Abstract
Introduction
Chronic rhinosinusitis (CRS) is strongly associated with significant impairment of quality of life (QoL) in children. The SN-5 questionnaire is an important assessment tool for pediatric CRS. This study aimed to evaluate potential prognostic factors for treatment of pediatric CRS within the Hebrew version of the SN-5 questionnaire.
Methods
A prospective study in pediatric otolaryngology unit. Patients were treated either surgically or pharmacologically. Following informed consent, parents of pediatric CRS patients completed the translated and validated Hebrew version (SN-5H) prior to treatment and after three months. We analyzed the results of both treatment arms according to success (achieving minimal clinically important difference; MCID).
Results
102 children aged 5–12 years and their caregivers participated (74 CRS patients and 28 controls without CRS). SN-5H items scores were significantly higher in CRS patients compared to controls (p < 0.001). Baseline activity scores were higher, while baseline emotional scores were lower in MCID( +) CRS patients, compared to MCID(-) CRS patients (p < 0.05). High emotional stress and low activity scores at baseline were associated with poorer odds to achieve MCID.
Conclusions
The SN-5H questionnaire is invaluable tool for assessing pediatric CRS patients. Psychosocial aspects of CRS significantly affect QoL and should be addressed in the office pre-treatment. The SN-5H can aid in highlighting patients in need for further reassurance and psychosocial support to manage expectations, and to improve QoL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CRS is strongly associated with significant impairment of quality of life (QoL), affecting physical fitness, social functioning, and mental health [1, 2]. The incidence of CRS in children is estimated at 2%–4%, according to the recent European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2020 [3]. The overall burden of pediatric ambulatory clinic visits per year in the United States, due to CRS, leads to over 5.6 million visits among patients from 0 to 20 years of age [4].
QoL questionnaires are a common assessment tool, both in adults and in children [5]. In adults, CRS-related questionnaires that assess QoL include, among others, the Sinonasal Outcome Test-22 (SNOT-22) [6], and the Nasal Obstruction Symptom Evaluation (NOSE) [7]. However, none of these questionnaires was designed for the pediatric population. The first CRS-specific QOL questionnaire that was validated in English for children is the Sinus and Nasal Quality of Life Survey (SN-5), published in 2003 [8]. It is designed for children, aged 2–12 years, and is composed of 5 items rated on a 7-point Likert scale, with an additional visual assessment score (VAS).
We sought to use the SN-5 questionnaire as a prognostic tool for successful treatment outcomes, defined as achievement of a minimal clinically important difference (MCID) following the course of treatment. We analyzed the questionnaires, by comparing MCID( +) and MCID(−) patients with CRS, in the setting of the largest pediatric sinonasal surgical unit in the country.
Materials and methods
Study design
Following an institutional review board (IRB) approval, we conducted a prospective study in the pediatric otolaryngology unit at Schneider's children medical center between 2018 and 2022. All the patients gave informed consent prior to participating in the study. After translating and validating the Hebrew version of the SN-5 (SN-5H), the participants were asked to complete the SN-5 questionnaire on two separate visits, three-months apart. Some of the patients underwent surgical intervention between both visits while other were treated medically with nasal steroids for the duration of two months. The allocation to each treatment arm depended upon physical and radiological findings, surgeon recommendations and parental preference. Details on the surgical procedures is depicted in Table 1.
Patients
All the participants were patients referred to our pediatric otolaryngology clinics. Inclusion criteria were 2–12 years of age, and parental/child demonstrating good communication skills and understanding in Hebrew (native speakers or fluent), both in conversation and in reading. Exclusion criteria were either parental/patient intellectual disability, cognitive impairment, or those without proficiency in Hebrew. The study group included children who were referred to our sinonasal clinic due to parental or patient complaints of sinonasal symptoms (purulent discharge, facial pain, nasal obstruction, rhinorrhea and/or cough), for more than 3 consecutive months. The control group included patients who were referred to the general pediatric ENT clinic for consultation due to non-sinonasal symptoms. All parents were asked to complete the SN-5H questionnaires on both clinic visits prior to the entering the doctor's office. The diagnosis of CRS was then made after reviewing the history of each patient and a thorough physical examination followed by flexible nasendoscopy, performed by a board-certified pediatric otolaryngologist, specialized in pediatric CRS surgeries. Treatment plan was discussed by the end of the 1st visit, following the surgeon's recommendations and parental preferences. After presenting the treatment options, the parents then elected either surgical or pharmacological regimens.
