Abstract
Background
Patient-reported outcome measures (PROMs) could play an important role in identifying patients’ needs and goals in clinical encounters, improving communication and decision-making with clinicians, while making care more patient-centred. Comprehensive evidence that PROMS are an effective intervention is lacking in single randomised controlled trials (RCTs).
Methods
A systematic search was performed using controlled vocabulary related to the terms: clinical care setting and patient-reported outcome. English language studies were included if they were a RCT with a PROM as an intervention in a patient population. Included studies were analysed and their methodologic quality was appraised using the Cochrane Risk of Bias tool. The protocol was registered with PROSPERO (CRD42016034182).
Results
Of 4302 articles initially identified, 115 underwent full-text review resulting in 22 studies reporting on 25 comparisons. The majority of included studies were conducted in USA (11), among cancer patients (11), with adult participants only (20). Statistically significant and robust improvements were reported in the pre-specified outcomes of the process of care (2) and health care (3). Additionally, five, eight and three statistically significant but possibly non-robust findings were reported in the process of care, health and patient satisfaction outcomes, respectively.
Conclusions
Overall, studies that compared PROM to standard care either reported a positive effect or were not powered to find pre-specified differences. There is justification for the use of a PROM as part of standard care, but further adequately powered studies on their use in different contexts are necessary for a more comprehensive evidence base.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
According to the ISOQOL Dictionary of Quality of Life and Health Outcomes Measurement, a patient-reported outcome (PRO) is ‘a measurement of any aspect of a patient’s health that comes directly from the patient, without interpretation of the patient’s response by a physician or anyone else [1]’. Patients’ experiential knowledge of the effects of any intervention is essential for the delivery of high-quality clinical care. All patients in clinical care are unique and therefore may experience different benefits or side effects from the same treatment [2], which cannot be captured by the mere assessment of traditional physiological outcomes. It is therefore important to ask patients about their preferences and values to set self-directed health goals and promote compliance with treatment.
The assessment of PRO requires instruments that are valid and reliable. These instruments are often termed PRO measures (PROMs). It is suggested that regular use of PRO instruments to collect patients’ health-related outcomes can affect the health and well-being of patients by improving patient–physician interaction, by focusing on the clinical encounter on patient-directed concerns and by promoting shared clinical decision-making [3]. PROMs are commonly used in comparative effectiveness research, comparative safety analysis and economic evaluations to inform resource allocation [4, 5], with contexts including the ongoing monitoring of PROs for patients with chronic diseases or in palliative care [6].
The existing evidence on the effectiveness of regular assessment of PROs comes from a variety of sources including observational studies and individual randomised controlled trials (utilising qualitative, quantitative and mixed methods) and gold standard literature reviews of randomised controlled trials (RCTs) [7]. The effectiveness of PROMs has been explored in a number of literature reviews [6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Despite having different aims, these synopses [6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] highlight that the evidence is equivocal. There are several potential reasons for this ambiguity in the field, such as the attempt to aggregate heterogeneous tools under the umbrella term of PROM (which inappropriately considers them equivalent) [26], assessment of different RCT outcomes by various different methods and at different times, as well as a lack of standardised procedures for the provision of PROM results to health care providers and methodological issues with primary studies [27].
The aim of this systematic review was to assess the evidence on the effectiveness of the use of PROMs as an intervention intended to support the representation of patient values and preferences in clinical encounters. This review of RCTs is not limited by disease, age of patient population, nor year of publication. In addition, this is the first systematic review to consider the statistical robustness of results reported, and differentiate between the use of PROMs with and without the formal presentation of completed PROMs to treating clinicians.
Methods
A detailed systematic review protocol outlining the search strategy; methods for relevance and full-text screening; data extraction form; quality assessment method; plan for data analysis, synthesis and statistical issues; sensitivity and subgroup analysis; publication bias and any conflicts of interest was developed and registered with PROSPERO, registration number: CRD42016034182 [28].
Search strategy
With the help of a health research librarian, a systematic search strategy was developed to search three major databases (PubMed, EMBASE and PsycINFO) from inception to February 2017.
The search was conducted using controlled vocabulary and keywords related to the terms: clinical care setting and patient-reported outcomes [28]. The search strategy for the Medline database is included in the Supplementary 1 and was modified to adapt to variations in indexing among the databases. Reference lists of relevant literature reviews [8,9,10,11,12,13, 21] were also screened to identify additional articles. Citation searches were performed in Scopus.
Eligibility criteria
A publication of a study was eligible for inclusion if it reported on a RCT that applied a PROM to patients with or without providing the patients’ PROM score (summary/profile/dimension) to health care providers as an intervention. The review was restricted to studies that were published and reported in English. There were no restrictions on types of PROM, the form in which the PROM was used as an intervention, the health condition being studied, the country or setting in which the study was conducted.
Trials were excluded if they applied PROMs only for screening of psychological disorders such as depression and anxiety, were in the palliative care setting, compared one type of PROM to another type of PROM, compared only paper application to computer application of the same or different PROMs applied PROMs assessing specific constructs such as pain.
