Abstract
Purpose
The TORG0503 study was undertaken to select a preferred platinum-based third-generation regimen for patients with completely resected non-small cell lung cancer (NSCLC). This study aimed to describe the quality of life (QOL) analysis of that study.
Methods
Patients with completely resected NSCLC were randomized to receive three cycles of docetaxel plus cisplatin (DC) or paclitaxel plus carboplatin (PC) on day 1 every 3 weeks. QOL was assessed at three time points (baseline, after two cycles, and after three cycles) using the Functional Assessment of Cancer Therapy–taxane (FACT-Taxane). The adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated by logistic regression analysis that was adjusted for the baseline score in the FACT-Taxane total score and each subscale to evaluate treatment (PC vs. DC) effectiveness.
Results
QOL data from 104 patients (DC, n = 56 patients; PC, n = 48) were analyzed. In the FACT-Taxane total score, the baseline-adjusted OR (95% CI) of not worse QOL for the DC group was 3.3 (1.4–8.3) compared with the PC group. In the taxane subscale, the baseline-adjusted OR (95% CI) was 6.2 (2.6–16.0).
Conclusion
Total QOL was maintained better in the DC group than in the PC group, especially the taxane subscale that consists of neurotoxicity and taxane components in spite of no treatment-related death in both arms between DC and PC. We might recommend DC as the control regimen for the next clinical trial from the viewpoint of QOL, similar to the primary outcomes in TORG0503.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer accounts for a great number of new cancer cases and cancer-related deaths worldwide, especially Asia [1]. Mortality patterns reflect incidence trends, declines in accelerating lung cancer and progression, coinciding with changes in medical practices related to cancer screening and/or treatment [2]. Non-small cell lung cancer (NSCLC) is the most common lung cancer type, and many patients with NSCLC are diagnosed at an advanced stage when treatments available cannot be curative [3]. Many technical, pharmacological, and service developments have been made in the staging and treatment of lung cancer over the past decade, but questions remain about how to best implement these [4]. The TAX 326 Study Group suggested that docetaxel plus cisplatin resulted in a more favorable overall response and survival and better tolerated and improved quality of life (QOL) than vinorelbine plus cisplatin for advanced NSCLC [5]. Additionally, the Japanese Taxotere Lung Cancer Study Group showed that docetaxel plus cisplatin resulted in greater clinical benefit in terms of the response rate with marked improvements in the overall and 2-year survival rates and QOL than did vindesine plus cisplatin as first-line treatment for stage IV NSCLC [6]. The adjuvant treatment for patients with completely resected NSCLC mainly includes platinum-based chemotherapy [7]. TORG0503 was undertaken to select a preferred platinum-based third-generation regimen for patients with completely resected NSCLC because of the few data from randomized trial regarding third-generation and platinum agents that have been used as the current standard regimen for advanced NSCLC [8]. In patients with completely resected NSCLC, the TORG0503 reported that relapse-free survival and overall survival were favorable with docetaxel plus cisplatin than with paclitaxel plus carboplatin [8].
Chemotherapy-induced nausea and vomiting and renal toxicity in patients receiving cisplatin have been improved with recent advances in supportive care; however, other side effects such as neurotoxicity and fatigue remain difficult to control. While overall survival has been considered the most important indicator of efficacy [9], QOL is increasingly recognized as a relevant endpoint. However, QOL remains an understudied outcome in patients with NSCLC [10]. In this study, we aimed to report the results of the QOL analysis of the TORG0503 study.
Materials and methods
Study design and patients
Details of the study design of TORG0503 have been previously published [8]. Patients with completely resected NSCLC were randomized to receive three cycles of docetaxel plus cisplatin or paclitaxel plus carboplatin on day 1 every 3 weeks.
Study setting
The study protocol was approved by the institutional review boards at all participating institutions. Written informed consent was obtained from all participants. All study procedures were conducted in accordance with the principles of the declaration of Helsinki and its later amendments.
