Abstract
Purpose
The Alcohol Quality of Life Scale (AQoLS) is a new patient-reported outcome 34-item questionnaire measuring health-related quality of life (HRQOL), specific to patients with an alcohol use disorder, developed from the patients’ perspective. This is the first report establishing evidence in support of measurement reliability and validity of the AQoLS.
Methods
A total of 285 randomly selected patients receiving interventions for alcohol use disorder in addiction specialised care settings in France were included in the study (response rate 80.1 %). Exploratory factor analysis was conducted to evaluate the hypothesised-during-development-stage dimensional structure of the AQoLS. Internal consistency of the total score and the dimensions subscores were assessed through Cronbach’s alpha coefficients. Construct validity was tested through correlations with the Short-Form 36 Health Survey (SF-36) and EuroQol 5 dimensions (EQ-5D).
Results
Exploratory analysis indicated seven observed dimensions which differed slightly from the 7 dimensions defined a priori in the framework hypothesised during the scale development: activities, relationships, living conditions, negative emotions, self-esteem, control and sleep. A major common factor allows the summing of the 34 items to obtain a total score. All the 34 items were acceptable. Cronbach’s alpha for the AQoLS total score was 0.96 and ranged from 0.8 to 0.9 for the dimensions subscores. Negative correlations between AQoLS and all dimensions of the SF-36, but general health and positive correlations between AQoLS and all items of the EQ-5D were shown. As expected, the correlations were mostly moderate in magnitude, low with scores referring to physical areas and the highest with the SF-36 MSC.
Conclusion
This study provides evidence of the measure’s psychometric properties in terms of construct validity and internal consistency. The “control” and “self-esteem” dimensions are of particular interest as these concepts are not captured in existing HRQOL. Further longitudinal validation of the scale is necessary to assess sensitivity to change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol use disorders (AUD) have the widest treatment gap (78.1 %) among psychiatric disorders worldwide [1]. This could be partially explained by the fear to be proposed an abstinence-oriented treatment [2]. These findings contributed to revisit the acceptability of strategies relying on reduction of alcohol consumption, supported by the recent modelling of harm reduction linked to reduction of alcohol consumption [3, 4]. This context, along with the recent first European approval of a pharmaceutical drug to reduce alcohol consumption in alcohol-dependent patients [3, 5, 6], highlights the need for non-drinking outcomes that could assess the efficacy of both abstinent and non-abstinent targeted interventions and allow comparison between them.
Moreover, these evolutions can be considered as part of the more global development of patient-centred approaches, considering the drinking goal as only a surrogate endpoint of the patient global state improvement, that could be preferably measured by non-drinking outcomes [7]. Even if alcohol consumption measures are adopted, its assessment can be difficult, with variations in reporting of alcohol consumed leading to different interpretations, especially when categorical variables are used and when the goal of treatment is not abstinence [8]. Categorical use could report with difficulty the nonlinear change in drinking patterns. The nonlinear change in drinking pattern has been recently taken into account by the Food and Drug Administration recommending a grace period when using categorical variables [9]. The use of continuous rather than categorical measures of alcohol consumption could increase the precision of the measurement and thus the likelihood of finding true and relevant changes, even if they are not consensual [10]. A recent review of alcohol outcome studies noted these difficulties, reporting that almost a quarter of reviewed studies failed to quantitatively assess alcohol use, the primary dependent variable, making it difficult or impossible to evaluate substance use changes pre- and post-intervention [10].
Recently, several non-drinking qualitative concepts have emerged to measure patients’ clinical states and outcomes: negatively related consequences, craving and health-related quality of life (HRQOL). The concept of negative alcohol-related consequences has the disadvantage of coming mostly from the expert’s point of view [11]. Craving has shown complexity in its assessment because of its moving nature, and conceptual and practical issues regarding its measurement are still debated [12].
Health-related quality of life (HRQOL) is a non-drinking outcome consensually recognised in the AUD field as well as in evidence-based medicine as a whole [13–15]. HRQOL could be particularly valuable to assess the benefit of treatment, especially in non-abstinent targeted interventions [16]. HRQOL reflects patients’ feelings and functioning and the impact of their health condition beyond simple symptom assessment [17]. Classically, HRQOL involves four domains: (1) physical state, including autonomy and physical abilities; (2) physical well-being, including pain and physical symptoms; (3) psychological state, including anxiety and depression; and (4) social relationships including the domains of family, friends and work [17]. However, most generic scales, such as the SF-36 and the EQ-5D, have not been developed from the patients’ but from the experts’ perspective; most of them have been developed primarily for use among patients with somatic diseases such as cardiovascular disorders or cancer, who experience different symptoms from those experienced in AUD [18, 19]. Moreover, as generic instruments are intended for use across a range of diseases, they are less able to capture the specific manifestations of a given disease, particularly complex disorders such as AUD. For example, they largely explore physical areas, such as self-reliance and pain, which do not seem the most relevant ones in AUD [20]. Although HRQOL is increasingly assessed in clinical trials in AUD, some generic scales could fail in exploring some specific relevant information in these patients [20]. For example, a systematic review of quality of life instrument used in clinical trials in alcohol dependence has shown that generic instruments often fail to show a difference between treatment groups, even where the efficacy of the experimental intervention on a drinking outcome or other outcomes has been demonstrated [20]. Similarly, social costs of AUD could be underestimated [21], in part due to the lack of relevant specific scales assessing important aspects of quality of life in this disorder. Until recently, no quality of life instrument had been developed specifically for patients with AUD, leaving some potentially relevant areas unexplored.
