Abstract
Purpose
Decline in physical function is common in older age, with important consequences for health-related quality of life, health care utilisation, and mortality. This study aimed to identify patterns of change in physical functioning (PF) for women in later life.
Methods
PF was measured longitudinally using the ten-item subscale of the Medical Outcomes Study 36-item Short Form Health Survey, for 10 515 participants of the Australian Longitudinal Study on Women’s Health, who completed at least two surveys between 1999 (aged 73–78 years) and 2011 (aged 85–90 years). Conditional and unconditional latent profile analysis was conducted separately for deceased and surviving subgroups of women to uncover latent patterns of change in PF scores over time.
Results
Four patterns of change were identified for women who were still alive in 2011 (N = 5928), and four similar classes for deceased women (N = 4587): (1) ‘poor PF’ representing women with low PF scores, (2) ‘moderate PF’, (3) ‘high PF’, and (4) ‘very high PF’, where scores remained very high. All patterns exhibited a decrease in PF over time. Factors which predict low PF included sedentary levels of exercise, obese and overweight BMI, difficulty managing on income, and lower education.
Conclusions
The results provided evidence for a gradual decrease in PF for all women with age; however, there was no evidence for an increased rate of decline prior to death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oldest old (aged 85 years and over) are the most rapidly expanding population segment in developed countries [1]. Decline in physical function is a common characteristic of older age, with important consequences for health-related quality of life, healthcare utilisation, aged care, and mortality [2, 3]. However, there is also potential for people to age without disability, or with disability ‘compressed’ towards the end of life. It is of interest to determine what proportions of oldest old are ageing ‘successfully’.
Rowe and Kahn [4] define successful ageing as having low probability of disease and disease-related disability, high cognitive and physical function capacity, and active engagement with life. Two theories related to successful ageing include ‘failure of success’, in which an increasing proportion of individuals surviving to late age will also have increased disability and disease, and an alternative hypothesis that greater survival to the highest ages is accompanied by postponement of physical and cognitive disability (compression of morbidity) [1, 5]. Another theory involves the notion of terminal decline, which proposes that late life change is primarily driven by processes closely tied to pathology and mortality rather than chronological age [6]. Within this concept, late life consists of a pre-terminal phase of relative stability or minor decline, and a terminal phase of rapid decline that ends with death. While these theories are not mutually exclusive, little is known about the dominant patterns of change in physical function in later life or in the period prior to death at older ages. A 2009 review found evidence that people are living longer than they did previously, and with less disability and fewer functional limitations [1]. For the young old (<85 years), evidence suggests a postponement of limitations and disabilities, despite a rise in chronic disease and conditions [1]. However, for the oldest old (>85 years), the evidence is less clear, especially since the data are sparse and inconsistent [1]. Some studies report no increase in physical and cognitive disability level [7], while others suggest the oldest old have worse health than their predecessors, and that increased survival is associated with increasingly worse health [8]. A recent analysis of several studies from different countries showed mixed results as to age-related patterns of disability, particularly at oldest ages where there are little data [9]. Other studies have also shown large variation in individual patterns of change in physical functioning [10]. Hsu and Jones [11] uncovered four groups of ageing trajectories in a cohort aged 60 and over: successful ageing, usual ageing, health declining, and care demanding. They also found that a higher percentage of the younger old were likely to belong to the successful ageing group than the oldest old (85 years or over). Beckett, Brock [12] found a faster estimated rate of decline in the period prior to death. However, few of these studies focus solely on the very old. It is important to develop a greater understanding of changes in physical functioning at oldest ages, and towards the end of life, with implications for planning public health, social and aged care.
Investigating change in physical functioning across advanced old ages in women is especially important, given that women have greater longevity than men, higher rates of disability [13], and make greater use of health services throughout their adult lives [1, 13]. Women are also less likely to recover from disability than men [12]. Longitudinal investigation is also important, since studies have shown that the average rate of functional decline tends to be greater than that predicted cross-sectional studies [12].
The Australian Longitudinal Study on Women’s Health (ALSWH) provides a particular opportunity to examine late life changes in physical functioning among a large cohort of older women. The aim of this study is to identify different patterns of change in physical functioning, with the hypothesis that, compared to women who maintain high levels of PF throughout later life, those who have worse patterns of PF will have more chronic disease and worse health risk behaviours (high BMI, smoking, lower physical activity), after adjusting for sociodemographic factors. To investigate the possibility of terminal decline, separate analyses were conducted for women who survived the first six studies, and those who died.