QOL measures: functional assessment of cancer therapy–taxane (FACT-Taxane)
The Japanese version of the Functional Assessment of Cancer Therapy–Taxane (FACT-Taxane) Questionnaire version 4.0 was developed to measure the health-related QOL of patients receiving taxane-containing chemotherapy [11]. The FACT-Taxane total score comprises the FACT-General (FACT-G) plus a 16-item Taxane subscale (TaxS). The FACT-G forms comprise subscales assessing physical well-being (PWB), social/family well-being (SFWB), emotional well-being (EWB), and functional well-being (FWB). Additionally, the TaxS combines the previously validated 11-item neurotoxicity subscale and five additional questions assessing symptoms of arthralgia, myalgia, and skin discoloration [12]. We made a QOL scoring system according to FACT-Taxane Scoring Guidelines (version 4) [13]. The QOL scales were linearly transformed, with higher scores indicating better QOL [12].
QOL assessments
Data were collected at three time points: baseline at the time of the study registration, after two cycles of chemotherapy (just before the third cycle), and the third assessment was conducted on the 22nd or 29th day after the third cycle, depending on the timing of outpatient clinic visits.
Statistical analysis
The outcomes were analyzed following a modified intention-to-treat (ITT) principle. For the modified ITT [14], we excluded patients who did not complete the FACT-Taxane questionnaire at baseline (after group allocation). The last observation carried forward (LOCF) method was used to manage missing data due to attrition [15]. Specifically, if an observation was missing, the last observed value was imputed at future time points where it was missing.
The National Cancer Institute of Canada Clinical Trials Group standard recommends a 10% change as the value for clinical significance [16]. The score changed from baseline by 10.0% in a direction indicating a worse QOL following three cycles of chemotherapy. During the three cycles of chemotherapy, from the start of treatment, we checked whether the scores showed worsening in the QOL scale by 10.0% from baseline after the third cycle of chemotherapy and classified as “worse.” Then, we checked whether the scores did not show worsening in the QOL scale by 10.0% from baseline after the third cycle of chemotherapy and classified as “not worse.” The reason behind using the 10% change as the value for clinical significance is given as follows. Considering the different stages of NSCLC, we used the 10% change as the value for clinical significance. While the study by Cella et al. [12] comprised of patients with stage IIIB/IV NSCLC, approximately half of the patients included in the present study had stage IIB or IIIA disease. Accordingly, we believe that the findings of the study by Cella et al. [12] may not be applicable to the patient population included in the present study. We used these reference values [16] as the approach described by the Canadian Cancer Trials Group represents a simple and practical method of analyzing, interpreting, and reporting health-related QOL data.
To determine the effects of the two treatment arms (docetaxel plus cisplatin and paclitaxel plus carboplatin), logistic regression analysis was used to determine the crude odds ratio (OR) with 95% confidence intervals (CIs) of the not worse QOL based on the response in the FACT-Taxane total score and subscales. We conducted logistic regression analysis that was adjusted for the baseline score in the FACT-Taxane total score and each subscale as a primary model.
Additionally, explanatory variables were adjusted for independent variables [baseline score of each QOL scale, two treatment arms (docetaxel plus cisplatin vs. paclitaxel plus carboplatin), age (continuous), sex (male vs. female), smoking status (yes vs. no), and cancer stages (IA/IB/IIA vs. IIB/IIIA)] using multivariate logistic regression analysis.
The goodness of fit of the model (P > 0.05) was checked using the Hosmer–Lemeshow test. As a part of sensitivity analysis, we analyzed the points of the missing data and analysis object. The multiple imputation (MI) method [17, 18] and the simple imputation (SI) method were used to manage missing data. In the SI, if an observation was missing, the lowest value was imputed at future time points where it was missing. Additionally, outcomes were analyzed using the per protocol set (PPS).
P < 0.05 (two-sided) was considered significant. Statistical analyses were conducted using SAS (Statistical Analysis Software 9.4, SAS Institute Inc., Cary, NC, USA) and Stata version 16.0 (Stata Corp., College Station, TX, USA).
Results
Participants
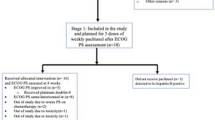
A flowchart of the randomized participation is presented in Fig. 1. In total, 111 patients were considered eligible and randomized: 58 patients in the docetaxel plus cisplatin group and 53 patients in the paclitaxel plus carboplatin group. Seven patients (docetaxel plus cisplatin, n = 2; paclitaxel plus carboplatin, n = 5) did not answer the FACT-Taxane questionnaire at baseline after group allocation, leaving 104 patients in this study who were analyzed for the modified ITT (docetaxel plus cisplatin, n = 56 (96.6%); paclitaxel plus carboplatin, n = 48 (90.6%).