Relevance and full-text screening
First, a title, abstract and keyword screening of initially identified articles was performed. In order to pilot the inclusion criteria, (see review protocol [28]), two authors (SI and RN) initially screened a random 10% of the search results. Discrepancies were discussed and inclusion criteria were modified accordingly. Full-text articles identified from this search were retrieved and discussed. Once agreement on the inclusion criteria was achieved, the primary author (SI) completed the relevance screening with the remaining studies. Next, the same authors (SI, RN) independently applied the inclusion criteria to full texts of potentially relevant studies (n = 115) to identify studies for final inclusion. Any discrepancies were resolved through discussion. While it was initially planned to contact authors of studies where there was doubt concerning eligibility, this was not necessary as all doubts were resolved by discussion. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed to ensure transparent and comprehensive reporting [29].
Data extraction, analysis, synthesis and statistical issues
The primary author extracted data from all studies including characteristics of the study design, the nature of the PROM, method of intervention and study outcomes.
Consistent with previous systematic reviews in this setting [9, 11, 12], study outcomes were classified into three categories: process of health care, health outcomes and satisfaction with health care. Outcomes relating to how the care was delivered (e.g. consultation time, discussion of quality of life (QOL)/health-related quality of life (HRQL), return visit referral to other health practitioners) were classified as ‘process of care’; monitoring of changes in a patient’s QOL/HRQL or in any symptoms were classified as ‘health outcomes’ and finally outcomes relating to patients’ satisfaction with health care or feasibility were classified as ‘satisfaction with health care’. Given the heterogeneous nature of the data (both for PROMs used and patient populations studied), it was not considered meaningful to perform a meta-analysis.
Positive results (i.e. in favour of the PROM intervention) were considered ‘robust’ when statistically significant differences in a pre-specified outcome were reported for a study which was adequately powered to determine them. Positive non-significant or significant results for an outcome that was not pre-specified and/or for which the study was not powered to determine were considered ‘non-robust’.
Quality assessment
Three authors (SI, RN, AS) performed methodological quality assessments of seven included studies independently using Cochrane’s Risk of bias tool [30]; any discrepancies were resolved by discussion. Thereafter, the primary author (SI) performed the quality assessment and discussed it with another team member (AS), both carefully considering the reasons for specified rankings.
Results
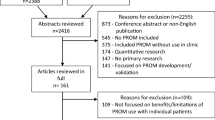
After removal of duplicates, 4302 articles were identified from database searches of which, 77 were found eligible for full-text screening. An additional 36 articles (of which 4 were included) were identified from previous literature reviews, and two articles were identified from other sources. After full-text screening of 116 articles, 22 RCTs met the inclusion criteria and were consequently included in this systematic review (Fig. 1) [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
Table 1 summarises the characteristics of included studies and Table 2 presents additional summary details of the RCTs, including the PROMs assessed, the intervention process and whether training was provided for health providers and/or patients. Based on the nature of the intervention evaluated, articles are grouped into two panels in Table 2: 18 studies in which all patients completed a PROM and compared the presentation of the PROM summary scores to clinicians vs. no presentation of summary scores (labelled ‘PROM ± summary’ studies) [31,32,33,34,35,36,37,38, 40, 41, 45,46,47,48,49,50,51,52]; 7 studies that compared patient completion of a PROM with standard care in the control group (i.e. no use of a PROM) (labelled ‘± PROM’ studies) [39, 42,43,44, 49]. Studies by Velikova [49] and Rosenbloom [44] compared more than two treatment arms and their data are thus represented in both tables and panels by specific comparisons. For example, the Velikova study (2004) RCT [49] compared PROM with presentation of results to health care providers vs. standard care, and PROM without presentation of results to health care providers vs. standard care.
Publication dates ranged from 1989 to 2016. Most of the studies took place in the USA [31, 32, 34, 35, 38, 40, 44,45,46, 48, 51, 52], followed by the UK [43, 49, 50], Netherlands [36, 37, 39], Australia [33, 41], Ireland [42] and Norway [47]. More than half of the studies (55%) included cancer patients [31,32,33, 35, 37, 39, 41, 42, 44, 47, 49, 52] and were performed in tertiary hospitals. Four studies [34, 40, 48, 50] were performed in tertiary care hospitals in sub-specialties other than cancer, and the remaining studies took place in GP/internal medicine/family physician offices [36, 38, 43, 45, 46, 51]. Six studies reported enrolling only new patients, five studies enrolled only patients who were previously known to clinicians and the remaining 11 studies did not specify. Sample size calculations to detect a specified effect size for named primary outcomes were reported in 11 studies [31, 32, 35,36,37, 40,41,42,43,44, 49]; three studies reported sample size calculations but their named primary outcome had multiple sub-components with no subsequent P value adjustments for multiple comparisons [39, 46, 47] and one performed only a post hoc power calculation [40].
