-cell lung cancer. The New England Journal of Medicine, 363(8), 733–742. https://doi.org/10.1056/NEJMoa1000678
Acknowledgements
The authors thank the members of the Laboratory of Cancer Rehabilitation for their support. This research would not have been possible without their leadership and cooperation.
Funding
This study was supported by the JSPS KAKENHI (grant number: 22K17603).
Author information
Authors and Affiliations
Contributions
TF and JN made substantial contributions to the conception and design. TF and JN were accountable for the collection and assembly of data. TF, JN, SM, JI, TO, TT, and KS performed the literature search and data analysis. TF and JN were major contributors in drafting and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fukushima, T., Suzuki, K., Tanaka, T. et al. Global quality of life and mortality risk in patients with cancer: a systematic review and meta-analysis. Qual Life Res (2024). https://doi.org/10.1007/s11136-024-03691-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11136-024-03691-3