Methods
Data
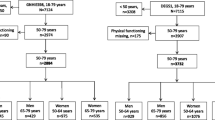
Data were from the 1921–26 birth cohort of the ALSWH. Women were sampled from the Medicare Australia database and invited to complete the baseline postal survey in 1996. Further details on the cohort have been published elsewhere [14]. This paper presents data collected for women who completed the baseline survey in 1996 (Survey 1—aged 70–75 years) and at least two three-yearly follow-up surveys (1999, 73–78 years), (2002, 76–81 years), (2005, 79–84 years), (2008, 82–87 years), and (2011, 85-90 years). Women may also have completed 6 monthly follow-up between 2011 and 2014 (aged 85–93 years). Women were described as survivors if they were still alive at the end of October 2012.
At each survey women completed the Medical Outcomes Study 36-item Short Form Health Survey version 1 (SF-36 Australian version) [15] which includes the physical function subscale (PF). PF includes ten items assessing a hierarchical range of difficulties on activities such as walking, climbing stairs, and carrying groceries and has been shown to exhibit internal consistency and convergent validity in for older community-dwelling adults [16]. Scores range from 0 to 100, with higher scores reflecting better physical functioning. The PF subscale has been shown to be an effective measure in older populations [17–19]. The PF subscale was chosen because it is a subscale which encapsulates vital activities of daily living (ADL) functioning which is likely to decline with age.
Surveys also include questions on marital status, education, area of residence, smoking, difficulty managing on income, and education. Body Mass Index (BMI) was calculated from self-reported weight and height and classified according to the World Health Organization categories [20], where BMI <18.5 was classified as underweight, BMI ≥18.5 and <25 as normal weight, BMI ≥25 and<30 as overweight, and BMI ≥30 was classified as obese. Exercise status was determined based on reported levels of activity, and women were classified as having either sedentary, low, moderate, or high physical activity levels based on estimated met:mins [21]. Women also self-reported diagnosed medical conditions including diabetes, arthritis, heart disease, hypertension, asthma, bronchitis/emphysema, stroke, osteoporosis, cancer (other than non-melanocytic skin cancer). All covariates were measured at baseline (except exercise status and arthritis, which were not asked until survey (2). A comorbidity count was created for each chronic disease, calculated as the reported number of other chronic conditions other than the disease of interest. Deaths were ascertained from the National Death Index [22].
Statistical analysis
Latent profile analysis (LPA) [23] was conducted using MPLUS [24] to uncover latent patterns of change (classes) in PF scores in two separate subsets of the data, over five surveys. The first data set (N = 5928) consisted of women who were still alive at Survey 6, and who had PF scores for at least two of Surveys 2–6. The second data set (N = 4587) consisted of deceased women who had non-missing PF scores for at least two out of the five most recent surveys (prior to death). Survey 1 was not included for the survivors to ensure a similar average length of longitudinal observation time to the deceased group. A sensitivity analysis was conducted including baseline observations for the survivor group, to confirm that this additional time point did not influence the findings. Between one and eight classes were trialled for each group of women, comparing models with equal and unequal variances for the PF scores across the classes. The ‘best’ model was chosen using the Bayesian information criterion [25], entropy (a measure of the degree of separation between the classes) [26], and the adjusted likelihood ratio test (LRT) [25], as well as the interpretability of the final classes and parsimony.
Conditional LPA was then conducted via one-step regression, which allows for estimation of covariate effects while simultaneously allowing the covariates to influence class membership. This approach also enables missing data on the covariates to be handled using full information maximum likelihood (FIML) estimation, assuming the missing values are missing at random (MAR). Covariates included in the conditional LPA were area of residence, BMI, difficulty managing on income, education, exercise status, marital status, smoking status, and whether or not a proxy completed the survey on the participant’s behalf. Time between the last returned survey and death was also included for the deceased group. Baseline age was initially included, but dropped from the models as it led to model non-identification. Class assignment between the adjusted (conditional) and unadjusted LPA models was cross-tabulated for each group, to investigate any change in latent class membership after covariate adjustment. Missing data due to death and non-death attrition were also estimated at each survey point, and the Satterthwaite t test (assuming unequal variances for the two groups) was applied to check for bias due to non-death attrition at Survey 3 and Survey 6.