A total of 23 PROMs were used in these 22 studies (Table 2). Reference to previous validation work for all PROMs was provided in the RCTs for each PROM in use. However, an evaluation of whether the PROM was a valid choice for the target population of the RCT in which it was in use was not reported. Cancer-specific (9) or generic tools (8) were most commonly applied; four studies used more than one PROM as an intervention [33, 36, 41, 49] [depression-specific and cancer-specific tools (2), generic and cancer-specific tools (1) and generic and diabetes-specific tools (1)] and one applied two arthritis-specific tools [40]. The most commonly utilised tool was the European Organisation for Research and Treatment of Cancer, Quality-of-Life Questionnaire Core 30 (EORTC QLQ-C30) (reported in three studies). The Beth Israel/UCLA Functional Status Assessment Questionnaire, the Hospital Anxiety and Depression Scale (HADS), the SF 36 and PedsQL generic were used in two studies each. The remaining PROM interventions were applied in one study only (Table 2).
In 18 studies [31,32,33, 36,37,38,39,40,41,42,43,44,45,46,47, 49,50,51], PROMs were self-administered by patients on paper (15) or via a computer touch screen (3); in three studies [34, 35, 48], PROMs were completed via telephone with assistance and one study [52] (on paediatric patients) varied the administration method according to the child’s age. In this latter trial; children younger than 7 years were allowed a parent-proxy PROM completion, children aged 7–13 years had combined child and parent-proxy PROM completion and children > 13 years self-administered the PROM.
PROMs were mostly completed in waiting rooms/clinics [32, 33, 37, 38, 40, 41, 44, 47, 49,50,51], followed by home/place of convenience [36, 39, 42, 45, 46], over the telephone [34, 48], first in clinic then subsequently over the telephone [35], a mixture of clinic and telephone interview [52], computer/paper-based completion according to patients’ preferences [31] or unspecified [43].
Of the 18 studies that compared presentation of PROM ± summary studies (completion of a PROM with presentation of results to clinicians vs. completion of a PROM alone), [34, 36,37,38, 45,46,47,48,49,50,51,52] provided training to clinicians/patients about interpretation of PROM scores. One [42] of the five studies that compared completion of a PROM with no PROM provided training to physicians about the layout of the PROM. The content of the training/education sessions provided to physicians and patients concerning the interpretation of the PROM varied substantially between studies (Table 2).
Table 3 summarises outcomes reported in the included studies classified into the three categories: process of care, health and satisfaction with health care. Pre-specified primary outcomes of studies are italicised.
In PROM ± summary studies, a process of care outcome was reported in nine studies [31, 32, 37,38,39,40, 45,46,47, 51], of which two were primary outcomes [32, 37]. In both studies that specified a process of care primary outcome intervention, patients reported a significant increase in the discussion of HRQL issues with their clinician [32, 37]. Four studies [31, 38, 47, 51] either did not report if their process of care outcomes were of primary or secondary interest, or claimed to power their study around a primary outcome that consisted of multiple sub-components. While these studies did report statistically significant results, their findings are considered non-robust due to the lack of adjustment for multiple comparisons.
Health outcomes were reported in 13 PROM ± summary studies [31, 33,34,35,36,37, 40, 41, 45,46,47, 49, 52], of which four were primary outcomes [31, 36, 41, 49]. A significant improvement in HRQL and psychosocial health was reported in the Basch [31] and De Wit [36] studies, respectively, whereas Velikova et al. [49] reported no significant difference in self-reported HRQL and McLachlan et al. [41] reported that the intervention did not significantly reduce the need for patient information regarding psychological and other health conditions. McLachlan et al. [41] reported a significant decrease in the spiritual needs of intervention patients, but without power for this comparison it was thus considered non-robust (Table 4). Cleeland [35] and Ruland [47] reported on studies whose outcomes had multiple sub-components, of which some showed significant improvement in intervention patients (Table 3). These effects were similarly considered non-robust given no P value adjustments for multiple comparisons (of sub-components) were made. Another seven studies [33, 34, 37, 40, 45, 46, 52] reported health outcomes without adequate power calculations for these comparisons, of which four reported significant non-robust results. In total, seven significant results for health outcomes were considered non-robust.
Satisfaction with health care was reported in seven [34, 36, 37, 40, 41, 50, 51] PROM ± summary studies as one of the outcomes of interest, with none of these studies explicitly specifying it as their primary or secondary outcome. Significantly more intervention patients were reported to be satisfied with their emotional support [37], overall care [36] and management of pain [51] (male patients only); with results categorised as non-robust. Three studies [34, 40, 41] reported no significant difference between the groups, and one [50] reported a greater (but non-significant) satisfaction with care in the control group.
In ± PROM studies (PROM completion with or without presentation of scores to clinicians vs. Standard Care), four studies [42, 44, 49] reported on process of care variables as one of their non-primary outcomes of interest. Of those, only Velikova [49] reported significantly (but non-robust) greater discussion of intervention patients’ HRQL issues.
Health outcomes were reported in five ± PROM studies [42,43,44, 49] as primary outcomes. Significant improvement in HRQL of intervention patients was reported by Velikova [49]. Four studies did not report any significant differences in health outcomes [42,43,44]. Health outcome variables were reported as secondary outcomes in Mills [42] and Velikova [49]. Mills [42] reported non-significant (and non-clinically meaningful) poorer lung cancer-specific QOL in the intervention group, whereas Velikova [49] reported non-robust significantly higher physical, emotional, functional well-being and HRQL of participants in the intervention group. The study by Hoekstra [39] reported on change in the prevalence and severity of several symptoms, some of which were reduced significantly, but the lack of adjustment for multiple comparisons rendered them non-robust.