The SN-5 questionnaire
The SN-5 questionnaire consists of 5 questions that evaluate different domains related to sinonasal disease during the 4 weeks prior to taking the questionnaire: nasal obstruction, sinus infection, allergy symptoms, emotional distress, and activity limitations (Fig. 1). Caregivers are asked to rate the child's symptoms on a 7-point Likert scale. After completing the five questions, participants are asked to mark a Visual Analog Scale (VAS) that evaluates the sinonasal specific overall QoL, scored from 0 to 10. The SN-5 questionnaire was previously translated to other languages [9, 10]. We sought to properly translate the SN-5 into Hebrew, while performing the necessary cultural and lingual adaptations.
Translation and cross-cultural adaptation
Following the recommendations of the WHO for the translation and adaptation of instruments [11], a forward–backward translation process was taken. A first version (V1) of translation (English to Hebrew) was constructed by an otolaryngologist who is a native Hebrew speaker and fluent in English as well. A panel of experts (two other otolaryngologists), who were also fluent in both Hebrew and English, were convened to identify and resolve the inadequacy in the translation (V1). Two versions were created (V2, V3), until a consensus was reached (V4). The final version (Fig. 2) was sent to a pediatric otolaryngologist whose native language is English and is also fluent in Hebrew for back-translation. Pre-test cognitive interviews with 20 participants were carried out to assess comprehensibility of the SN-5H questionnaire.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 27.0 (IBM Corp, Armonk, NY, USA). A p-value cut-off point of 0.05 at 95% confidence interval (CI) was used to determine statistical significance. Cronbach’s alpha was calculated for internal consistency. Test–retest reliability was assessed using the intraclass correlation coefficient (ICC, p < 0.05). External validity was assessed by comparing SN-5H and VAS scores between the study group and the controls, by using the Mann–Whitney U test. The minimum sample size was estimated at 33 patients, with an expected Cronbach alpha correlation coefficient of 0.7, as the outcome of interest[12]. Student's T-tests were also used in subgroup analysis, to compare between patients in the different treatment groups.
Results
CRS versus controls analysis
One hundred and two children participated in the study during 2018–2022, among them, 62% were boys, and the rest (38%) were girls. The study group included 74 patients, while the controls were 28 in total. The average age was 8.3 ± 2.3 years (range 5.3–12) years, for the entire cohort. No differences in age were seen between the study group and the controls (p = 0.11). Among CRS patients 46% underwent surgical procedures. The rest of the patients were treated pharmacologically with topical steroids via nasal sprays There were 3 patients who were loss to follow up in the CRS group (4%). Results are depicted in Table 1.
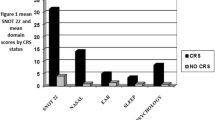
The overall SN-5H scores (sum of 5 domains) for the study and control groups were 21.5 ± 4.2 and 8.8 ± 3.6, respectively, p < 0.001. The individual domains were also compared between both groups and were all significant (p < 0.001). The VAS scores for the study and control groups were 3.6 ± 1.5 and 7.8 ± 1.3, respectively, p < 0.001. Results are depicted in Fig. 1. Significant correlation was seen between SN-5H and VAS scores (r = 0.74), assessed with Spearman's coefficient (CI = 0.64–0.82, p < 0.001). Internal validity (test–retest reproducibility), was assessed among the controls and among patients who did not undergo surgery, demonstrated good reliability (ICC = 0.86; CI = 0.78–0.91, p < 0.001). The internal consistency of the SN-5H, as measured by Cronbach’s alpha, was 0.84 for the entire cohort (CI = 0.79–0.88, p < 0.001). Results are depicted in Fig. 3.
CRS subgroup analysis according to MCID
We defined the minimal clinically important difference (MCID) as the difference in sum of scores between 1st and 2nd visit that was greater than 3 (according to the maximal improvement seen in the control group between the first and second visit; i.e. sum2-sum1, which was no greater than 3 points). In the study group, 75% in the surgical group and 59% in the nonsurgical group achieved MCID between 1st and 2nd visits, respectively (p = 0.2, data not shown). Next, we compared SN-5H scores between patients according to MCID status (those who achieved it and those who did not). We looked for both baseline scores and changes between 1st and 2nd visit in CRS patients. We analyzed each treatment arm (surgical and non-surgical) separately (Tables 2, 3). There were no differences between surgical and non-surgical groups in terms of gender, age distribution or baseline SN-5H scores (data not shown).
The activity scores
In each treatment arm we compared baseline SN-5H scores between those who achieved MCID and those who did not. Compared to the other 4 items that comprise the SN-5H score, only the baseline (1st visit) activity scores were significantly different between MCID ( +) and MCID (−) patients, regardless of the treatment arm. In the surgical group, the baseline activity scores for MCID( +) and MCID(−) patients were 5.1 and 2.1, respectively (p-0.03). In the nonsurgical group, the baseline (1st visit) activity scores were 4.9 and 2.3, for MCID( +) and MCID(−) patients, respectively (p-0.02). Data is depicted in Tables 2, 3 and Fig. 4.