In order to estimate the effects of each chronic disease on class membership, three-step multinomial regression models were performed. The conditional latent classes were regressed on each disease, adjusted for comorbidity count.
Finally, the leading causes of death were identified for each of the (unadjusted) latent patterns for the deceased group. A post hoc analysis was conducted for the survivor group, estimating mean PF scores at all subsequent 6 monthly follow-up (6MF) surveys. Multiple imputation was utilised to fill in missing 6MF scores for surviving women [27], using SAS Proc MI and Proc MIANALYZE [28].
Results
A total of 12 432 women completed the baseline survey in 1996. Between 1996 and 2011, 5962 women died, and 4587 of these women had PF data for at least two surveys prior to death. The average time between death and the most recent survey in the deceased group was 3.9 years (std. 3.2 years). A total of 6470 women survived, and 5928 of these women completed at least two surveys between 1999 (Survey 2) and 2011 (Survey 6). Baseline characteristics for each group are displayed in Table 1.
The PF scale was answered well by the women in the study, and the Cronbach’s alpha for the PF scale at baseline was 0.91. Missing data rates for the PF scale were mostly due to cohort attrition (Table 1). Missingness due to death was assumed a priori to be missing not at random (NMAR), and this was accounted for analysing deceased and surviving women separately. Missing not due to death was assumed to be MAR for the LPA. However, a check for bias due to non-death attrition at Survey 3 showed a significant difference in Mean (and 95 % CI) baseline PF scores for S3 respondents [66.57 (66.04, 67.09)] and non-respondents [58.32 (57.21, 59.42)] with Satterthwaite t test statistic −13.21 and p < 0.0001. At Survey 6, these differences in baseline PF were 70.42 (69.73, 71.11) and 65.20 (64.34, 66.05) Satterthwaite t test statistic −9.33, and p < 0.0001.
For both groups of women, a four class model that described the latent pattern of change in PF was selected (Table 2). These groups are displayed in Fig. 1 (deceased group) and Fig. 2 (survivor group). The first pattern in each group represents women with low PF scores (17 and 15 % of women for the deceased and survivor group, respectively), indicating poor physical functioning which worsens over time. The second pattern is ‘moderate’ PF (41 and 38 % of women for the deceased and survivor group, respectively), with scores beginning around 60 and decreasing over time to around the level of functioning that the low PF class started with. The third pattern is high PF (31 and 33 % of women for the deceased and survivor group, respectively). Women in this class have scores that start in the 80s, but decrease over time to below the level of functioning that the moderate PF class started with. Finally, the fourth pattern is very high PF (11 and 14 % of women for the deceased and survivor group, respectively), with women scoring very high on PF across all time points, although for the survivors there was a small decrease by Survey 6. The variance of the PF scores (Table 1) increased over time for the moderate, high and very high patterns of change. Model entropy for the deceased group was 0.79, and for the survivor group was 0.77. The class structure of the adjusted LPA models was very similar to the unadjusted, with no clinically significant changes in their meaning. Table 3 shows cross-tabulation between class membership in the unadjusted versus the adjusted LPA for the deceased and surviving women, demonstrating only small changes in class membership after the covariates were added.
Results for the adjusted LPA models are presented in Table 4. Exercise status was the strongest predictor of latent PF class for the survivors group. Those in the ‘high’ exercise class (compared to sedentary women) had an odds ratio (OR) of only 0.04 (95 % CI 0.02, 0.06) for belonging to the low PF class compared to the very high PF class. In fact, compared to sedentary women, even women who reported ‘low’ levels of exercise were much better off, being 81 % less likely to belong to the low PF class than the very high PF class. BMI was also a very strong predictor of latent PF class for surviving women. Compared to normal weight women, overweight and obese women more likely to belong to any PF class compared to the very high PF class. In particular, obese women had an OR 26.21 (95 % CI 15.06, 45.63) of belonging to the low PF class, and overweight women had an OR 5.02 (95 % CI 3.68, 6.86). Being underweight, however, was not a predictor of latent PF class. Compared to non-smokers survivors, being an ex-smoker was not a significant for predictor of latent PF class membership; however, smokers were almost three times more likely (OR 2.93, 95 % CI 1.55, 5.53) to belong to the low PF class, and also more likely to belong to the moderate PF class, than the very high PF class. Surviving women who reported having a proxy fill out their survey were four times more likely than women who did not use a proxy (OR 4.40, 95 % CI 1.43, 13.49) to belong to the low than very high PF class. Greater levels of education were generally associated with better physical functioning for survivors, and surviving women who reported no difficulty managing on income were less likely to belong to the low or moderate PF classes. Finally, ARIA was non-significant, and marital status was weakly significant, in predicting PF class for the survivor group.