Study flow chart. *Modified intention-to-treat: patients who did not complete the FACT-Taxane questionnaire at baseline after group allocation were excluded. **Per protocol set. Patients included in the modified ITT analysis who did not complete the FACT-Taxane questionnaire after three cycles were excluded
Patient characteristics
The baseline characteristics of the patients are shown in Table 1. The median age in the docetaxel plus cisplatin group was 63.0 years and that in the paclitaxel plus carboplatin group was 59.5 years, approximately 60% of the patients were male, and approximately 70% were smokers. Additionally, approximately half of the patients had stage IIB or IIIA disease, and most patients had histology-confirmed adenocarcinoma, and approximately 20% of the patients had performance status 1.
The QOL scores at baseline are shown in Table 1. The mean (standard deviation) of FACT-Taxane total score and PWB, SFWB, EWB, FWB, and TaxS scores at baseline for the docetaxel plus cisplatin group and paclitaxel plus carboplatin group were 132.8 (17.8) and 132.6 (16.5), 19.9 (5.4) and 19.7 (4.9), 21.0 (5.2) and 21.0 (4.5), 15.7 (5.6) and 14.6 (5.5), 16.3 (5.6) and 17.4 (5.7), and 59.9 (5.6) and 59.9 (4.3), respectively. No significant differences in FACT-Taxane total score and subscales were found.
QOL response
Tables 2 and 3 show the number of QOL response (not worse or worse) of patients, including odds ratios (crude, baseline-adjusted, and multivariate model) with 95% CI between the two treatment arms (docetaxel plus cisplatin vs. paclitaxel plus carboplatin). Regarding the fitness of the model, the logistic regression model showed a significantly good fit in FACT-Taxane total score and all subscales (Hosmer–Lemeshow test, P > 0.05). QOL responses according to treatment are shown in Fig. 2.
Baseline-adjusted odds ratios between the two treatment arms (docetaxel plus cisplatin vs. paclitaxel plus carboplatin). The goodness of fit of all models was determined using the Hosmer–Lemeshow test (P > 0.05). *Odds ratios with 95% confidence intervals (CI) were adjusted for the baseline score in QOL
We report the results of the baseline-adjusted model of the primary model in the FACT-Taxane total score and subscales (PWB, SFWB, EWB, FWB, and TaxS). In the FACT-Taxane total score, the adjusted OR of not worse QOL for the docetaxel plus cisplatin group was 3.1 (95% CI, 1.4–7.5) compared with the paclitaxel plus carboplatin group.
In the subscales, adjusted ORs of not worse QOL for the docetaxel plus cisplatin group were 1.3 (95% CI, 0.6–2.9) in PWB, 2.5 (95% CI, 1.1–6.3) in SFWB, 0.9 (95% CI, 0.4–2.3) in EWB, 0.8 (95% CI, 0.3–1.8) in FWB, and 6.2 (95% CI, 2.6–16.0) in TaxS compared with those for the paclitaxel plus carboplatin group.
In the sensitivity analysis, the supplementary table shows the baseline-adjusted ORs with 95% CI following LOCF, MI, and PPS. The baseline-adjusted ORs following MI, PPS, and SI were considered to have a similar tendency in the ORs following LOCF.
Discussion
We analyzed the QOL response of patients with completely resected NSCLC by comparing docetaxel plus cisplatin versus paclitaxel plus carboplatin. This QOL analysis clearly showed the superior global QOL (FACT-Taxane total score) of patients with NSCLC who received chemotherapy with docetaxel plus cisplatin (Table 2 and Fig. 2). In the subscales, symptoms related to the treatments (TaxS) were severe and clearly differed between the docetaxel plus cisplatin group than in the paclitaxel plus carboplatin group. These differences may be due to the greater neurotoxicity of paclitaxel plus carboplatin than docetaxel plus cisplatin, as shown in the present study. Although higher rates of nausea, anorexia, and vomiting have been reported with the use of docetaxel plus cisplatin, sensory neuropathy and arthralgia are reported as being more severe with the use of paclitaxel plus carboplatin [8]. Neurotoxicity may affect the global QOL of patients.