Activity and Emotional scores among surgical and non-surgical patients. 1st visit activity and emotional scores are presented for both surgical and non-surgical patients, according to MCID achievement. MCID = minimal clinically important difference greater than 3 between 1st and 2nd clinic visit. *Statistically significant (p-value < 0.005) compared to MCID(−) patients in the same treatment arm
The emotional scores, sum of scores and VAS
In both surgical and nonsurgical groups, higher baseline emotional scores were seen in patients who did not reach MCID (4.8, 4.3, respectively), compared to MCID ( +) patients in these groups (3.9, 3.2, respectively), although it was not statistically significant. In both treatment arms, baseline VAS scores and baseline sum of scores were higher in MCID( +) patients, compared to their MCID(-) counterparts. Data is depicted in Tables 2, 3 and Fig. 4.
Discussion
Due to the overall burden of the disease, CRS patients suffer from significant QoL impairment [13]. Moreover, as discrepancies between clinical and radiological findings are common in CRS [14], QoL assessments greatly contribute and influence decision-making in doctors who treat these patients[15]. In children, health related QoL is of great importance, and should be regularly assessed and addressed by both treating physicians and their caregivers [16]. Nevertheless, it is generally accepted that children cannot reliably self-report, and caregivers are usually the ones that fill out the QoL questionnaires on their behalf [17]. The SN-5, was the first validated questionnaire in children with CRS, published in 2003 by Kay & Rosenfeld [10]. It is an invaluable assessment tool for pediatric CRS, that was previously shown to be correlated with radiological findings and post-surgical results in children [18].
In our study, we assessed the validity of the SN-5H, by comparing the individual item-scores as well as the overall scores between CRS patients and controls. The sum of scores for CRS patients was 2.4 times higher, and their VAS score was 50% lower, compared to the control group. These results demonstrate an overall poorer QoL in CRS patients compared to controls. Both validity and reliability scores for the SN-5H were high, similar to previously translated versions published in the literature [9, 10].
Following translation, we sought to find initial prognostic factors that can predict successful treatment, by achieving a minimal clinically important difference (MCID). The MCID was first introduced and defined by Jaeschke [19]. The two methodological approaches to assess the MCID are classified as distribution-based or anchor-based methods [20]. Similar to the SN-5 questionnaire in children, the validated 22-question Sinonasal Outcomes Test (SNOT-22) is a widely adopted instrument to evaluate chronic rhinosinusitis (CRS) treatment outcomes in adults. The MCID of SNOT-22 has previously been defined as 8.9 points using 3-month postoperative scores [6]. Unlike SNOT-22, the SN-5 does not have a defined and well-accepted MCID. Soler et al. [21], defined the MCID as 1. A recent systematic review by Ni et al. [22], demonstrated a significant difference in both MCID scores and criteria across different studies that implemented the SN-5 questionnaire. Using a distribution-based method, we calculated the MCID by looking at the standard deviation (SD). One widely accepted approach is to define the MCID as equal to 0.5 SD in the preoperative results of the study group [23]. In our dataset, this approach yields MCID as 2.1. However, when looking at the change in SN-5 scores between the first and second visits in the control group, the maximal change was 3. To assess for temporal changes following an intervention, rather than chance, we decided to define the MCID based on the clinical dataset (MCID of 3).
The decision on treatment plan, specifically surgical versus pharmacological, is complex, and is dependent upon physician preference and experience, clinical findings on physical examination and parental preference. It is therefore beyond the scope of this research. To adjust for the different treatment arms, we looked at each one separately, even though baseline characteristics (age, gender, co-morbidities, severity of CRS disease according to physical examination, radiological findings and SN-5H scores) between the surgical and non-surgical patients at baseline did not vary.
Several prognostic factors were seen, mainly the activity and emotional scores. High activity score represents increased burden of CRS disease, that limits the child's abilities to take part in physical activities. In this study, the activity score at baseline was significantly different between MCID ( +) and MCID (−) patients. It has an important clinical value as an indicator for the severity of CRS disease in children. The activity score was significantly higher at baseline in MCID ( +) patients. Following treatment, a significant reduction in activity scores were seen in both treatment arms, representing the improvement in the burden of CRS disease.