In order to respond to these methodological, clinical and epidemiological issues, we developed the first patient-reported outcome (PRO) measure of HRQOL, specific to patients with AUDs, the Alcohol Quality of Life Scale (AQoLS) [22]. The AQoLS has been developed from the patients’ perspective, using focus groups and individual interviews with subjects with current or remitted AUD in France and the UK. The development followed industry-recognised standards for patient-reported outcome instruments used in clinical trials [23], including an iterative process for deriving the underlying conceptual framework and extensive patient input throughout the development process to ensure content validity. This methodology ensures that the AQoLS reflects patients’ concerns and can be used to explore the global impact of AUD on patients’ HRQOL. The methodology employed for the development of the scale development is described in detail elsewhere [24]. Briefly, an initial, conceptual framework for the AQoLS was developed from a previous systematic review of HRQOL instruments conducted by Luquiens et al. [10]. A discussion guide for the running of the focus groups relied on this framework to help exploration of potential HRQOL concepts in the concept elicitation stage. To reduce country-specific characteristic bias of the new measure, all developmental stages of the AQoLS were conducted simultaneously in the UK (UK) and France. The content was developed from the qualitative analysis of focus groups in the UK and France involving 38 English and French patients with current or remitted AUD of varying levels of severity. Patients were asked to share the impact that alcohol had had or was having on their life. The sessions were transcribed, a thematic analysis was used to identify key areas of impact of AUD, and draft items were developed to capture these issues. The draft items of the AQoLS underwent expert review to ensure clinical and cross-cultural applicability. AQoLS has previously shown good face and content validity through two iterative rounds of cognitive debriefing interviews conducted with 31 patients with current or remitted AUD in both countries [24].
This is the first report establishing evidence in support of measurement reliability and validity of the AQoLS.
Methods
Setting and patient sample
This validation study is part of a larger survey taking place in eight European countries, including France [25, 26].The study is a personal interview-based investigation among patients with AUD aged 18–64 years to gain an understanding of the medical needs in this population. The study presented in this article is a nested-study aiming to validate the French version of the AQoLS.
Patients were recruited in French addiction specialised care settings. Six centres were randomly selected from a register of 161 addiction specialised care sites across the country. The random sampling method was chosen to enhance the representativeness of our sample in order to reflect the treatment system for AUD in France. A total of 285 outpatients currently treated for an AUD were included in the study. All adult subjects aged 18–64 years in the specialised care settings who had AUD (either current or remitted) and who were undergoing treatment for their disorder were eligible to be part of the study. The study was proposed systematically to each patient attending a medical appointment in the investigation site on a preselected day and treated for AUD. The included patients were then proposed an additional appointment to complete the study assessments. Patients signed a written and informed consent form after having being explained the purpose of the study. The study met the French requirements for observational studies and was approved by the Comité Consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé (CCTIRS) on 24 April 2015, Number 13.222.
Assessments
Patient demographics and disease state
Sociodemographic characteristics were collected from patients, including age, gender and employment. Patients also completed the AUDs Identification Test Consumption (AUDIT-C), a brief screening questionnaire which reliably identifies patients who have current AUD [27] (range 0–12). A score ≥4 (men) or 3 (women) is considered as a proxy for AUD [28].
AQoLS
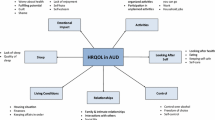
Patients included in the validation study self-completed the AQoLS on a single occasion in the health care setting, during a dedicated appointment, without assistance with completion of the questionnaire. The AQoLS is a self-completed questionnaire composed of 34 items and 7 a priori dimensions hypothesised during the development stage: activities (items 1–6), relationships (items 7–14), living conditions (items 15–18), negative emotions (items 19–23), looking after self (items 24–27), control (items 28–32) and sleep (items 33–34). A 4-point Likert-type response scale [“not at all” (0), “a little” (1), “quite a lot” (2) and “very much” (3)] was selected to balance responder burden and the potential measurement sensitivity of the items. The recall period is 4 weeks.