In the American Society of Clinical Oncology adjuvant therapy guideline for resected NSCLC, adjuvant cisplatin-based chemotherapy is recommended as the standard of care with respect to overall survival, disease-free survival, and adverse events [19]. In TORG0503, similar results had been reported for docetaxel plus cisplatin favored in terms of relapse-free survival and overall survival [8], whereas our analysis revealed that QOL is one of the patient-reported outcomes (PROs), which was better in the docetaxel plus cisplatin group than in the paclitaxel plus carboplatin group. A previous study reported that the integration of QOL analyses into clinical trials should be considered because maintenance of a good QOL strengthens the clinical efficacy of the treatment being investigated [20]. Although QOL surveys are conducted in many clinical trials, the results of the QOL analyses have not been reported. The results of TORG0503 regarding QOL might be significant.
In our analysis, the TaxS scale could clearly show a difference between treatment arms. Adverse events followed the benefit attributes [9]. In a previous study, assessments of longitudinally clinician-collected Common Terminology Criteria for Adverse Events better predict unfavorable clinical events, whereas patient reports better reflect daily health status, which are complementary, each providing clinically meaningful information [21]. Current methods for detecting adverse events in clinical trials are acknowledged to lack sensitivity, and concerning symptoms might well come to light earlier in the drug development cycle if patient reporting was the standard practice [22]. Our reports of the scale of treatment-related symptoms could provide more useful information.
Health-related QOL domains are important when evaluating how a clinical trial population feels or functions as a result of a specific disease or its treatment [23]. Current practices in reporting health-related QOL outcomes from randomized controlled trials remain highly variable, both with regard to the quality of reporting and the patterns of data analysis and presentation [24]. Our evaluation of patient QOL during the TORG0503 study may support the results of these previous studies.
This study has other limitations [8]. First, missing data in the QOL investigation were institution-dependent. However, the randomization of study treatments was stratified by institution, and the influence of selection bias might not be large. Both treatment arms had comparable patient characteristics and baseline QOL scores (Table 1). Additionally, a previous study showed that a modified ITT analysis allowed a systematically less biased approach to evaluate the effects of an intervention [25]. Second, because the primary endpoint of the TORG0503 study was relapse-free survival, the sample size for QOL analysis might not be sufficient. However, significant differences were found among the treatments in the QOL analysis. Finally, we did not collect QOL data regarding the long-term toxicity of FACT-Taxane chemotherapy. As long-term neurotoxicity impacts the QOL of patients receiving adjuvant chemotherapy, such data should have been collected in the present study.
Conclusion
The QOL analysis of the TORG0503 study clearly demonstrated that QOL was maintained better in the docetaxel plus cisplatin group than in the paclitaxel plus carboplatin group, especially in TaxS that consists of neurotoxicity and taxane components even if no treatment-related death occurred in both arms (docetaxel plus cisplatin or paclitaxel plus carboplatin). We might recommend docetaxel plus cisplatin as the control regimen for the next clinical trial from the viewpoint of QOL, similar to the primary outcomes of the TORG0503 study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2022). Cancer statistics, 2022. CA: A Cancer Journal for Clinicians, 72(1), 7–33. https://doi.org/10.3322/caac.21708