A study by Chmielik et al. [24], assessed QoL in pediatric CRS, with the use of the Child Health Questionnaire Parent Form (CHQ-PF-50). In their study, the greatest reduction in QoL in CRS patients was in the perception of general health, equivalent to the VAS score in the SN-5H, which resembles our results. Interestingly, physical fitness, which corresponds to the activity score in SN-5H, was relatively less affected by the disease. The results of the present study highlight the importance of the activity score as an independent marker for successful treatment. The higher the score, the better the chances for a clinically significant post-treatment improvement in QoL. In patients with a lower activity score at baseline it is more valuable to discuss expectations in length so to manage then during the treatment. Without recognizing these patients a priori, it will be difficult for both the patient and the treating doctor to match high expectations. We therefore recommend the "routine screening" of CRS patients with the SN-5H, before beginning of any course of treatment.
In both treatment groups, the emotional scores at baseline were higher in MCID (−) patients, while MCID ( +) patients in both groups demonstrated a reduction in emotional (stress) scores following treatment. These scores often represent the parental concerns about the child disease and associated morbidities. These results highlight the importance of addressing emotional stress and overall concerns of parents, which is of outmost importance for any treatment to achieve successful outcomes. It is essential to acknowledge fears and dilemmas, answer questions, and to provide information and reassurance to caregivers, to create the much-needed trust [25].
Higher sum of scores and lower VAS scores both correlate with increased overall burden of CRS disease in children, as seen in the previous sections. However, when implemented only on CRS patients, baseline values of the VAS and sum of scores were not significantly different between MCID ( +)/ MCID (−) patients and could not provide prognostic information for successful treatment.
Conclusions
Our results demonstrate the importance of the psychosocial aspects that affect QoL in parents and caregivers to CRS children. The SN-5H is an invaluable assessment tool for the severity of CRS disease. It is also a screening tool for a treating physician to be reminded of psychosocial aspects of CRS, related to QoL. These should be addressed prior to and throughout the course of treatment. Managing expectations, and addressing emotional stress is imperative for any treatment to successful.
Data Availability
The data that support the findings of this study are available on request from the corresponding author [O.Z].
References
Cunningham, M. J., Chiu, E. J., Landgraf, J. M., & Gliklich, R. E. (2000). The health impact of chronic recurrent rhinosinusitis in children. Archives of otolaryngology--head & neck surgery, 126(11), 1363–1368.
Rudnick, E. F., & Mitchell, R. B. (2006). Improvements in quality of life in children after surgical therapy for Sinonasal disease. Otolaryngology-Head and Neck Surgery, 134(5), 737–740. https://doi.org/10.1016/j.otohns.2005.12.033
Fokkens, W. J., Lund, V. J., Hopkins, C., Hellings, P. W., Kern, R., Reitsma, S., Toppila-Salmi, S., Bernal-Sprekelsen, M., Mullol, J., Alobid, I., Terezinha Anselmo-Lima, W., Bachert, C., Baroody, F., von Buchwald, C., Cervin, A., Cohen, N., Constantinidis, J., De Gabory, L., Desrosiers, M., Diamant, Z., … Zwetsloot, C. P. (2020). European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology, 58(Suppl S29), 1–464. https://doi.org/10.4193/Rhin20.600
Gilani, S., & Shin, J. J. (2017). The burden and visit prevalence of pediatric chronic rhinosinusitis. Otolaryngology-Head and Neck Surgery, 157(6), 1048–1052. https://doi.org/10.1177/0194599817721177
Garratt, A., Schmidt, L., Mackintosh, A., & Fitzpatrick, R. (2002). Quality of life measurement: Bibliographic study of patient assessed health outcome measures. BMJ (Clinical research ed.), 324(7351), 1417. https://doi.org/10.1136/bmj.324.7351.1417
Hopkins, C., Gillett, S., Slack, R., Lund, V. J., & Browne, J. P. (2009). Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology, 34(5), 447–454.
Stewart, M. G., Witsell, D. L., Smith, T. L., Weaver, E. M., Yueh, B., & Hannley, M. T. (2004). Development and validation of the nasal obstruction symptom evaluation (nose) scale. Otolaryngology-Head and Neck Surgery, 130(2), 157–163. https://doi.org/10.1016/j.otohns.2003.09.016
Kay, D. J., & Rosenfeld, R. M. (2003). Quality of life for children with persistent sinonasal symptoms. Otolaryngology-Head and Neck Surgery, 128(1), 17–26. https://doi.org/10.1067/mhn.2003.41
Gargula, S., Luscan, R., Drummond, D., Denoyelle, F., Couloigner, V., Leboulanger, N., & Simon, F. (2021). French translation and validation of the Sinus and Nasal Quality of Life Survey (SN-5) in children. International Journal of Pediatric Otorhinolaryngology, 145, 110706.