Exercise status was the strongest predictor of latent PF class membership in the deceased group; however, the OR point estimates for these women were smaller than for the survivors. Those in the ‘high’ exercise class (compared to sedentary women) had OR 0.02 (95 % CI 001, 0.03) for belonging to the low PF class compared to the very high PF class. Again, even low levels of exercise were beneficial in comparison with being sedentary, reducing the odds of belonging to the low or moderate PF class compared to the very high PF class. BMI was also a strong predictor in the deceased group, and again the effect size was not as large as for the survivors. Compared to those of normal weight, overweight women had OR 2.59 (95 % CI 1.8, 3.73) and obese women had OR 18.59 (95 % CI 9.05, 38.21) for belonging to the low PF class compared to the very high PF class. Unlike the survivors, ex-smokers were significantly more likely to belong to the low PF and moderate PF class than the very high PF class, but the effect for smokers was non-significant. Deceased women who reported no difficulty managing on income were less likely to belong to the low, moderate, or high PF classes compared to women who reported difficulty managing on income. Area of residence, marital status, education, and use of a proxy were not significant for the deceased women. Finally, each year increase in time between the final returned survey and death was associated with decreased odds of belonging to the low, moderate, and high PF classes compared to the very high PF class for the deceased group.
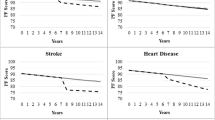
Table 5 displays the results for the separate multinomial regression models performed for each chronic disease (adjusted of comorbidity count), for both groups of women. Compared to women without arthritis, having arthritis was the strongest predictor of belonging to the low PF class compared to the very high PF class, for both survivors (OR 11.1, 95 % CI 8.23, 14.98) and deceased women (OR 7.46, 95 % CI 5.43, 10.43). The ORs were also significant, but smaller in magnitude, for the moderate and high PF classes, and in each case the effect size was larger in the survivor group. Stroke, osteoporosis, and heart disease were the next largest associations, with ORs of between 4 and 5 for belonging to the low PF class—in each case the effect size was larger for the deceased group. Having these diseases was also associated with greater, but smaller, odds of belonging to the moderate and high PF classes. In descending order, the remaining significant effects for belonging to the low PF class were diabetes, hypertension, bronchitis/emphysema and asthma. Cancer was not significant for the surviving group, but was associated with greater odds of belonging to the low and moderate PF classes in the deceased group.
The main causes of death were similar across all latent patterns of change, with cancer (23.5 %), ischaemic heart disease (18.5 %), cerebrovascular disease (11.1 %), Alzheimer’s/dementia (6.4 %), and other heart disease (5.9 %) being the top five causes overall (see Fig. 3).
Results of the post hoc analysis of the subsequent (post Survey 6) PF scores for the survivors are displayed in Table 6. The numbers of women in each six-month follow-up (6MF) decrease due to death and other attrition, with an additional 238 women having died by 6MF4. Scores for women in the low PF pattern remain low, potentially reflecting measurement floor. Scores for the moderate, high and very PF patterns declined further over time, with scores for women in the very high PF pattern now being closer to the intercept for the moderate PF pattern.