Additional HRQOL scales
Additional HRQOL scales were amended to the clinical research form after the beginning of the inclusion period. Inclusions were ended up in 3 of 6 investigation centres at the amendment time. All 60 remaining patients to be included in the three other centres systematically self-completed the additional HRQOL scales (SF-36 and EQ-5D) in addition to AQoLS, during the same dedicated interview as mentioned above. Characteristics of this subsample of patients were described.
The Short-Form 36 Health Survey (SF-36) was derived from the Medical Outcome Study [29], an observational study that began in 1986 among subjects with cardiac impairment. It is a 36-item questionnaire that includes eight dimensions: physical functioning, physical role limitation, emotional role limitation, bodily pain, mental health, social functioning, vitality and general health perception. It also includes two component summaries: mental (MCS) and physical (PCS). It was validated in a French version among 147 alcohol-dependent patients. In this population, test–retest intraclass coefficients for a 0–10-day interval were in the range 0.65–0.79, whereas the Cronbach’s alpha coefficient indicated good internal consistency (range 0.70–0.89) [30].
The EuroQol 5 dimensions (EQ-5D) is a generic, preference-based instrument for describing and assessing HRQOL in terms of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension comprises three levels, resulting in a total of 243 unique health states. Among 52 alcohol-dependent patients, it showed only trivial to medium responsiveness at 18 months [31] and probably requires large patient samples in order to detect meaningful clinical differences. The EQ-5D has not been explicitly tested for measurement properties in individuals with AUD in any language. The standardised extended version of EQ-5D was designed for the collection of health state values using a VAS rating scale—a vertical 20-cm visual analogue scale with the endpoints labelled best imaginable health state at the top and worst imaginable health state at the bottom having numeric values of 100 and 0, respectively.
Statistical analysis
Descriptive analysis of the population demographics and disease severity was performed.
As content and face validities were documented in a previous publication, we report here, according to the COSMIN checklist with a 4-point scale [32]: internal consistency, structural validity and construct validity and hypothesis testing.
The total score was obtained by summing all items, and the theoretical range was therefore 0–102; method to assess the appropriateness of calculating a total score is described below. The AQoLS total score and domain scores were described in the study population. Distribution of responses per item was studied to assess acceptability and upper and lower limit effects of the scale.
Validity
Structural validity
Only patients who had completed all items of AQoLS were included in the structural validity analysis. There was no data imputation. AQoLS is based on a reflective model. Exploratory factor analysis was conducted to explore the dimensional structure of the AQoLS. The optimal number of factors was identified from a preliminary principal component analysis, using Cattell’s scree plot inspection for the point of inflexion [33]. A scree plot is the plotting of the eigenvalues of the correlation matrix in decreasing order, with the components as the X-axis and the corresponding eigenvalues as the Y-axis. A first substantial dimension on the plot would support graphically the appropriateness of calculating a total score summing all the items. A factor analysis with varimax rotation was performed with the number of factors identified from the principal component analysis. Items were attributed to the dimension for which they had the highest loads; exceptions were: (1) when two loads were close, the a priori dimension could be kept, or (2) when several loads were close, the item could be attributed to the more relevant dimension.
Item–dimension correlations were calculating, omitting the item from its dimension, in order to avoid artificially inflated correlation (Spearman test) [34].
Construct validity and hypothesis testing
For assessment of construct validity, Spearman correlations between the AQoLS scores and EQ-5D dimension scores (i.e. item level scores), EQ-5D health state score, and SF-36 dimension and summary scores were calculated. We are interested in analysing the detailed content of EQ-5D, as our scale is challenging the relevance of this scale in our population of interest. As level of agreement between EQ-5D items and another health-related quality of life scale (i.e. SF-36) has already been reported, we chose to focus on the item level scores [35]. Given that an increase in AQoLS or EQ-5D scores reflects a worse quality of life, while an increase in SF-36 reflects a better quality of life, we hypothesised a negative correlation between AQoLS total score and SF-36 scores and positive correlation between AQoLS total score and EQ-5D items scores. We expected a moderate correlation between AQoLS total score and the role functioning/emotional, energy/fatigue, emotional well-being, social functioning, general health, mental component summary scores (MCS) of the SF-36, and between AQoLS total score and activity, anxiety scores and health state visual analogue index of EQ-5D, but lower correlation between AQoLS total score and dimensions reporting exclusively physical areas, namely: physical functioning, physical role limitation, bodily pain and physical component summary (PCS) of SF-36, and mobility, self-care, pain/discomfort of EQ-5D.
