Abstract
Purpose
Low self-rated health (SRH) has been found to be associated with increased risk of type 2 diabetes (T2D) and with mortality. We examined the possible interaction between SRH and diabetic state on all-cause mortality in a large cohort of elderly subjects, followed for 14 years.
Methods
During the years 2000–2004, survivors of the nationwide longitudinal Israel Study of Glucose Intolerance, Obesity and Hypertension were interviewed and examined for the third follow-up. The 1037 participants (mean age 72.4 ± 7.2 years) were asked to rate their health as: excellent, good, fair, poor, or very poor. Glucose categories were as follows: Normoglycemic, Prediabetes, T2D and Undiagnosed diabetes. Survival time was defined as the time from interview to date of death or date of last vital status follow-up (August 1, 2013). Multivariate Cox proportional hazards models were performed in order to assess whether SRH interacts with glycemic state in the association with mortality.
Results
A better SRH was reported by those with undiagnosed than known diabetes, and best for normoglycemic and prediabetic individuals. While all individuals with fair or poor/very poor SRH were at increased risk of mortality compared to those with excellent/good SRH, in the known diabetic individuals a greater hazard was observed in the excellent/good SRH (HR 3.32, 95 % CI 1.71–6.47) than in those with fair or poor/very poor SRH (HR 2.19, 95 % CI 1.25–3.86), after adjusting for age, sex, ethnic origin, marital status, education, BMI, physical activity, CVD, tumors, and creatinine level (p for interaction = 0.01).
Conclusions
Self-rated health is not a sensitive tool for predicting mortality in elderly men and women with known T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Several studies [1–8], including two meta-analysis [9, 10], have reported an association between low self-rated health (SRH) and mortality, after adjusting for various clinical, behavioral and sociodemographic risk factors. In an Israeli sample, low SRH was associated with 4-year, but not 9-year mortality [11].
The association between type 2 diabetes (T2D), defined as fasting plasma glucose ≥126 mg/dl or 2-h plasma glucose ≥200 mg/dl, and increased risk of mortality is well known. A number of studies have shown an increased risk of mortality among individuals with prediabetes; specifically with impaired fasting glucose (IFG), defined as fasting plasma glucose of 110–125 mg/dl [12–16]; impaired glucose tolerance (IGT), defined as 2-h plasma glucose of 140–199 mg/dl [14–16]; and HbA1c in the prediabetes range [17]. Undetected diabetes was shown to confer an increased risk of mortality, though the association did not remain statistically significant after adjusting for demographic and clinical factors and SRH [18].
Low compared to high SRH has been found to associate with increased risk of T2D, after adjusting for age and sex [19], and with increased risk of IGT in men but not women, after controlling for lifestyle factors and biomedical variables [20].
The objective of this study was to explore the possible interaction between SRH (from excellent to very poor) and diabetic state, i.e., normoglycemic, prediabetic and known and unknown diabetic men and women, on all-cause mortality in a large cohort of elderly subjects, followed for 14 years.
Methods
Design and study population
This is a follow-up study of the survivors of the Israel Study of Glucose Intolerance, Obesity and Hypertension (The Israel GOH Study) [21], an ongoing nationwide longitudinal study of individuals selected in 1967 from the Israel Central Population Registry according to sex (50 % male and female), age (33 % from each 10-year increment of subjects born between 1912 and 1941) and four ethnic groups (European-American origin, North African, Yemenite, and other Middle Eastern). A deliberate increase in the sampling fraction of the Yemenite-born group was undertaken in order to enable a balanced evaluation of morbidity and mortality. During the year 2000–2004, survivors of the original cohort were interviewed and examined for the third follow-up. The second follow-up was conducted during the early 1980s [21].
Of the 1970 individuals who were the target population for the current study, 756 were not included: 254 were deceased, 278 were unable to be traced (probably due to being institutionalized or living with their children), 177 refused to undergo re-examination and 47 were too ill to participate. In addition, 177 individuals were excluded from the analysis because they were only interviewed by telephone and did not undergo blood tests to validate their glycemic state (see flow diagram, Fig. 1). We compared the 1037 participants in the current analysis with the 679 non-participants alive at the begining of follow-up (in the year 2000); the latter were 2 years older on average of female predominance (61 %), and with a greater cumulative mortality rate (39.8 % as compared to 28.0 % in the participants).