Discussion
In this study, we utilised repeated measures latent profile analysis, a longitudinal application of latent profile analysis (LPA) to assess dominant patterns of change in PF scores among a large cohort of women, including women who died between the ages of 70–75 years and 85–90 years, and women who survived to an older age. This approach allows for investigation of latent longitudinal patterns [29–31] and is therefore appropriate for identification of potential subgroups women with different patterns of change in physical functioning. Unlike other longitudinal models, such as latent growth curve models, this repeated measures LPA approach does not impose a priori a functional form to the longitudinal trajectory. Rather it allows the change to be modelled in whatever form it naturally occurs in each latent class [23]. Using this approach, four latent patterns of change in PF were identified in each group of women, most characterised by a decrease in PF scores over time, although some women maintained very high scores.
The patterns of change described were not that different between deceased and surviving women. As such, there was no evidence within this cohort of a ‘terminal decline’ where one would expect PF trajectories to decline more steeply for the deceased group than for the survivors. Rather, our results provide evidence for a gradual decline in PF for all women. The post hoc analysis further supports this, in that for the survivor group the average PF scores within each pattern continued to decrease over time. Botoseneanu [32] similarly studied a large cohort of men and women, stratifying analyses according to survival and found a nonlinear declining trajectory of physical function scores. They found that women who survived longer had a higher intercept for measures of function, similar to this study. However, unlike this study, the women with shorter survival times also had a steeper decline in function.
Gerstorf and Ram state that there is a need to examine whether and how ‘terminal-like declines’ turn into true terminal decline, and how people transition from a ‘preterminal phase of relative stability into a terminal phase of rapid decline that ends with death’ [6]. This transition may occur 2–8 years prior to death (in the case of cognitive decline) [33, 34], or 3–5 years for decline in well-being [35]. In our data, the observed patterns of decline were steady over time and similar for both subgroups of women, and thus provided no evidence of a transition to terminal decline. However, it is possible that the 3-yearly survey intervals were not sufficiently close in time to capture such a transition. As more 6 monthly survey waves become available, future work will further investigate the possibility of terminal transition. A further limitation in our data is that women who were most frail may be less likely to complete later surveys. This non-death attrition should be considered when interpreting the results.
Another theory relative to QoL in older persons is ‘compression of morbidity’, where healthy life expectancy increases relative to life expectancy, and morbidity is therefore confined to a shorter period with less lifetime disability [5, 36]. This compression is desirable since it implies that older people will spend a greater proportion of their remaining years in good health. In regard to investigating the existence of such compression, Fries states that ‘there is a lack of good data on the “quality of life,” and efforts at systematic accumulation of such data must be accelerated’ and that ‘studies need to identify and quantitate inter-individual variation for specific marker variables at particular ages’ [5]. The current study addresses both of these points. The presence of the ‘high’ and ‘very high’ PF classes for both the deceased and survivor groups shows that, at least for some older women, a compression of morbidity has occurred. These women began the study with good physical function (aged 70–75 years) and maintained very high or high levels through to age 85–90 years. However, the presence of the low PF class demonstrates that there is also a subgroup of the population for whom compression of morbidity does not occur. These women entered the study with poor physical functioning and remained so for the duration of the study. This class was present in the survivors as well as the deceased group, demonstrating that the morbidity was not merely an indicator of terminal decline, but a long-term state of poor health across 15 years. Fries states that the policy implications of morbidity compression are that health enhancement programs should begin early and continue throughout adult life, and that ageing programs directed only at the aged will have less impact than those addressing the same problems earlier in life [5]. We suggest such programs should be tailored to different subpopulations: aiming to maintain good health for those who have not yet experienced morbidity, and aiming to reverse poor health in those with morbidity (since for some of these women, at least, this morbidity may last for over a decade).
The reduction in PF over time evident in all groups of women is an important finding. In order to achieve successful ageing, functional status needs to be maximised, and even ‘modest reductions in the capacity to perform physical functions may prevent full participation in productive and recreational activities of daily life’ [4]. Factors that predicted low patterns of change in PF in this study included sedentary levels of exercise, obese and overweight BMI, difficulty managing on income, and lower education. These results reflect other findings in the literature. A study by Xu et al. [37.] of adults aged 65 years and older in the United States Health and Retirement Survey found that lower education, income, and net worth were associated with health trajectories characterised by impairment or rapid deterioration. Of the significant factors, the variables most readily changed via intervention are BMI and exercise. Our results suggest that even low levels of exercise, compared to being sedentary, may be beneficial for older women. However, the direction of this association would merit further investigation—it may also be the case that low physical function causes women to become sedentary. Older women should also be encouraged to maintain a healthy BMI, with attention to nutrition, physical activity, and maintenance of lean body mass. Healthy weight and maintenance of physical activity are also important for prevention of arthritis [38] which was the condition with the strongest effect on PF classes. Smoking was a significant factor in both the survivor and decedents group with continued smoking being more important to survivors, and ex-smoking being important among the decedents. Smoking is a very strong risk factor for earlier death among these women, whereas the mortality effect for ex-smokers decreased with the length of time since quitting [39]. Difficulty managing on income may reflect both financial resources, and more general socio-economic disadvantage. Along with the result for education, this finding indicates a need to focus on lower socio-economic groups and to ensure adequate financial support (e.g. pensions, and medical insurance) as these women age.