Internal consistency
We believe that a total score could be useful to assess interventions efficacy in alcohol use disorder and that subscores could also inform more precisely on efficacy of targeted interventions on a particular domain and could be particularly interesting in assessing targeted interventions. Therefore, internal consistency was assessed for each dimension of the AQoLS and for the total score using Cronbach’s coefficient alpha.
All the analyses were performed using R 3.0.3 software.
Results
Patient sample
A total of 285 French patients treated for an AUD in a specialised care setting were included in this analysis. This sample size fits with the current guidelines regarding validation of scales [36]. Sixty per cent of all specialised care facilities contacted via phone refused to be part in the study because they refrain from studies in general. Near 20 % of patients refused to participate because lack of time and reluctance to attend an additional appointment for the study completion (Fig. 1). The sample was composed of 65 % male patients. Mean age was 48 years. This demographic profile is close to the one usually observed in the selected settings. Mean AUDIT-C score was 6. All women and all men had an AUDIT-C score above 3 or 4 respectfully, considered positive for the current year. Unemployment due to medical or other reasons was observed in 33 % of the included patients. The subsample of patients who completed the other HRQOL scale had very similar characteristics: 65 % male patients, mean age 50, mean AUDIT-C score was 6, all patients had an AUDIT-C score above 3 (female) or 4 (male), and unemployment rate was 30 %.
AQoLS score distribution
An AQoLS total score could only be calculated in 236 of the 285 patients (82 %) due to missing data. The missing data rate for each item varied from 2 to 4 %. All items were, therefore, well accepted by patients. The total range of responses from 0 to 3 was used for each item of the AQoLS, indicating no lower or upper limit effect. The AQoLS total mean score was 45.3 (SD 22.1), with a range 0–90 (0–102 is the maximum possible score range) Score 0 was obtained for 4 % of the completers (n = 9), and score 102 was obtained for 0 % of the completers (n = 0) (Fig. 2; Table 1).
Validity
Structural validity
The question of dimensionality of the construct is addressed in two different ways. These two steps are based on exploratory analysis. First, a scree plot is shown (Fig. 3). The preliminary principal component analysis indicated a substantive principal dimension accounting for 42 % of the variance, thus indicating the appropriateness of a total AQoLS score summing the items. Seven dimensions were graphically identified before the point of inflexion, accounting for a total of 60 % of the variance.
Table 2 presents the 7-factor solution from the factor analysis with varimax rotation. Twenty-one of the 34 items had the highest loading on their a priori dimension. Several items presented loadings close in two dimensions; we chose to include them in the dimension which seemed more meaningful, even if the loading was lower in the corresponding dimension (differences in loading between the highest and the chosen dimension <0.11). A minimum loading of 0.5 is often recommended when exploring item component or dimension loading [37]. Items with lower loadings were identified and considered for rejection. Five items (3, 7, 13, 19, 25) demonstrated low value loadings across more than one dimension (range 0.26–0.47: 3—restricted in places; 7—cut myself off; 13—sex; 25—appearance; 19—shame). However, these items were considered important to patients and they have high clinical relevance and do not overlap with the other items; therefore they were retained in the AQoLS. Moreover, missing data rates for these items are similar to the other items (n = 11, 9, 12, 9, 8, ≤ 4 % for all five items).
-
Two a priori dimensions have been fully confirmed: “control”, and sleep with all items having the highest loading on these factors.
-
The loadings of some items differed from the a priori structure.
Specifically, four dimensions were partially confirmed. The “negative emotions” dimension grouped the two items related to worry. Three other items were a posteriori related to the “activities” dimension: item 15—household affairs, item 25—appearance and item 26—general health. One was removed from “activities” to “relationships”: item 1—everyday activities. Two items were a posteriori related to the “relationship” dimension: item 1—everyday activities and item 27—risky situations. Item 24—appetite was a posteriori related to the “living conditions” dimension.
-
Finally, the a priori dimension “looking after self” was not observed. Respective items were related to “living condition” (item 24—appetite), “activities” (items 25—appearance and item 26—general health) and “relationships” (item 27—risky situations).
A posteriori, a seventh dimension could be described and labelled “self-esteem”, grouping the following items: item 12—trust, item 14—friends, item 19—shame, item 20—contempt and item 21—wasting my life.
Construct validity
Table 3 shows the mean scores on the SF-36 and EQ-5D. As explained in the methods section, in both cases, the sample size is smaller due to the inclusion of the scales in the clinical research form after the beginning of the inclusions (n = 60).
Missing data per item was ≤7 % for all SF-36 and EQ-5D items except for 5 of the 36 SF-36 items: 4.1, 5.1, 5.2, 9b and 11d (range of missing data 8–11 %). Acceptability of these two scales’ items was then lower than AQoLS’ items acceptability.