The Institutional Review Board of the Sheba Medical Center approved the current study, based on the third follow-up of the GOH cohort. All participants consented in writing.
Interview and laboratory examinations
Participants were interviewed by trained nurses in regional clinics and were asked about their lifestyle habits, medical history, use of medications, and health maintenance organization affiliation. They were asked to rate their health as: excellent, good, fair, poor, or very poor. Height, weight, waist circumference, and three blood pressure measurements were taken during the interview. Venous blood was drawn after a 12-h fast for glucose and lipids (total serum cholesterol, LDL- and HDL-cholesterol, and triglycerides) and creatinine. In the non-diabetic patients, an oral glucose tolerance test (OGTT) was performed with 100 g of glucose and blood glucose was determined after 2 h.
Plasma glucose was determined by enzymatic UV test (hexokinase method) performed on Beckman-Coulter AU5800 analyzer. Creatinine levels were determined using Beckman-Coulter kit, based on kinetic color test with picric acid (Jaffé method) and performed on Beckman-Coulter AU5800 analyzer. All laboratory analyses were performed by the Metabolic Laboratory of Sheba Medical Center.
The following definitions were applied: Normoglycemic—not using glucose lowering medications and fasting plasma glucose (FPG) <5.6 mmol/L (100 mg/dl), and, when available, 2-h blood glucose <7.8 mmol/L (140 mg/dl); Prediabetes—FPG 5.6–6.9 mmol/L (100–125 mg/dl) and/or, when available, 2-h blood glucose 7.8–11.0 mmol/L (140–199 mg/dl); Diabetes (T2D)—reported diabetes (substantiated by documentation of prescriptions for oral glucose lowering medications or insulin, or medical reports); Undiagnosed diabetes—2-h blood glucose of ≥11.1 mmol/L (200 mg/dl) or fasting glucose ≥7.0 mmol/L (126 mg/dl); cardiovascular disease was considered with a reported history of myocardial infarction, stroke, transient ischemic attack, or peripheral vascular disease. Obesity was defined as BMI ≥ 30 kg/m2.
Statistical analyses
All analyses were performed using SAS software. Mean ± SD was calculated for continuous variables, and frequencies were presented for discrete variables. Comparison of the study variables according to the four glycemic categories (normoglycemic, prediabetes, undiagnosed T2D, and T2D) was made using the Chi-square test and Cochran–Armitage trend test for discrete variables and ANOVA test for continuous variables. Survival time was defined as the time from interview to date of death or date of last follow-up (August 1, 2013). Univariate and multivariate Cox proportional hazards regression models were performed. The variable of interest—self-rated health, glycemic group, and other variables were tested independently. Explanatory variables that reached p < 0.2 in the univariate analysis were included in the multivariate model in order to assess whether self-rated health is an independent and significant explanatory variable of mortality adjusting for glycemic group and other independent variables. Interaction between self-rated health and glycemic group was also explored and found to be significant. To test the proportional hazard assumption, we generated time-dependent variables by creating interaction terms for the independent variables and time and included them in the model. Results of the interaction are presented according to Knol and VanderWeele recommendation [22]. The assumption of proportionality was not found to be statistically significant (p = 0.9). All tests of significance were two tailed. A value of p < 0.05 was considered statistically significant.
Results
Of the 1037 individuals included (mean age 72.4 ± 7.2; range 58–94) in the present study, 269 (25.9 %) were known to have diabetes. Laboratory tests revealed that 110 (10.6 %) had undiagnosed diabetes and 321 (31.0 %) were prediabetic. Table 1 presents the baseline characteristics of the cohort according to glycemic group. Higher proportions of undiagnosed diabetes were observed in men than women (13.1 vs. 8.1 %, respectively); among the ethnic origins, it was highest among individuals of Yemenite origin (13.4 %; 31/231). More individuals with undiagnosed diabetes reported being physically active than did the others, yet they had the highest rates of current smoking and obesity. The known diabetics had the oldest mean age, the lowest proportions reporting physical activity and current smoking, and the highest rates of hypertension and cardiovascular disease.