The ALSWH is a large long running study with good external validity. A comparison with the 1996 Australian Census indicates women who returned S1 were roughly representative of similar aged women in the Australian population [40]. Ongoing comparisons of the cohort against the national population suggest that the cohort is a good representation of older women, although there are some biases. Compared to the general population, women in the ALSWH cohort have had a relative survival advantage of 9.5 % (95 % confidence interval, 8.3–10.7 %), mainly due to baseline differences in demographic characteristics and health behaviours [41], and are unlikely to affect measures of association [42]. The results of the study are largely generalisable to Australian women in these age ranges.
However, against these strengths, non-death attrition and missing PF data are limitations of this study. Missing PF data due to death are likely to be not missing at random (NMAR); however, this was dealt with by performing the analysis separately for deceased and surviving women. We assumed that the remaining missing PF data were MAR. However, assessment of bias due to attrition demonstrated that non-respondents at Surveys 3 and 6 have different PF scores at baseline compared to respondents. If we were to investigate the PF score in a cross-sectional analysis, then the MAR assumption is unlikely to be plausible. However, simultaneously including all previous measures of PF in the LPA models, as well as including other covariates which likely also predict missing PF data (e.g. area of residence, BMI, education, exercise group, marital status, managing on income, smoking status, and use of a proxy) in the one-step regressions, diminishes the effect of bias of missing PF data on class membership. The model assumption that missing PF values are MAR then become more plausible—and therefore we are satisfied that we have dealt with missing PF values appropriately.
Given this long and steady decrease in PF scores over many years, the results of this study suggest an opportunity to potentially prevent this decline. A number of studies have shown that preventive activities for older people can reduce functional decline [43]. Important points for intervention identified in this study include maintenance of physical activity and healthy BMI, and prevention and management of chronic conditions such as stroke. The results also demonstrate an ongoing need to support women with reduced functional capacity.
Conclusion
This study identifies different patterns of change in physical functioning among a large cohort of older women over a long follow-up period. Most women will experience some decline in PF as they age. However, we also identify a group of women whose PF remains higher over time. The results highlight the importance of physical activity and maintenance of healthy weight to minimise decline in PF, and the need to focus on particular risk groups.
References
Christensen, K., Doblhammer, G., Rau, R., et al. (2009). Ageing populations: The challenges ahead. Lancet, 374, 1196–1208.
Litwin, H., Shira, A., & Schmotkin, D. (2012). Self-reported functional status among the old-old: A comparison of two Israeli cohorts. Journal Aging Health, 24(5), 846–862.
Sibbritt, D. W., Byles, J. E., & Regan, C. (2007). Factors associated with decline in physical functional health in a cohort of older women. Age and Ageing, 36, 382–388.
Rowe, J. W., & Kahn, R. L. (1997). Successful aging. The Gerontologist, 37(4), 433–440.
Fries, J. F. (1982). The compression of morbidity. The Milbank Memorial Fund Quarterly, 61(3), 397–419.
Gerstorf, D., & Ram, N. (2013). Inquiry into terminal decline: Five objectives for future study. Gerontologist, 53(5), 727–737.
Ahacic, K., Parker, M. G., & Thorslund, M. (2003). Mobility limitations 1974–1991: Period changes explaining improvement in the population. Social Science and Medicine, 57, 2411–2422.
Wang, Z., Zeng, Y., Jeune, B., et al. (1997). A demographic and health profile of centenarians in China. In J. M. Robine, et al. (Eds.), Longevity: to the limits and beyond (pp. 91–104). New York: Springer.