Comparisons on satisfaction with care for ± PROM studies were reported in three publications of RCTs [42, 44], none of which were positive or statistically significant, thus there was no significant evidence that intervention patients were more satisfied than their comparator group regarding the health care that they received.
Feasibility data (including physician satisfaction) on acceptance and the perceived usefulness of the PROM intervention tools were collected in nine studies [32,33,34, 37, 40, 45, 49, 50, 52] with largely positive results (Table 3).
Methodological quality was evaluated using the Cochrane Collaboration’s Risk of Bias tool [30], with detailed assessment reported in Supplementary 2. The potential for bias was assessed in the domains of random sequence generation, allocation concealment, detection (blinding of outcome assessment), attrition (incomplete outcome data) and reporting (selective outcome reporting). The risk of introducing systematic error was found to be high in two studies on random sequence generation [46, 48], two studies on allocation concealment [46, 48], none on detection bias, two on attrition bias [40, 46] and six on reporting bias [34, 40, 46,47,48, 50]. Some studies had missing information, noted by the categorisation of domains as uncertain. Information regarding the likelihood of detection bias (blinding of outcome assessors/data analyst) and allocation concealment was missing in a large number of studies, 13 [32,33,34,35,36, 39,40,41, 43,44,45, 48, 50] and 10 [34, 37,38,39,40, 43,44,45, 50, 51], respectively.
We considered the potential for performance bias (blinding of participants and personnel) difficult to avoid due to the nature of the PROM interventions. However, in one study, authors acknowledged that they were able to blind patients and staff to the study hypothesis [44], but not to the interventions, and as such were considered to have low risk of performance bias.
Apart from performance bias, reporting bias was the most common domain, being present in six studies [34, 40, 46,47,48, 50]. Of those, two were conducted in the internal medicine units: one in each of an arthritis, obstetrics and gynaecology and neurology clinic, and one in a tertiary care cancer hospital.
Discussion
This systematic review of results from RCTs evaluating the use of PROMs in clinical practice categorised the reported comparisons into two groups. While in the first group of 18 studies [31,32,33,34,35,36,37,38, 40, 41, 45,46,47,48,49,50,51,52], the intervention participants completed the PROM and had their PROM results presented to the clinicians providing their clinical care (PROM ± summary), in the remaining seven studies [39, 42,43,44, 49] participants in the intervention group were simply asked to complete the PROM (± PROM). Reported results were grouped in one of three outcome categories: process of care, health and satisfaction with care (Table 4). Analysis of tabulated results led to the following findings: more positive results were reported for health outcomes, compared to those for the process of care or satisfaction with care; PROM interventions worked better when PROM results were provided to clinicians and the inclusion of PROM training to clinicians prior to a trial commencement appeared to result in no obvious differences in positive results.
Reviewed studies focused predominantly on statistically significant results, without typically mentioning whether they were clinically meaningful. If the results were positive but non-significant, there was no consideration in the publication of whether this may have been the result of a smaller than necessary sample size. Equally, when results were positive and significant, there was no discussion of whether this was possibly due to an inflated Type I error resulting from multiple comparisons. This concurs with results from the methodological quality assessment, indicating that the most common form of bias was that of reporting bias, regardless of the study context. Indeed, when considering characteristics of studies that may have been more prone to bias in any one (or more) domains, no one type of study appeared more prone.
All positive results were reported in this systematic review, regardless of significance, with Table 4 presenting a summary of the results differentiated by robustness, which includes studies reporting no evidence of a difference in treatment groups.
For the 18 studies classified as PROM ± summary, six [31, 32, 36, 37, 41, 49] were powered to detect an effect for their pre-specified primary outcome (two process of care [32, 37] and four health outcome variables [31, 36, 41, 49]), with the remaining studies either not pre-specifying a primary outcome, not reporting on power calculations for pre-specified outcomes or reporting that power calculations were performed for outcomes with multiple sub-components without evidence of this. Of the four studies with health outcomes as their primary focus, two reported significant results in favour of the intervention group [31, 36]. Non-significant improvement was reported in one study for the intervention group [41] and for the control group in another study [49]. Comparing the characteristics of studies reporting significant vs. non-significant effects, there were no identifiable differences in sample size, disease area, clinician training and mean age of participants or risk of bias.
Among seven studies [39, 49, 4244, 49] in the group classified as ± PROM, none reported a process of care primary outcome. Five studies [42,43,44, 49] reported a health outcome of primary interest with only one [49] reporting results in favour of the intervention, three reporting no significant evidence of a difference [43, 44] and one reporting a non-significant poorer effect in the intervention patients [42]. Studies that failed to show any significant difference or reported poorer effects in intervention patients either stated that they did not achieve their desired sample size [42, 44] or did not state this but appeared to have a relatively small sample size [43].