Table 1 and supplementary Fig. 1 show that normoglycemic and prediabetic individuals rated their health similarly, while known diabetic individuals rated it worse (11.1 and 11.6 % vs. 3.1 % for excellent SRH, respectively). A similar pattern was seen for “good” SRH. The SRH distribution of those with undiagnosed diabetes was between those with known diabetes and those without diabetes.
Altogether, 291 individuals died over the 14-year follow-up, 73 in the normoglycemic (21.7 %), 86 in the prediabetic (26.8 %), 25 in the undiagnosed diabetic (22.7 %), and 107 in the T2D group (39.8 %), p < 0.0001. Univariate analysis of each characteristic and its association with mortality showed that in addition to older age and male gender, sedentary lifestyle, hypertension, and the j-shaped BMI, which are classical risk factors, also higher creatinine levels, presence of CVD, renal disease, and malignant tumors, were associated with mortality. Being married and of higher education were significantly associated with lower mortality (supplementary Table 1). Known diabetes, but not undiagnosed diabetes or prediabetes, associated significantly with mortality [hazard ratio (HR) = 1.95, 95 % CI 1.41–2.70], compared to the normoglycemic individuals. Both fair and poor/very poor SRH were positively associated with mortality, conferring twofold to fourfold mortality risk, when compared to excellent/good SRH. Ethnic origin and smoking were not associated with mortality in this cohort.
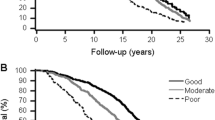
A multivariate analysis (Table 2) showed that known diabetes as compared to normoglycemia conferred a non-statistically significant increased mortality risk of 26 % in the presence of all other study variables (95 % CI 0.87–1.26). Fair/poor and very poor SRH, as compared to excellent/good, were associated with increased mortality risk of 48 % (95 % CI 1.09–2.03), after controlling for demographic characteristics, lifestyle habits, and comorbidities. We found a significant interaction between the two factors, SRH and glycemic group, which was entered into the final model (Table 3). Except for known diabetic individuals, individuals with fair or poor/very poor SRH were at increased risk of mortality compared to those with excellent or good SRH. Only the known diabetic individuals were at increased risk of mortality regardless of their SRH (HR = 3.32, 95 % CI 1.71–6.47, and HR = 2.19, 95 % CI 1.25–3.86, for “Good” and “Poor” SRH, respectively). Moreover, among diabetic individuals, those with excellent/good SRH were at greater risk of mortality than those with fair or poor/very poor SRH, after adjusting for age, gender, ethnic origin, marital status, education, BMI, physical activity, CVD, tumors, and creatinine level (Fig. 2). The finding of the above interaction is expressed by the ratio of the HRs being greater than 1 for the normoglycemia (ratio of 2.41, p = 0.002), prediabetes (ratio of 1.46, p = 0.15), and undiagnosed diabetes (ratio of 2.19, p = 0.1) individuals, whereas for the known diabetic individuals this ratio was less than 1 (0.66, p = 0.14). This inverse pattern, regarding the association between SRH and mortality among individuals with known diabetes, remained when stratifying by gender (not shown).
Discussion
In this study of an elderly population with mean age >70 years, the combined category of fair, poor, or very poor self-rated health, denoted as “poor SRH”, was associated with increased mortality risk. This association held for individuals with normoglycemia, prediabetes, and undiagnosed diabetes, but not for individuals with known diabetes, supporting a significant interaction between SRH and glycemic group (p for interaction 0.01). Both SRH known diabetic groups showed a high and statistically significant mortality risk (HR = 3.32, 95 % CI 1.71–6.47; HR = 2.19, 95 % CI 1.25–3.86, for excellent/good and for poor SRH, respectively). Nevertheless, the difference in the mortality risk between these two later groups is not statistically significant (p = 0.14).
Our finding that SRH was not associated with mortality among individuals with T2D contrasts with results from the nested analysis of persons with diabetes from the European Prospective Investigation into Cancer and Nutrition study. Wennberg and colleagues found that low SRH was associated with a higher risk of mortality among men, but not women, after controlling for established risk factors [23].
The increased risk of mortality among individuals with undiagnosed T2D concurs with a Danish study [24]. In this study, increased 5-year mortality was observed among individuals newly diagnosed with diabetes who rated their health as less than excellent, compared to those who rated their health as excellent [24].