As hypothesised and given that an increase in AQoLS or EQ-5D scores reflect a worse quality of life, while an increase in SF-36 reflects a better quality of life, we showed a negative correlation between AQoLS total score and SF-36 scores and positive correlation between AQoLS total score and EQ-5D items scores. Only the SF-36 general health subscore was positively correlated to AQoLS total score, reflecting that an improvement of general health assessed with EQ-5D was correlated to a worsening of quality of life assessed with the AQoLS. As hypothesised, moderate correlations were found between the AQoLS total score and role functioning/emotional dimension, energy/fatigue dimension, emotional well-being dimension of the SF-36 and ranged from 0.4 (emotional well-being) to 0.6 (energy/fatigue). Similarly and as expected, MCS was highly correlated with AQoLS total score (r = 0.7), and PCS correlation magnitude with AQoLS total score was very low (r = 0.1). In return, correlations between AQoLS total score, social functioning and general health dimensions were lower than hypothesised (respectively, r magnitude = −0.2 and 0.3) and correlations with some dimensions exploring SF-36 physical areas of the SF-36 (i.e. physical functioning, role functioning/physical and pain) were higher than hypothesised, even if they remained moderate (r = −0.5).
Regarding EQ-5D, as hypothesised, moderate correlations were found between AQoLS total score and activity and anxiety items (r = 0.5) and low correlations were found between AQoLS total score and pain and self-care items (magnitude ≤0.2). In return, correlation between AQoLS and health state visual analogue scale of the EQ-5D was low (0.2), and correlation between AQoLS total score and mobility item was moderate (0.4).
Internal consistency
Cronbach’s coefficient alpha for the AQoLS total score was 0.96, which shows excellent internal consistency. Cronbach’s coefficients for the seven a posteriori dimensions ranged from 0.8 to 0.9.
Item–dimension correlations
Item–dimension correlations ranged 0.15 (item 8—“negative emotions”) to 0.83 (items 33 and 34—“sleep”). Spearman correlation coefficients between items and dimensions of the AQoLS showed the largest correlation coefficient for each item with its a posteriori corresponding dimension, except for items 14 (relationships) and 24 (sleep).
Discussion
This validation study of the AQoLS shows a 7-dimension structure of the AQoLS, with 34 acceptable items, in a population of French patients with current or remitted AUD. Our data indicated 7 observed dimensions which differed slightly from the 7 dimensions defined a priori: “activities”, “relationships”, “living conditions”, “negative emotions”, “self-esteem”, “control” and “sleep”. Nearly two-third of the items loaded on their a priori dimension, and the others were redistributed on the others a posteriori dimensions, leading in particular to the emergence of the “self-esteem” dimension. Future use of this scale will clarify its behaviour and on the clinical relevance of dimensions’ subscores. The structure analysis with a major dimension allows the summing of the 34 items to obtain a total score. The AQoLS showed excellent internal consistency.
Methodological quality of reporting of internal consistency, structural validity and hypothesis testing including convergent validity was excellent according to the COSMIN checklist [38].
Two unexplored dimensions
Two dimensions, control and self-esteem, are of particular interest because these aspects of patient HRQOL have been unexplored to date by the HRQOL instruments previously used in patients with AUD.
Control was an a priori dimension emerging from the content analysis of the focus groups during the AQoLS development. Control has high clinical relevance in addiction field: “loss of control” is a hallmark of addictions [39, 40]. Addiction has been described as a loss of control of drug intake, resulting from impaired prefrontal cortex function and long-term drug exposure [41]. Control has also been described as the core cognitive dysfunction in addictive behaviours [42–44]. Although instruments assessing drinking-related control exist [45], control is not currently included in HRQOL instruments as a separate dimension. The advantage of the AQoLS is that it was developed using patients’ input, which allows the inclusion of concepts identified by patients themselves as dimensions of HRQOL and which have previously only been explored from the expert point of view. Control is no longer only a symptom described from the expert perspective, or a prognostic factor, but a difficulty reported by patients as having a significant impact on their quality of life, which they were willing to improve.