A total of 15 comparisons (in all identified studies) were on HRQL/health status/PROM score outcomes [31, 33, 34, 36, 37, 42,43,44,45,46, 49, 52]. One key observation noted in over half of these studies was the lack of discussion on what constitutes a clinically important difference. A predefined ‘clinically important’ and statistically significant difference in HRQL was reported in only three comparisons [31, 49]. The Mills study [42] reported non-significant non-clinically important poorer HRQL in intervention patients, but while the Detmar study [37] found no significant difference in health status measured by SF-36; a significantly larger percentage of people in the intervention group had clinically meaningful improved SF-36 scores [53]. The Velikova et al. PROM ± summary comparison did not find any significant and clinically important HRQL differences [49]. The remaining studies (8/15) [33, 34, 36, 43,44,45,46, 52], simply referred to P values to oppose or support the PROM intervention without reference to whether differences were considered to be clinically meaningful. While P values can provide important evidence of a difference in average outcome scores, they indicate only the probability that study findings such as those reported (or more extreme) could have occurred due to chance alone if there really was no difference in the two groups in the underlying population [54, 55]. As such, they lack the ability to inform clinicians of whether (in general) the difference really matters to their patients, i.e. if it was clinically meaningful [54, 55].
There are a large number of validated PROMs available for use (generic and disease specific) and their selection for a particular clinical population can be challenging [56]. The fact that different PROMs are often designed for and used in different populations means that the recommendation of one particular PROM over another in any given scenario is generally not possible. Given that studies in this review reported some positive and robust effects of PROM interventions, there is likely to be value in the use of PROMs in clinical care.
Thirteen studies provided training sessions to clinicians (and patients/families in some cases) on the interpretation and understanding of the PROM [34, 36,37,38, 42, 45,46,47,48,49,50,51,52]. Contrary to our expectation that clinicians would be more engaged in the use of the PROMs if training was provided [57,58,59], there appeared to be no obvious difference in positive outcomes when this took place. Some studies assessed the feasibility of PROM use, but none evaluated changes in clinicians’ perceptions before and after the intervention and thus the role of the clinician in the use of PROMs may require further study to be understood.
Compared to the previous literature [6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], this systematic review provides more contemporary evidence on the use of PROMs in RCTs and additional information about the use of PROMs in children, by including two RCTs with patients under 18 years old. The fact that only two paediatric studies [36, 52] met the inclusion criteria and were included in this review highlights the fact that little has been done to understand the value of PROMs in this context. It is well documented that children often struggle to communicate their health issues with parents and clinicians, and so a rationale for the use of PROMs to provide a voice for children is strong. Wolfe et al. [52] reported improvements in children’s and parents’ perceptions of talking to doctors, and in parents’ understanding of their child’s feelings. Clinicians also found PROM reports by children provided useful and new information in many cases [52]. While the study by Wolfe [52] did not report significant effects on primary health outcomes from the PROM intervention, a post hoc analysis of survivors beyond 20 weeks showed significant improvement in the emotional subscale of the PROM and in overall sickness scores. These findings were even stronger in children aged 8 years and older who were more likely to have completed the PROM without a proxy. Hemmingsson [60] recommended the use of self-reporting paediatric PROMs whenever possible, or observable components of HRQL when parent-proxy assessment is unavoidable, e.g. for young children. The De Wit [36] study on diabetic adolescents also reported a positive effect of PROMs on patients’ HRQL and patient satisfaction with health care. Also, provided that haemoglobin A1C levels were kept under control, a positive effect of PROMs on psychological outcomes was reported [36].
Methodological quality was evaluated using the Risk of Bias tool [30]. Detection bias occurs when outcome assessors are not blinded to the group allocation and study hypothesis. Although the studies in this review are considered pragmatic trials, blinding of the data analyst could have been achieved relatively easily.
This review has several methodological strengths. To minimise bias and to ensure that the systematic review was conducted in a transparent manner, a review protocol was submitted to PROSPERO. There are several benefits of developing a review protocol a priori as outlined in the PRISMA statement on reporting items for systematic review and meta-analysis protocols [61], and they include assurance that methods are replicable and in line with current recommendations. A comprehensive systematic search of three major databases was performed to reduce the possibility of missing relevant studies. An especially wide time frame, age range of target population and disease type were considered.
We acknowledge that there are some limitations of this review. Firstly, the inclusion criteria were restricted to studies published in the English language only. While this means we may have missed some important studies, it is unlikely that our conclusions would change given that previously documented systematic reviews included articles in multiple languages (in French, German, Italian, Russian or Spanish) and yielded similar results [9, 10]. Secondly, the relevance screening was performed by a single author, and data were extracted by the same author, but the title, abstract and keywords of a random 10% of the search were independently screened and inclusion criteria were modified after discussion with a second author (RN) and a more cautious (inclusive) approach was taken when screening. We acknowledge that single data extraction could result in missed items, and thus could affect the conclusions of a review. However, in an effort to minimise issues with interpretation, double extraction for the quality assessment and risk of bias analysis was performed. Thirdly, unlike previous systematic reviews, we excluded trials that claimed to use PROMs for screening of psychological conditions. Studies on psychological and mental health conditions were excluded from our systematic review. Patient-reported outcomes in these contexts are typically used for the purpose of diagnosing psychological or mental health conditions. Given that our focus was on the use of PROMs to effectively incorporate patient values and preferences in clinical encounters, these studies did not meet with the aim of our systematic review. Therefore, despite a much larger time frame for inclusion, there were fewer studies in this review compared to previous systematic reviews [6, 9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Our justification for this decision was our interest in the use of PROMs as a behavioural intervention and not as a screening tool. Given that the results of screening tools are typically reviewed by clinicians as part of a process to identify a disease at an earlier stage for secondary prevention, and thus identify patients who require follow-up, their use in this context is not optional. Hence, the use of PROMs as part of diagnostic tools for mental health disorders is different from proposing their use to inform patient-centred decision-making related to the choice of approach to care or treatment, and thus does not add to our evidence base.