Chatterji, S., Byles, J., Cutler, D., et al. (2014). Health, functioning, and disability in older adults - present status and future implications. The Lancet, 14(6), 1462–1468.
Gill, T. M., Guo, Z., & Allore, H. G. (2008). Subtypes of disability in older persons over the course of nearly 8 years. Journal of the American Geriatrics Society, 56, 436–443.
Hsu, H.-C., & Jones, B. L. (2012). Multiple trajectories of successful aging of older and younger cohorts. Gerontologist, 52(6), 843–856.
Beckett, L. A., Brock, D. B., Lemke, J. H., et al. (1996). Analysis of change in self-reported physical function among older persons in four population studies. American Journal of Epidemiology, 143(8), 766–778.
Seidel, D., Jagger, C., Brayne, C., et al. (2009). Recovery in instrumental activities of daily living (IADLs): findings from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Age and Ageing, 38(6), 663–668.
Dobson, A, Hockey,R., Brown W.J., et al. (2015) Cohort Profile Update: Australian Longitudinal Study on Women’s Health. International Journalof Epidemiology. Published online 30/6/2015.
Ware, J. E., Kosinski, M., & Keller, S. D. (1994). SF-36 physical and mental health summary scales: A user’s manual. Boston, MA: The Health Institute.
Bohannon, R. W., & DePasquale, L. (2010). Physical functioing scale of the Short-Form (SF) 36: Internal consistency and validity with older adults. Journal of Geriatric Physical Therapy, 33, 16–18.
Lyons, R. A., Perry, H. M., & Littlepage, B. N. C. (1994). Evidence for the validity of the short-form 36 questionnaire (SF-36) in an elderly population. Age and Ageing, 23(3), 182–186.
Bartsch, L., Butterworth, P., Byles, J., et al. (2011). Examining the SF36 in an older population: Analysis of data from the Dynamic Analyses to Optimise Ageing (DYNOPTA) project. Quality of Life Research, 20(8), 1227–1236.
Taft, C., Karlsson, J., & Sullivan, M. (2001). Do SF-36 summary component scores accurately summarize subscale scores? Quality of Life Research, 10(5), 395–404.
World Health Organization. Obesity and Overweight. Fact sheet 311 [WHO Web site]. 2011 March, 2013 6 February, 2014]; http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
Russell, A., j. Ball, W.J. Brown, et al. Physical activity - Survey 2 and latr surveys. 2001 [cited 2016 9th June].
Powers, J., Ball, J., Adamson, L., et al. (2000). Effectiveness of the National Death Index for establishing the vital status of older women in the Australian Longitudinal Study on Women’s Health. Australian and New Zealand Journal Public Health, 24, 526–528.
Collins, L.M. and S.T. Lanza, Latent Class and Latent Transition Analysis with Applications in the Social, Behavioural and Health Sciences Wiley Series in Probability and Statistics, ed. D.J. Balding, et al. 2010, Hoboken, New Jersey: John Wiley & Sons.
Muthén, B. and Muthén L.K. (1998–2012) MPLUS User’s Guide. Seventh Edition. Muthén & Muthén: Los Angeles, CA.
Nylund, K. L., Asparouhov, T., & Muthen, B. (2007). Deciding on the number of classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo simulation study. Structual Equation Modeling, 14(4), 535–569.
Celeux, G., & Soromenho, G. (1996). An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification, 13, 195–212.
Women’s Health Australia, Technical Report 37. 2014, University of Newcastle and University of Queensland: http://www.alswh.org.au/images/content/pdf/technical_reports/report_37_alswh.pdf.
SAS Institutute Inc., SAS/STAT Software, Version 9.4. http://www.sas.com/. Cary, NC.
Silverwood, R. J., Nitsch, D., Pierce, M., et al. (2011). Characterizing longitudinal patterns of physical activity in mid-adulthood using latent class analysis: results from a prospective cohort study. American Journal of Epidemiology, 174(12), 1406–1415.
Lanza, S. T., & Collins, L. M. (2006). A mixture model of discontinuous development in heavy drinking from ages 18 to 30: The role of college enrollment. Journal of Studies on Alcohol, 67(4), 552–561.