Similarly, instruments that assess self-esteem are available; the most commonly used being the Rosenberg Self-Esteem Scale [46]. Self-esteem has recently been shown to be lower in alcohol-dependent patients than in healthy controls [47]. Moreover, self-esteem has been shown to be significantly associated with non-HRQOL, using the World Health Organisation Quality of Life-BREF measure, among remitted alcohol-dependent patients [48]. Self-esteem has previously been described as a dimension of a non-HRQOL instrument in the Life Situation Survey [49], which was later validated in a population of alcohol-dependent patients [50]. However, general non-HRQOL instruments, such as the Life Situation Survey, are reputed to be less robust than HRQOL ones, and their use as outcomes in clinical trials are not recommended. Self-esteem has also been previously included as a dimension in a HRQOL instrument designed for use among children, the German generic quality of life instrument for children (the KINDL) [51], but, to our knowledge, not in any HRQOL used among patients with AUD. Quotations from patients’ from one of the focus groups consulted in the development of the AQoLS included: “I got a quarter bottle of vodka and just necked it. Afterwards I felt guilty that I’d actually done it because I’d been actually trying to prove to myself and be strong for myself that I can do this. And when I had that relapse I felt really dirty on myself that I’d done it.” [United Kingdom-Focus Group 3_Remitted AUD]. This quotation illustrates how harsh patients were about themselves and how low their self-esteem was. Patients included these feelings as part of their impacted HRQOL and noticed self-esteem to be sensitive to change over the course of the disease. Assessing self-esteem in HRQOL could be an informative outcome in patients with AUD, reflecting patients’ concerns. It thus seems relevant to include self-esteem as a distinct dimension of HRQOL in the AQoLS, even though not defined a priori.
These newly identified domains in measuring and monitoring HRQOL during assessment and treatment can add important value to patient recovery [52]. The patient-centred approach adopted throughout the development process of AQoLS could contribute to the recognition of the importance of the patient perspective in the treatment and outcome assessment in AUD, and help avoid a paternalistic style of health care, thereby reducing the residual stigma still impacting the health care system in this field [53].
Construct validity
Support for the construct validity of the AQoLS was gained through negative correlations between AQoLS and all dimensions of the SF-36 but general health and positive correlations between AQoLS and all items of the EQ-5D. As expected, the correlations were mostly moderate in magnitude, low with scores referring to physical areas and the highest with the SF-36 MSC. This suggests that the AQoLS and traditional other HRQOL instruments are not superimposed. This could be explained by the totally different methods in item generation between AQoLS-patient-centred and the other scales, which have not benefitted from patients’ input in their development process [19]. Particularly, we observed a low correlation between AQoLS and the social functioning subscore of SF-36 despite a relationship dimension in AQoLS. The difference in instructions, namely “your physical health or emotional problems” in the SF-36 and “your relationship with alcohol” in the AQoLS, could explain this discrepancy: knowing that alcohol dependence is a highly self-stigmatised disorder [53], some patients could not consider that their relation to alcohol is not part of their physical or mental health. Another lead is that the explicit instruction regarding alcohol helps patients in identifying the impacted domains. As hypothesised, the highest correlation was found between the AQoLS and the mental summary score of the SF-36, which is the score that most often demonstrates improvement in AUD clinical trials [20]. This is an encouraging argument to support a possible sensitivity to change of the AQoLS in this population. Even if patients with alcohol use disorders usually consider their condition as more psychological than physical [30], the moderate correlation with some subscores of the SF-36 and items of the EQ-5D exploring physical areas could be explained by the fact that mental distress can impact physical well-being, for instance in major depressive disorders [54]. However, no question explored precisely these physical areas. More surprisingly, the SF-36 general health subscore was positively (i.e. inversely) correlated to AQoLS. This could be due to a response shift of some severe patients on their general health assessment [55]; a response shift in health-related quality of life measurements refers to a change in the meaning of one’s self-evaluation of a target construct as a result of a change in the respondent’s internal standards of measurement (scale recalibration, in psychometric terms). This can be illustrated by the fact that people with a severe chronic illness report a level of quality of life neither inferior nor better than that of less severely ill patients or healthy people, as measured by some generic health-related quality of life instruments [56]. Construct validity was explored in a smaller sample (n = 60), and even if significance is not the issue of interest in construct validity, results are to be taken with caution due to possible lack of power.
Limitations
Representativeness of the sample is limited due to the refusal to participate of same randomly selected centres. The major limitation of this validation study is that it is cross-sectional and does not allow testing of the instrument’s responsiveness. Therefore, further dynamic validation of the scale is planned. The construct validity of the scale was evaluated in a restricted sample size due to the inclusion in the clinical research form of the other HRQOL scales in a second step, and the comparison of the AQoLS with other related concepts like alcohol-related consequences should be undertaken. The AQoLS was validated using the French version of the scale. A validation of the English version should be performed in the future; however, the simultaneous development of the scale in French and English means that the properties of the two versions are likely to be similar. The AQoLS has also been adapted for use in South Korea, Japan and China [57], as well as Germany, and validations of these language versions are planned. Finally, the length of the scale could limit its practicality; moreover, Cronbach’s alpha >0.9 could translate redundancy in some items. The development of a short version should improve these issues and is planned.