Conclusions
Overall, positive findings in favour of the PROM intervention were reported on 21 occasions but the reported effects were robust in only five cases, i.e. statistically significant and adequately powered. Despite explicit CONSORT guidelines [27], many trials on PROM interventions failed to pre-specify their primary and secondary outcomes and/or adequately power their comparisons for clinically meaningful differences. Despite this, the combined evidence appears to support the use of PROMs to improve communication and decision-making in clinical practice. It is vital that future trials on PROM interventions follow CONSORT guidelines and continue to contribute robust evidence on the use of PROMs in clinical practice.
Abbreviations
- ± PROM studies:
-
Studies that compared patient completion of a PROM with standard care in the control group
- PROM ± summary studies:
-
Studies in which all patients completed a PROM and compared the presentation of PROM summary scores to clinicians vs. no presentation of summary scores
- FDA:
-
Food and drug administration
- HRQL:
-
Health-related quality of life
- PRO:
-
Patient-reported outcomes
- PROM:
-
Patient-reported outcome measure
- QOL:
-
Quality of life
- RCT:
-
Randomised controlled trial
- SR:
-
Systematic review
References
Mayo, E. N., et al. (2015). ISOQOL dictionary of quality of life and health outcomes measurement. First Edition.
Varadhan, R., Segal, J. B., Boyd, C. M., Wu, A. W., & Weiss, C. O. (2013). A framework for the analysis of heterogeneity of treatment effect in patient-centered outcomes research. Journal of Clinical Epidemiology, 66, 818–825.
Greenhalgh, J., & (Feb (2009). The applications of PROs in clinical practice: What are they, do they work, and why? Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care & Rehabilitation, 18(1), 115–123.
Ahmed, S., Berzon, R. A., Revicki, D. A., Lenderking, W. R., Moinpour, C. M., Basch, E., et al. (2012). The use of patient-reported outcomes (PRO) within comparative effectiveness research: Implications for clinical practice and health care policy. Medical Care, 50(12), 1060–1070.
Wu, A. W., Snyder, C., Clancy, C. M., & Steinwachs, D. M. (2010). Adding the patient perspective to comparative effectiveness research. Health Affairs, 29(10), 1863–1871.
Antunes, B., Harding, R., Irene, J. H., & on behalf EUROIMPACT (2014). Implementing patient-reported outcome measures in palliative care clinical practice: A systematic review of facilitators and barriers. Palliative Medicine, 28(2), 158–175.
Burns, P. B., Rohrich, R. J., & Chung, K. C. (2011). The levels of evidence and their role in evidence-based medicine. Plastic and Reconstructive Surgery, 128(1), 305–310. https://doi.org/10.1097/PRS.0b013e318219c171.
Greenhalgh, J., & Meadows, K. (1999). The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: A literature review. Journal of Evaluation in Clinical Practice, 5(4), 401–416.
Espallargues, M., Valderas, J. M., & Alonso, J. (2000). Provision of feedback on perceived health status to health care professionals: A systematic review of its impact. Medical Care, 38(2), 175–186.
Gilbody, S. M., House, A. O., & Sheldon, T. (2002). Routine administration of health related quality of life (HRQoL) and needs assessment instruments to improve psychological outcome—A systemic review. Psychological Medicine, 32(8), 1345–1356.
Kotronoulas, G., Kearney, N., Maguire, R., Harrow, A., Di Domenico, D., Croy, S., et al. (2014). What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. Journal of Clinical Oncology, 32(14), 1480–1501.
Valderas, J. M., Kotzeva, A., Espallargues, M., Guyatt, G., Ferrans, C. E., Halyard, M. Y., et al. (2008). The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Quality of Life Research, 17(2), 179–193.
Marshall, S., Haywood, K., & Fitzpatrick, R. (2006). Impact of patient-reported outcome measures on routine practice: A structured review. Journal of Evaluation in Clinical Practice, 12(5), 559–568.
Alsaleh, K. (2013). Routine administration of standardized questionnaires that assess aspects of patients’ quality of life in medical oncology clinics: A systematic review. Journal of the Egyptian National Cancer Institute, 25(2), 63–70. https://doi.org/10.1016/j.jnci.2013.03.001.
Boyce, M. B., & Browne, J. P. (2013). Does providing feedback on patient-reported outcomes to healthcare professionals result in better outcomes for patients? A systematic review. Quality of Life Research, 22(9), 2265–2278. https://doi.org/10.1007/s11136-013-0390-0.
Chen, J., Ou, L., & Hollis, S. J. (2013). A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Services Research. https://doi.org/10.1186/1472-6963-13-211.