Calvo-Henríquez, C., Valencia-Blanco, B., Boronat-Catalá, B., Maza-Solano, J., Díaz-Anadón, Á., Kahn, S., Moure-Gonzalez, J. D., Faraldo-García, A., & Martinez-Capoccioni, G. (2020). Cross-cultural adaptation of the sinus and nasal quality of life survey (SN-5) to Spanish. International Journal of Pediatric Otorhinolaryngology. https://doi.org/10.1016/j.ijporl.2020.110425
World Health Organization (2015). Process of Translation and Adaption of Instruments. http://www.who.int/substance_abuse/research_tools/translation/en
Bobak, C. A., Barr, P. J., & O’Malley, A. J. (2018). Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Medical Research Methodology, 18(1), 93. https://doi.org/10.1186/s12874-018-0550-6
Alobid, I., Benítez, P., Bernal-Sprekelsen, M., Roca, J., Alonso, J., Picado, C., & Mullol, J. (2005). Nasal polyposis and its impact on quality of life: Comparison between the effects of medical and surgical treatments. Allergy, 60(4), 452–458. https://doi.org/10.1111/j.1398-9995.2005.00725.x
Wabnitz, D. A., Nair, S., & Wormald, P. J. (2005). Correlation between preoperative symptom scores, quality-of-life questionnaires, and staging with computed tomography in patients with chronic rhinosinusitis. American Journal of Rhinology, 19(1), 91–96.
Tahamiler, R., Canakcioglu, S., Ogreden, S., & Acioglu, E. (2007). The accuracy of symptom-based definition of chronic rhinosinusitis. Allergy, 62(9), 1029–1032. https://doi.org/10.1111/j.1398-9995.2007.01397.x
Eiser, C., & Morse, R. (2001). A review of measures of quality of life for children with chronic illness. Archives of disease in childhood, 84(3), 205–211. https://doi.org/10.1136/adc.84.3.205
Solans, M., Pane, S., Estrada, M. D., Serra-Sutton, V., Berra, S., Herdman, M., Alonso, J., & Rajmil, L. (2008). Health-related quality of life measurement in children and adolescents: A systematic review of generic and disease-specific instruments. Value in health : The journal of the International Society for Pharmacoeconomics and Outcomes Research, 11(4), 742–764. https://doi.org/10.1111/j.1524-4733.2007.00293.x
Rudnick, E. F., & Mitchell, R. B. (2006). Improvements in quality of life in children after surgical therapy for sinonasal disease. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 134(5), 737–740. https://doi.org/10.1016/j.otohns.2005.12.033
Jaeshke, R., Singer, J., & Guyatt, G. (1989). Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials, 10, 407–415.
Mouelhi, Y., Jouve, E., Castelli, C., & Gentile, S. (2020). How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods. Health and Quality of Life Outcomes, 18(1), 136.
Soler, Z. M., Rosenbloom, J. S., Skarada, D., Gutman, M., Hoy, M. J., & Nguyen, S. A. (2017). Prospective, multicenter evaluation of balloon sinus dilation for treatment of pediatric chronic rhinosinusitis. International Forum of Allergy and Rhinology, 7(3), 221–229.
Ni, J. S., Kompelli, A. R., Nguyen, S. A., Schlosser, R. J., Clemmens, C., & Soler, Z. M. (2018). The sinus and nasal quality of life survey (SN-5) in the Management of Pediatric Chronic Rhinosinusitis: A systematic review and meta-analysis. International Journal of Pediatric Otorhinolaryngology, 111, 162–169.
Norman, G. R., Sloan, J. A., & Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care, 41, 582–592.
Chmielik, L. P., Mielnik-Niedzielska, G., Kasprzyk, A., Stankiewicz, T., & Niedzielski, A. (2021). Health-related quality of life assessed in children with chronic rhinitis and sinusitis. Children (Basel, Switzerland), 8(12), 1133.
Rolfe, A., Cash-Gibson, L., Car, J., Sheikh, A., & McKinstry, B. (2014). Interventions for improving patients' trust in doctors and groups of doctors. The Cochrane Database of Systematic Reviews, 2014(3), CD004134.
Funding
Nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Ethical approval
This study protocol was reviewed and approved by Rabin Medical Center IRB committee, R20-1922.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zavdy, O., Golan, G., Yaniv, D. et al. Novel prognostic factors for successful treatment of pediatric chronic rhinosinusitis using the sinus and nasal quality of life survey (SN-5H). Qual Life Res 32, 2541–2549 (2023). https://doi.org/10.1007/s11136-023-03421-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03421-1