In the present study, those with prediabetes rated their health similarly as did those with normoglycemia, and those with undiagnosed diabetes rated their health worse, yet better than did those with diabetes. These findings concur with results of a Swedish study that showed poor SRH to be associated with increased glucometabolic disturbance [25], as well as with an earlier Israeli study that showed higher SRH among individuals with undetected than known diabetes [26]. Analysis of the 4770 midlife adults participating in the US Health and Retirement study found SRH to be a significant predictor of onset of chronic conditions including diabetes [27]. However, a recently published population study reported poorer SRH among people with known hypothyroidism, diabetes, and hypertension than among people who were not aware that they had these conditions [28].
The outcome in the current study was all-cause mortality, and not diabetes-related or cardiovascular mortality. We were thus not able to investigate whether the association between diabetes and all-cause mortality is attributed to cardiovascular mortality. An analysis based on 700,000 participants of the American National Health Interview Survey, followed for up to 20 years, showed SRH to strongly predict death from diabetes [29]. More recently, a systematic review and meta-analysis reported an association between poor SRH and cardiovascular mortality in populations with and without prior cardiovascular disease [30].
No association was found between the two glycemic groups: prediabetes and undiagnosed diabetes, and mortality. The category of prediabetes in the current study included both impaired fasting glucose (IFG: defined as 100–125 mg/dl) and impaired glucose tolerance (IGT). This finding is in line with a study that used the same definition and found IFG not to be associated with increased mortality [31]. Another study that used this broader classification of IFG reported an increased risk of mortality in a non-obese population only [32].
We found a positive association between diabetes morbidity and all-cause 14-year mortality, as was already well documented in the literature. In the present study, diabetic individuals were divided according to the duration of their illness, where known diabetics are most probably of longer duration, and those who were undiagnosed prior to the current study were incident diabetics, probably with a shorter duration of the disease. In this elderly cohort, SRH was a significant and independent predictor of mortality, after controlling for anthropometrics, lifestyle habits, sociodemographic characteristics, and comorbidities, concurring with previous studies. The lack of association found in our study between SRH and mortality among the known diabetic patients may be due to the presence of other illness complications or other residual confounding factors not measured in this study, such as cognitive decline. Possible other explanations may lie in that self-perception of general health in long-standing diabetics may reflect to a lesser extent the true risk of mortality.
This study has a number of limitations. The inclusion of only 60 % of the target cohort may have introduced a selection bias. Nevertheless, when analyzing the 177 individuals excluded (due to lack of laboratory test results for blood glucose, consequent to the acquisition of information by telephone interview), the same association between SRH and vital status was observed as for the study sample (46 % and 47 % of those with poor SRH died by the end of follow-up, respectively), minimizing concerns for selection bias.
A 100 g oral glucose load was used in this study, rather than the recommended 75 g, due to its greater stimulation of insulin response [33]. The same protocol was applied for comparability with tests performed at previous time points of the 25-year follow-up of this cohort [21]. Differences in glucose loads have been shown to have little effect on blood glucose levels [33]. Nevertheless, data from the DECODE study group regarding prevalence of undiagnosed T2D, using standard 75 g glucose OGTT protocols, were similar to ours [34].
There are some strengths to be mentioned regarding our study: the validation of the exposure to baseline glycemic group in all included participants by laboratory testing, assuring high validity of exposure information. In addition, there was no loss to follow-up for the outcome of mortality in our study, minimizing concerns for information bias.
In summary, this study, of elderly men and women, found an interaction, demonstrating that SRH associated with mortality in all glycemic groups except for individuals with known diabetes. Thus, although SRH assessment is an inexpensive screening tool, it should be used cautiously when evaluating an elderly diabetic patient. Further research should investigate the perception of health and health-related behaviors of patients diagnosed with diabetes.
References
Mavaddat, N., Parker, R. A., Sanderson, S., Mant, J., & Kinmonth, A. L. (2014). Relationship of self-rated health with fatal and non-fatal outcomes in cardiovascular disease: A systematic review and meta-analysis. PLoS ONE, 9(7), e103509.
Okamoto, K., Momose, Y., Fujino, A., & Osawa, Y. (2008). Gender differences in the relationship between self-rated health (SRH) and 6-year mortality risks among the elderly in Japan. Archives of Gerontology and Geriatrics, 47, 311–317.