Greenhalgh, J., Dalkin, S., Gooding, K., Gibbons, E., Wright, J., Meads, D., et al. (2017). Functionality and feedback: A realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Health Services and Delivery Research, 5(2), 1–280.
Etkind, S. N., Daveson, B. A., Kwok, W., Witt, J., Bausewein, C., Higginson, I. J., et al. (2015). Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: Does it make a difference? A systematic review. Journal of Pain and Symptom Management, 49(3), 611–624. https://doi.org/10.1016/j.jpainsymman.2014.07.010.
Gutteling, J. J., Darlington, A. S., Janssen, H. L., Duivenvoorden, H. J., Busschbach, J. J. V., & De Man, R. A. (2008). Effectiveness of health-related quality-of-life measurement in clinical practice: A prospective, randomized controlled trial in patients with chronic liver disease and their physicians. Quality of Life Research, 17(2), 195–205. https://doi.org/10.1007/s11136-008-9308-7.
Lambert, M. J., Whipple, J. L., Hawkins, E. J., Vermeersch, D. A., Nielsen, S. L., & Smart, D. W. (2003). Is it time for clinicians to routinely track patient outcome? A meta-analysis. Clinical Psychology: Science and Practice, 10(3), 288–301. https://doi.org/10.1093/clipsy/bpg025.
Luckett, T., Butow, P. N., & King, M. T. (2009). Improving patient outcomes through the routine use of patient-reported data in cancer clinics: Future directions. Psycho-oncology, 18(11), 1129–1138.
Guyatt, G. H., Van Zanten, S. V., Feeny, D. H., & Patrick, D. L. (1989). Measuring quality of life in clinical trials: A taxonomy and review. CMAJ: Canadian Medical Association Journal, 140(12), 1441–1448.
Williams, K., Sansoni, J., Morris, D., Grootemaat, P., & Thompson, C. (2016). Patient-reported outcome measures: Literature review. Sydney: ACSQHC.
Boyce, M. B., Browne, J. P., & Greenhalgh, J. (2014). The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research. BMJ Quality and Safety, 23(6), 508–518. https://doi.org/10.1136/bmjqs-2013-002524.
Duncan, E. A. S., & Murray, J. (2012). The barriers and facilitators to routine outcome measurement by allied health professionals in practice: A systematic review. BMC Health Services Research. https://doi.org/10.1186/1472-6963-12-96.
Valderas, J. M., & Alonso, J. (2008). Patient reported outcome measures: A model-based classification system for research and clinical practice. Quality of Life Research, 17(9), 1125–1135. https://doi.org/10.1007/s11136-008-9396-4.
Schulz, K. F., Altman, D. G., & Moher, D. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology, 63(8), 834–840. https://doi.org/10.1016/j.jclinepi.2010.02.005.
Ishaque, S., Salter, A., Karnon, J., Chan, G., Nair, R. (2016). A systematic review of randomized clinical trials evaluating the use of patient reported outcome measures (PROMs) to improve patient outcomes. PROSPERO CRD42016034182. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016034182.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. https://doi.org/10.1186/2046-4053-4-1
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928.
Basch, E., Deal, A. M., Kris, M. G., Scher, H. I., Hudis, C. A., Sabbatini, P., et al. (2016). Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. Journal of Clinical Oncology, 34(6), 557–565.
Berry, D. L., Blumenstein, B. A., Halpenny, B., Wolpin, S., Fann, J. R., Austin-Seymour, M., et al. (2011). Enhancing patient-provider communication with the electronic self-report assessment for cancer: A randomized trial. Journal of Clinical Oncology, 29(8), 1029–1035.
Boyes, A., Newell, S., Girgis, A., McElduff, P., & Sanson-Fisher, R. (2006). Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? European Journal of Cancer Care, 15(2), 163–171.
Calkins, D. R., Rubenstein, L. V., Cleary, P. D., Davies, A. R., Jette, A. M., Fink, A., et al. (1994). Functional disability screening of ambulatory patients—A randomized controlled trial in a hospital-based group practice. Journal of General Internal Medicine, 9(10), 590–592.
Cleeland, C. S., Wang, X. S., Shi, Q., Mendoza, T. R., Wright, S. L., Berry, M. D., et al. (2011). Automated symptom alerts reduce postoperative symptom severity after cancer surgery: A randomized controlled clinical trial. Journal of Clinical Oncology, 29(8), 994–1000.
De Wit, M., Delemarre-van De Waal, H. A., Bokma, J. A., Haasnoot, K., Houdijk, M. C., Gemke, R. J., et al. (2008). Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: A randomized controlled trial. Diabetes Care, 31(8), 1521–1526.
Detmar, S. B., Muller, M. J., Schornagel, J. H., Wever, L. D. V., & Aaronson, N. K. (2002). Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. Journal of the American Medical Association, 288(23), 3027–3034.
Goldsmith, G., & Brodwick, M. (1989). Assessing the functional status of older patients with chronic illness. Family Medicine, 21(1), 38–41.
Hoekstra, J., de Vos, R., van Duijn, N. P., Schade, E., & Bindels, P. J. (2006). Using the symptom monitor in a randomized controlled trial: The effect on symptom prevalence and severity. Journal of Pain & Symptom Management, 31(1), 22–30.