Conclusion
The AQoLS is a new instrument to assess HRQOL among patients with AUD from the patients’ perspective and presents good psychometric properties. The scale is the only HRQOL instrument specific to patients with AUDs and was developed based on patients’ personal input. This characteristic should allow a more precise and relevant assessment of HRQOL in patients with AUD. The “control” and “self-esteem” dimensions are of particular interest as these concepts are not captured in existing HRQOL instruments used for patients with AUDs. The AQoLS can be used in the context of clinical research. It can provide information about the efficacy of therapeutic interventions regardless of the drinking goal and demonstrate the interventions’ relevance from the patients’ perspective. The development of a short version is planned. The AQoLS also could be used in pharmaco-economic analysis to refine current estimates of the societal burden of AUD. The utility of the AQoLS in routine clinical practice remains to be investigated. Future use of the AQoLS should enable further validation with respect to sensitivity to change.
References
Kohn, R., et al. (2004). The treatment gap in mental health care. Bulletin of the World Health Organization, 82(11), 858–866.
SAMHSA. (2012). Results from the 2011 National survey on drug use and health: Mental health findings. In NSDUH Series H-45. U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality.
EMA. (2010). Guideline on the development of medicinal products for the treatment of alcohol dependence. European Medicines Agency.
Rehm, J., & Roerecke, M. (2013). Reduction of drinking in problem drinkers and all-cause mortality. Alcohol and Alcoholism, 48(4), 509–513.
EMA-CHMP. (2012). Selincro Assessment report.
EuropeanMedicinesAgency. (2013). Selincro (nalmefene): EU summary of product characteristics.
Luquiens, A., & Aubin, H. J. (2014). Patient preferences and perspectives regarding reducing alcohol consumption: Role of nalmefene. Patient Prefer Adherence, 8, 1347–1352.
Sobell, L. C., et al. (2003). Assessing drinking outcomes in alcohol treatment efficacy studies: Selecting a yardstick of success. Alcoholism, Clinical and Experimental Research, 27(10), 1661–1666.
FDA. (2015). Alcoholism: Developing drugs for treatment guidance for industry draft guidance. Center for Drug Evaluation and Research (CDER).
Robinson, S. M., et al. (2014). Alcohol and drug treatment outcome studies: New methodological review (2005–2010) and comparison with past reviews. Addictive Behaviors, 39(1), 39–47.
Miller, W.R., & Longabaugh, R. The drinker inventory of consequences (DrInC) An instrument for assessing adverse consequences of alcohol abuse. Test Manual. In Project MATCH Monograph Series. 1995, National Institute on Alcohol Abuse and Alcoholism.
Tiffany, S. T., Carter, B. L., & Singleton, E. G. (2000). Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction, 95(Suppl 2), S177–S187.
Donovan, D., et al. (2005). Quality of life as an outcome measure in alcoholism treatment research. Journal of Studies on Alcohol, 15, 119–139. (discussion 92–93).
Zubaran, C., & Foresti, K. (2009). Quality of life and substance use: Concepts and recent tendencies. Current Opinion in Psychiatry, 22(3), 281–286.
Drummond, M. F., et al. (2005). Methods for the economic evaluation of health care programmes (3rd ed.). Oxford: Oxford University Press.
Raistrick, D., Heather, N., & Godfrey, C. (2006). Review of the effectiveness of treatment for alcohol problems: NHS, National Treatment Agency for Substance Misuse. NHS, National Treatment Agency for Substance Misuse.
Leplege, A. (2011). Measurements of quality of life, construction and validation of an instrument. Soins, 759, 35–37.
McHorney, C. A., Ware, J. E, Jr, & Raczek, A. E. (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31(3), 247–263.
EuroQolGroup. (1990). EuroQol—A new facility for the measurement of health-related quality of life. Health Policy, 16, 199–208.
Luquiens, A., et al. (2012). Quality of life among alcohol-dependent patients: How satisfactory are the available instruments? A systematic review. Drug and Alcohol Dependence, 125(3), 192–202.
Jeanrenaud, C., et al. (2003). Le coût social de l’abus d’alcool en Suisse. Institut de recherches économiques et régionales—Université de Neuchatel.
Luquiens, A., et al. (2012). Development of a patient reported outcome measure for alcohol use disorder, in ISBRA. Japan: Sapporo.
FDA. (2009). Guidance for industry. Patient-reported outcome measures: Use in medical product development to support labeling claims.
Luquiens, A., et al. (2014). Development of the Alcohol Quality of Life Scale (AQoLS): A new patient-reported outcome measure to assess health-related quality of life in alcohol use disorder. Quality of Life Research, 24(6), 1471–1481.
Rehm, J., et al. (2015). People with alcohol use disorders in specialized care in eight different European countries. Alcohol and Alcoholism, 50(3), 310–318.
Rehm, J., et al. (2015). General practitioners recognizing alcohol dependence: A large cross-sectional study in 6 European countries. The Annals of Family Medicine, 13(1), 28–32.