McEwen, L. N., Kim, C., Haan, M. N., Ghosh, D., Lantz, P. M., Thompson, T. J., & Herman, W. H. (2009). Are health-related quality-of-life and self-rated health associated with mortality? Insights from Translating Research Into Action for Diabetes (TRIAD). Primary Care Diabetes, 3, 37–42.
Bopp, M., Braun, J., Gutzwiller, F., & Faeh, D. (2012). Swiss National Cohort Study Group. Health risk or resource? Gradual and independent association between self-rated health and mortality persists over 30 years. PLoS ONE, 7, e30795.
Giltay, E. J., Vollaard, A. M., & Kromhout, D. (2012). Netherlands self-rated health and physician-rated health as independent predictors of mortality in elderly men. Age and Ageing, 41, 165–171.
Lima-Costa, M. F., Cesar, C. C., Chor, D., & Proietti, F. A. (2012). Self-rated health compared with objectively measured health status as a tool for mortality risk screening in older adults: 10-year follow-up of the Bambuí Cohort Study of Aging. American Journal of Epidemiology, 175, 228–235.
Halford, C., Wallman, T., Welin, L., Rosengren, A., Bardel, A., Johansson, S., et al. (2012). Effects of self-rated health on sick leave, disability pension, hospital admissions and mortality. A population-based longitudinal study of nearly 15,000 observations among Swedish women and men. BMC Public Health, 12, 1103.
Tamayo-Fonseca, N., Quesada, J. A., Nolasco, A., Melchor, I., Moncho, J., Pereyra-Zamora, P., et al. (2013). Self-rated health and mortality: A follow-up study of a Spanish population. Public Health, 127, 1097–1104.
Berger, N., Van der Heyden, J., & Van Oyen, H. (2015). The global activity limitation indicator and self-rated health: Two complementary predictors of mortality. Archives of Public Health, 73(1), 25.
Desalvo, K. B., & Muntner, P. (2011). Discordance between physician and patient self-rated health and all-cause mortality. The Ochsner Journal, 11, 232–240.
Benyamini, Y., Blumstein, T., Lusky, A., & Modan, B. (2003). Gender differences in the self-rated health-mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? Gerontologist, 43, 396.
Henry, P., Thomas, F., Benetos, A., & Guize, L. (2002). Impaired fasting glucose, blood pressure and cardiovascular disease mortality. Hypertension, 40, 458–463.
Wen, C. P., Cheng, T. Y., Tsai, S. P., Hsu, H. L., & Wang, S. L. (2005). Increased mortality risks of pre-diabetes (impaired fasting glucose) in Taiwan. Diabetes Care, 28, 2756–2761.
McMaster University Evidence Based Practice Center. (2014). Diagnosis, prognosis and treatment of impaired glucose tolerance and impaired fasting glucose. Evidence Report 128. http://archive.ahrq.gov/downloads/pub/evidence/pdf/impglucose/impglucose.pdf. Accessed May 23, 2014.
Barr, E. L., Zimmet, P. Z., Welborn, T. A., Jolley, D., Magliano, D. J., Dunstan, D. W., et al. (2007). Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation, 116, 151–157.
Huang, Y., Cai, X., Chen, P., Mai, W., Tang, H., Huang, Y., & Hu, Y. (2014). Associations of prediabetes with all-cause and cardiovascular mortality: A meta-analysis. Annals of Medicine, 46(8), 684–692.
Trivin, C., Metzger, M., Haymann, J. P., Boffa, J. J., Flamant, M., Vrtovsnik, F., et al. (2015). Glycated hemoglobin level and mortality in a nondiabetic population with CKD. Clinical Journal of the American Society of Nephrology, 10(6), 957–964.
Hiltunen, L. (2005). Ten-year mortality and glucose tolerance status in an elderly Finnish population. Diabetes Research and Clinical Practice, 69, 81–87.
Wennberg, P., Rolandsson, O., Van der A, D. L., Spijkerman, A. M., Kaaks, R., Boeing, H., et al. (2013). Self-rated health and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition-InterAct study: A case-cohort study. BMJ Open, 3, e002436.