Kazis, L. E., Callahan, L. F., Meenan, R. F., & Pincus, T. (1990). Health status reports in the care of patients with rheumatoid arthritis. Journal of Clinical Epidemiology, 43(11), 1243–1253.
McLachlan, S. A., Allenby, A., Matthews, J., Wirth, A., Kissane, D., Bishop, M., et al. (2001). Randomized trial of coordinated psychosocial interventions based on patient self-assessments versus standard care to improve the psychosocial functioning of patients with cancer. Journal of Clinical Oncology, 19(21), 4117–4125.
Mills, M. E., Murray, L. J., Johnston, B. T., Cardwell, C., & Donnelly, M. (2009). Does a patient-held quality-of-life diary benefit patients with inoperable lung cancer? Journal of Clinical Oncology, 27(1), 70–77.
Qureshi, N., Standen, P. J., Hapgood, R., & Hayes, J. (2001). A randomized controlled trial to assess the psychological impact of a family history screening questionnaire in general practice. Family Practice, 18(1), 78–83.
Rosenbloom, S. K., Victorson, D. E., Hahn, E. A., Peterman, A. H., & Cella, D. (2007). Assessment is not enough: A randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psycho-oncology, 16(12), 1069–1079.
Rubenstein, L. V., Calkins, D. R., Young, R. T., Cleary, P. D., Fink, A., Kosecoff, J., et al. (1989). Improving patient function: A randomized trial of functional disability screening. Annals of Internal Medicine, 111(10), 836–842.
Rubenstein, L. V., McCoy, J. M., Cope, D. W., Barrett, P. A., Hirsch, S. H., Messer, K. S., et al. (1995). Improving patient quality of life with feedback to physicians about functional status. Journal of General Internal Medicine, 10(11), 607–614.
Ruland, C. M., Holte, H. H., Røislien, J., Heaven, C., Hamilton, G. A., Kristiansen, J., et al. (2010). Effects of a computer-supported interactive tailored patient assessment tool on patient care, symptom distress, and patients’ need for symptom management support: A randomized clinical trial. Journal of the American Medical Informatics Association, 17(4), 403–410.
Street, R. L., Gold, W. R., & McDowell, T. (1994). Using health status surveys in medical consultations. Medical Care, 32(7), 732–744.
Velikova, G., Booth, L., Smith, A. B., Brown, P. M., Lynch, P., Brown, J. M., et al. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. Journal of Clinical Oncology, 22(4), 714–724.
Wagner, A. K., Ehrenberg, B. L., Tran, T. A., Bungay, K. M., Cynn, D. J., & Rogers, W. H. (1997). Patient-based health status measurement in clinical practice: A study of its impact on epilepsy patients’ care. Quality of Life Research, 6(4), 329–341.
Wasson, J., Hays, R., Rubenstein, L., Nelson, E., Leaning, J., Johnson, D., et al. (1992). The short-term effect of patient health status assessment in a health maintenance organization. Quality of Life Research, 1(2), 99–106.
Wolfe, J., Orellana, L., Cook, E. F., Ullrich, C., Kang, T., Geyer, J. R., et al. (2014). Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: Results from the PediQUEST randomized controlled trial. Journal of Clinical Oncology, 32(11), 1119–1126.
Measuring Impact—SF-36. (2018). Accessed September 2, 2018, from http://www.measuringimpact.org/s4-sf-36.
Skelly, A. C. (2011). Probability, proof, and clinical significance. Evidence-Based Spine-Care Journal, 2(4), 9–11.
Hays, R. D., & Woolley, J. M. (2000). The concept of clinically meaningful difference in health-related quality-of-life research: How meaningful is it? PharmacoEconomics, 18(5), 419–423.
El Gaafary, M. (2016). A guide to PROMs methodology and selection criteria. In Y. El Miedany (Ed.), Patient reported outcome measures in rheumatic diseases (pp. 21–58). Cham: Springer.
Cappelleri, J. C., & Bushmakin, A. G. (2014). Interpretation of patient-reported outcomes. Statistical Methods in Medical Research, 23(5), 460–483. https://doi.org/10.1177/0962280213476377.
Coon, C. D., & Cappelleri, J. C. (2016). Interpreting change in scores on patient-reported outcome instruments. Therapeutic Innovation and Regulatory Science, 50(1), 22–29. https://doi.org/10.1177/2168479015622667.
Dreyer, R. P., Jones, P. G., Kutty, S., et al. (2016). Quantifying clinical change: Discrepencies between patient’s and providers’ perspectives. Quality of Life Research, 25(9), 2213–2220.
Hemmingsson, H., Ólafsdóttir, L. B., & Egilson, S. T. (2017). Agreements and disagreements between children and their parents in health-related assessments. Disability and Rehabilitation, 39(11), 1059–1072.
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ: British Medical Journal. https://doi.org/10.1136/bmj.g7647.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This review does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed Consent was not applicable to this review as no primary data were collected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ishaque, S., Karnon, J., Chen, G. et al. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs). Qual Life Res 28, 567–592 (2019). https://doi.org/10.1007/s11136-018-2016-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-2016-z