Goodman, L. A. (1974). The analysis of systems of qualitative variables when some of the variables are unobservable. Part I - A modified latent structure approach. American Journal of Sociology, 79, 1179–1259.
Botoseneanu, A., Allore, H. G., Gahbauer, E. A., et al. (2013). Long-term trajectories of lower extremity function in older adults: Estimating gender differences while accounting for potential mortality bias. The Journal of Gerontology A: Biology Science Medical Science, 68(7), 861–868.
Sliwinski, M. J., Stawski, R. S., Hall, R. B., et al. (2006). On the importance of distinguishing pre-terminal and terminal cognitive decline. European Psychologist, 11, 172–181.
Wilson, R. S., Beck, T. L., Bienias, J. L., et al. (2007). Terminal cognitive decline: Accelerated loss of cognition in the last years of life. Psychosomatic Medicine, 69, 131–137.
Gerstorf, D., Ram, N., Mayraz, G., et al. (2010). Late-life decline in well-being across adulthood in Germany, the United Kingdom, and the United States: Something is seriously wrong at the end of life. Psychology and Aging, 25, 477–485.
Fries, J. F., Bruce, B., & Chakravarty, E. (2011). Compression of morbidity 1980-2011: A focused review of paradigms and progress. Journal of Aging Research, 261702, 1–10.
Xu, X., Liang, J., Botoseneanu, A., et al. (2014). Socioeconomic stratification and multidimensional health trajectories: evidence of convergence in later old age. The Journal of Gerontology B: Psychological Science Social Science, 70(4), 661–671.
Heesch, K.C., Miller, Y.D., & Brown, W.J. (2007) Relationship between physical activity and stiff or painful joints in mid-aged women and older women: a 3 year prospective study. Arthritis Resaerch and Therapy, 9(2), R34.
Jamrozik, K., McLaughlin, D., McCaul, K., et al. (2011). Women who smoke like men die like men who smoke: Findings from two Australian cohort studies. Tobacco Control, 20, 258–265.
Brown, W. J., Dobson, A. J., Bryson, L., et al. (1999). Women’s Health Australia: On the progress of the main cohort studies. Journal of Womens Health and Gender-Based Medicine, 8(5), 681–689.
Hockey, R., Tooth, L., & Dobson, A. (2011). Relative survival: a useful tol to assess generalisability in longitudinal studies of health in older persons. Emerging Trends in Epidemiolgy, 8(1), 3.
Brilleman, S., Pachana, N., & Dobson, A. (2010). The impact of attrition on the representativeness of cohort studies of older people. BMC Medical Research Methodology, 10, 71.
Beswick, A. D., Rees, K., Dieppe, P., et al. (2008). Complex interventions to improve physical function and maintain independent living in elderly people: A systematic review and meta-analysis. Lancet, 371, 125–135.
Acknowledgments
This research was conducted as part of the Australian Longitudinal Study on Women’s Health (ALSWH), the University of Newcastle and the University of Queensland. We are grateful to the Australian Government Department of Health and Ageing for funding, to the staff of ALSWH, and to the women who provided the survey data. Researchers in the Research Centre for Generational Health and Ageing at the University of Newcastle are members of the Hunter Medical Research Institute (HMRI), Newcastle. We acknowledge the assistance of the Data Linkage Unit at the Australian Institute of Health and Welfare (AIHW) for undertaking the data linkage to the National Death Index (NDI). GDM is funded by the Australian Research Council Future Fellowship (FT120100812).
Funding
The ALSWH is funded by the Australian Government Department of Health and Ageing. Gita D Mishra is funded by the Australian Research Council Future Fellowship (FT120100812). Lucy Leigh is a PhD Candidate receiving an Australian Postgraduate Award Scholarship
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lucy Leigh, Julie E Byles, Gita D Mishra declare no conflicts of interest.
Ethical approval
All procedures (surveys) performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Lucy Leigh and Julie E. Byles are joint first authors.
Rights and permissions
About this article
Cite this article
Leigh, L., Byles, J.E. & Mishra, G.D. Change in physical function among women as they age: findings from the Australian Longitudinal Study on Women’s Health. Qual Life Res 26, 981–991 (2017). https://doi.org/10.1007/s11136-016-1422-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1422-3