Testa, U., Castelli, G., & Pelosi, E. (2018). Lung cancers: Molecular characterisation, clonal heterogeneity and evolution, and cancer stem cells. Cancers, 10(8), 248. https://doi.org/10.3390/cancers10080248
Jones, G. S., & Baldwin, D. R. (2018). Recent advances in the management of lung cancer. Clinical Medicine, 18(Suppl 2), s41–s46. https://doi.org/10.7861/clinmedicine.18-2-s41
Fossella, F., Pereira, J. R., von Pawel, J., Pluzanska, A., Gorbounova, V., Kaukel, E., Mattson, K. V., Ramlau, R., Szczesna, A., Fidias, P., Millward, M., & Belani, C. P. (2003). Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 Study Group. Journal of Clinical Oncology, 21(16), 3016–3024. https://doi.org/10.1200/JCO.2003.12.046
Kubota, K., Watanabe, K., Kunitoh, H., Noda, K., Ichinose, Y., Katakami, N., Sugiura, T., Kawahara, M., Yokoyama, A., Yokota, S., Yoneda, S., Matsui, K., Kudo, S., Shibuya, M., Isobe, T., Segawa, Y., Nishiwaki, Y., Ohashi, Y., Niitani, H., et al. (2004). Phase III randomised trial of docetaxel Plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: The Japanese Taxotere Lung Cancer study Group. Journal of Clinical Oncology, 22(2), 254–261. https://doi.org/10.1200/JCO.2004.06.114
Lim, J. U., & Yeo, C. D. (2022). Update on adjuvant therapy in completely resected NSCLC patients. Thoracic Cancer, 13(3), 277–283. https://doi.org/10.1111/1759-7714.14277
Kubota, K., Kunitoh, H., Seto, T., Shimada, N., Tsuboi, M., Ohhira, T., Okamoto, H., Masuda, N., Maruyama, R., Shibuya, M., & Watanabe, K. (2020). Randomized phase II trial of adjuvant chemotherapy with docetaxel plus cisplatin versus paclitaxel plus carboplatin in patients with completely resected non-small cell lung cancer: TORG 0503. Lung Cancer, 141, 32–36. https://doi.org/10.1016/j.lungcan.2019.11.009
Sugitani, Y., Sugitani, N., & Ono, S. (2020). Quantitative preferences for lung cancer treatment from the patients’ perspective: A systematic review. Patient, 13(5), 521–536. https://doi.org/10.1007/s40271-020-00434-7
Liao, K., Wang, T., Coomber-Moore, J., Wong, D. C., Gomes, F., Faivre-Finn, C., Sperrin, M., Yorke, J., & van der Veer, S. N. (2022). Prognostic value of patient-reported outcome measures (PROMs) in adults with non-small cell lung cancer: A scoping review. BMC Cancer, 22(1), 1076.
FACIT. org. Retrived February 18, 2022, from https://www.facit.org/measures/FACT-Taxane
Cella, D., Peterman, A., Hudgens, S., Webster, K., & Socinski, M. A. (2003). Measuring the side effects of taxane therapy in oncology: The functional assesment of cancer therapy-taxane (FACT-taxane). Cancer, 98(4), 822–831. https://doi.org/10.1002/cncr.11578
FACIT. org. Retrived March 1, 2022, from https://www.facit.org/measures-scoring-downloads/fact-taxane-scoring-downloads
Dossing, A., Tarp, S., Furst, D. E., Gluud, C., Wells, G. A., Beyene, J., Hansen, B. B., Bliddal, H., & Christensen, R. (2016). Modified intention-to-treat analysis did not bias trial results. Journal of Clinical Epidemiology, 72, 66–74. https://doi.org/10.1016/j.jclinepi.2015.11.003
Lydersen, S. (2019). Last observation carried forward. Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, Ny Raekke. https://doi.org/10.4045/tidsskr.19.0061
Osoba, D., Bezjak, A., Brundage, M., Zee, B., Tu, D., Pater, J., Quality of Life Committee of the NCIC CTG. (2005). Analysis and interpretation of health-related quality-of-life data from clinical trials: Basic approach of the National Cancer Institute of Canada Clinical Trials Group. European Journal of Cancer, 41(2), 280–287. https://doi.org/10.1016/j.ejca.2004.10.017
Carpenter, J., & Kenward, M. (2013). Multiple imputation and its application. John Wiley & Sons.
Barnes, S. A., Lindborg, S. R., & Seaman, J. W. (2006). Multiple imputation techniques in small sample clinical trials. Statistics in Medicine, 25(2), 233–245.