Andersson, S., Ekman, I., Friberg, F., Daka, B., Lindblad, U., & Larsson, C. A. (2013). The association between self-rated health and impaired glucose tolerance in Swedish adults: A cross-sectional study. Scandinavian Journal of Primary Health Care, 31, 111–118.
Modan, M., Halkin, H., Almog, S., Lusky, A., Eshkol, A., Shefi, M., et al. (1985). Hyperinsulinemia: A link between hypertension obesity and glucose intolerance. Journal of Clinical Investigation, 1985(75), 809–817.
Knol, M. J., & VanderWeele, T. J. (2012). Recommendations for presenting analyses of effect modification and interaction. International Journal of Epidemiology, 41, 514–520. doi:10.1093/ije/dyr218.
Wennberg, P., Rolandsson, O., Jerdén, L., Boeing, H., Sluik, D., Kaaks, R., et al. (2012). Self-rated health and mortality in individuals with diabetes mellitus: Prospective cohort study. BMJ Open, 2, e000760.
de Fine Olivarius, N., Siersma, V., Nielsen, A. B., Hansen, L. J., Rosenvinge, L., & Mogensen, C. E. (2010). Predictors of mortality of patients newly diagnosed with clinical type 2 diabetes: A 5-year follow up study. BMC Endocrine Disorders, 10, 14.
Leosdottir, M., Willenheimer, R., Persson, M., & Nilsson, P. M. (2011). The association between glucometabolic disturbances, traditional cardiovascular risk factors and self-rated health by age and gender: A cross-sectional analysis within the Malmö Preventive Project. Cardiovasc Diabetol, 10, 118.
Dankner, R., Geulayov, G., Olmer, L., & Kaplan, G. (2009). Undetected type 2 diabetes in older adults. Age and Ageing, 38(1), 56–62.
Latham, K., & Peek, C. W. (2013). Self-rated health and morbidity onset among late midlife U.S. adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 68, 107–116.
Jørgensen, P., Langhammer, A., Krokstad, S., & Forsmo, S. (2015). Diagnostic labelling influences self-rated health. A prospective cohort study: The HUNT Study, Norway. Family Practice, 32(5), 492–499.
Benjamins, M. R., Hummer, R. A., Eberstein, I. W., & Nam, C. B. (2004). Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Social Science and Medicine, 59, 1297–1306.
Mavaddat, N., Parker, R. A., Sanderson, S., Mant, J., & Kinmonth, A. L. (2014). Relationship of self-rated health with fatal and non-fatal outcomes in cardiovascular disease: A systematic review and meta-analysis. PLoS ONE, 9, e103509.
Deedwania, P., Patel, K., Fonarow, G. C., Desai, R. V., Zhang, Y., Feller, M. A., et al. (2013). Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: Findings from a population-based cohort study. International Journal of Cardiology, 168, 3616–3622.
Shah, R. V., Abbasi, S. A., Yamal, J. M., Davis, B. R., Barzilay, J., Einhorn, P. T., et al. (2014). Impaired fasting glucose and body mass index as determinants of mortality in ALLHAT: Is the obesity paradox real? The Journal of Clinical Hypertension (Greenwich), 16, 451–458.
Cerasi, E., Ependic, S., & Luft, R. (1973). Dose-response relation between plasma-insulin and blood-glucose levels during oral glucose loads in prediabetic and diabetic subjects. Lancet, 1, 794–797.
DECODE Study Group. (2003). Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care, 26, 61–69.
Acknowledgments
This work was supported by the Sapir Pais Foundation and Israeli Ministry of Health.
Author contributions
Dankner was responsible for study concept and design. Dankner and Chetrit were responsible for acquisition of subjects and data. Dankner, Chetrit, Olmer, and Kaplan participated in analysis and interpretation of data and preparation of the manuscript.
Sponsor’s role
The sponsor had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dankner, Chetrit, Olmer, and Kaplan have no conflict of interest to disclose with the supporting institutions.
Informed consent
This study was approved by the institutional review board. All participants signed an informed consent form.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dankner, R., Olmer, L., Kaplan, G. et al. The joint association of self-rated health and diabetes status on 14-year mortality in elderly men and women. Qual Life Res 25, 2889–2896 (2016). https://doi.org/10.1007/s11136-016-1291-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1291-9