Kris, M. G., Gaspar, L. E., Chaft, J. E., Kennedy, E. B., Azzoli, C. G., Ellis, P. M., Lin, S. H., Pass, H. I., Seth, R., Shepherd, F. A., Spigel, D. R., Strawn, J. R., Ung, Y. C., & Weyant, M. (2017). Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-Cell lung cancers. Journal of Clinical Oncology, 35, 2960–2974. https://doi.org/10.1200/JCO.2017.72
Oizumi, S., Kobayashi, K., Inoue, A., Maemondo, M., Sugawara, S., Yoshizawa, H., Isobe, H., Harada, M., Kinoshita, I., Okinaga, S., Kato, T., Harada, T., Gemma, A., Saijo, Y., Yokomizo, Y., Morita, S., Hagiwara, K., & Nukiwa, T. (2012). Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: Quality of life analysis of northeast Japan Study Group 002 trial. The Oncologist, 17(6), 863–870. https://doi.org/10.1634/theoncologist.2011-0426
Basch, E., Jia, X., Heller, G., Barz, A., Sit, L., Fruscione, M., Appawu, M., Iasonos, A., Atkinson, T., Goldfarb, S., Culkin, A., Kris, M. G., & Schrag, D. (2009). Adverse symptom reporting by patients versus clinicians: Relationships with clinical outcomes. Journal of the National Cancer Institute, 101(23), 1624–1632. https://doi.org/10.1093/jnci/djp386
Basch, E. (2010). The missing voice of patients in drug-safety reporting. New England Journal of Medicine, 362(10), 865–869. https://doi.org/10.1056/NEJMp0911494
United States Food and Drug Administration (FDA). (2009). Guidance for industry. U.S. Department of Health and Human Services. Patient-reported outcome measures: Use in medical product development to support labeling claims. Retrived March 1, 2020, from https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf.
Brundage, M., Bass, B., Davidson, J., Queenan, J., Bezjak, A., Ringash, J., Wilkinson, A., & Feldman-Stewart, D. (2011). Patterns of reporting health-related quality of life outcomes in randomized clinical trials: Implications for clinicians and quality of life researchers. Quality of Life Research, 20, 653–664.
Currow, D. C., Plummer, J. L., Kutner, J. S., Samsa, G. P., & Abernethy, A. P. (2012). Analyzing PHASE III studies in hospice/palliative care: A solution that sits between intention-to-treat and per protocol analyses: The palliative-modified ITT analysis. Journal of Pain and Symptom Management, 44(4), 595–603. https://doi.org/10.1016/j.jpainsymman.2011.10.028
Acknowledgements
We thank the participants in this trial and Hiroyuki Kashiro for the administrative support.
Funding
This work was supported by TORG. The research fund was provided to TORG by Sanofi Pharmaceutical Co. Ltd under the research contract. The funders did not have any involvement in the design of the study; collection, analysis, and interpretation of the data; writing of the article; or decision to submit the article for publication. Additionally, this study was supported by the Ministry of Education, Culture, Sports, Science and Technology in Japan Grant-in-Aid for Scientific Research Grant C in 2020 (Grant No. 20K07835).
Author information
Authors and Affiliations
Contributions
Conceptualization: KK, HK, HO, and AM. Data curation: HK, TS, MT, TO, RM, HO, and KK. Formal analysis: AM. Funding acquisition: AM and KK. Methodology: AM, KK, HK, and KY. Project administration: KK. Software and Validation: AM. Supervision: KK. Visualization: KK, KY. Roles/Writing—original draft: AM. Writing—review & editing: KK, KY, HK, TS, MT, TO, RM, HO, and KK.
Corresponding author
Ethics declarations
Conflict of interest
Professor Kaoru Kubota reports grants and personal fees from Boehringer Ingelheim and Ono and personal fees from Chugai MSD, AstraZeneca, Eli Lilly, Daiichi Sankyo, and Bristol Myers Squibb, outside the submitted work. The authors report no other conflicts of interest in this work.
Ethical approval
This study was conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of all participating institutes in agreement for medical research involving human subjects.
Consent to participate
All patients signed informed consent before any study-related procedures.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuda, A., Yamaoka, K., Kunitoh, H. et al. Quality of life with docetaxel plus cisplatin versus paclitaxel plus carboplatin in patients with completely resected non-small cell lung cancer: quality of life analysis of TORG 0503. Qual Life Res 32, 2629–2637 (2023). https://doi.org/10.1007/s11136-023-03424-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03424-y