Bush, K., et al. (1998). The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of Internal Medicine, 158(16), 1789–1795.
Bradley, K. A., et al. (2003). Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): Validation in a female Veterans Affairs patient population. Archives of Internal Medicine, 163(7), 821–829.
Ware, J. E, Jr, & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30(6), 473–483.
Daeppen, J. B., et al. (1998). MOS-SF-36 in evaluating health-related quality of life in alcohol-dependent patients. American Journal of Drug and Alcohol Abuse, 24(4), 685–694.
Gunther, O., et al. (2007). The EQ-5D in alcohol dependent patients: Relationships among health-related quality of life, psychopathology and social functioning. Drug and Alcohol Dependence, 86(2–3), 253–264.
Mokkink, L. B., et al. (2012). COSMIN checklist manual. Amsterdam: EMGO Institute for Health and Care Research.
Cattell, R. B. (1966). The scree plot test for the number of factors. Multivariate Behavioral Research, 1(2), 245–276.
Streiner, D. L., Norman, G. R., & Cairney, J. (2014). Health measurement scales: A practical guide to their development and use. Oxford: Oxford University Press.
Gabbe, B. J., et al. (2015). Level of agreement between patient-reported EQ-5D responses and EQ-5D responses mapped from the SF-12 in an injury population. Population Health Metrics, 13, 14.
Rouquette, A., & Falissard, B. (2011). Sample size requirements for the internal validation of psychiatric scales. International Journal of Methods in Psychiatric Research, 20(4), 235–249.
De Vet, H., et al. (2011). Measurement in medicine: A practical guide. Cambridge: Cambridge University Press.
Terwee, C. B., et al. (2011). Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Quality of Life Research, 21(4), 651–657.
Volkow, N. D., & Baler, R. D. (2014). Addiction science: Uncovering neurobiological complexity. Neuropharmacology, 76, 235–249.
Volkow, N. D., et al. (2010). Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage, 49(3), 2536–2543.
Piazza, P. V., & Deroche-Gamonet, V. (2013). A multistep general theory of transition to addiction. Psychopharmacology (Berl), 229(3), 387–413.
Noel, X., Brevers, D., & Bechara, A. (2013). A neurocognitive approach to understanding the neurobiology of addiction. Current Opinion in Neurobiology, 23(4), 632–638.
Muraven, M., & Shmueli, D. (2006). The self-control costs of fighting the temptation to drink. Psychology of Addictive Behaviors, 20(2), 154–160.
Wilcox, C. E., et al. (2014). Cognitive control in alcohol use disorder: Deficits and clinical relevance. Reviews in the Neurosciences, 25(1), 1–24.
Donovan, D. M., & O’Leary, M. R. (1978). The Drinking-Related Locus of Control Scale. Reliability, factor structure and validity. Journal of Studies on Alcohol, 39(5), 759–784.
Rosenberg, M. (1965). Society and the adolescent self image. New Jersey: Princeton University Press.
Tikka, D. L., et al. (2014). Socio-emotional factors in alcohol dependence. Indian Journal of Psychological Medicine, 36(2), 153–157.
Hibbert, L. J., & Best, D. W. (2011). Assessing recovery and functioning in former problem drinkers at different stages of their recovery journeys. Drug and Alcohol Review, 30(1), 12–20.
Chubon, R. (1999). Manual for the life situation survey. Department of neuropsychiatry and behaviorial Science, University of South Carolina.
Foster, J. H., Marshall, E. J., & Peters, T. J. (2000). Application of a quality of life measure, the life situation survey (LSS), to alcohol-dependent subjects in relapse and remission. Alcoholism, Clinical and Experimental Research, 24(11), 1687–1692.
Ravens-Sieberer, U., & Bullinger, M. (1998). Assessing health-related quality of life in chronically ill children with the German KINDL: First psychometric and content analytical results. Quality of Life Research, 7(5), 399–407.
Ugochukwu, C., et al. (2013). The importance of quality of life in patients with alcohol abuse and dependence. Harvard Review of Psychiatry, 21(1), 1–17.
Wallhed Finn, S., Bakshi, A. S., & Andreasson, S. (2014). Alcohol consumption, dependence, and treatment barriers: Perceptions among nontreatment seekers with alcohol dependence. Substance Use & Misuse, 49(6), 762–769.
Hsu, D.T., et al. (2015). It still hurts: Altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry.
Schwartz, C. E., et al. (2013). Guidelines for secondary analysis in search of response shift. Quality of Life Research, 22(10), 2663–2673.
Sprangers, M. A., & Schwartz, C. E. (1999). Integrating response shift into health-related quality of life research: A theoretical model. Social Science and Medicine, 48(11), 1507–1515.
Whalley, D., et al. (2014). Cultural adaptation of the Alcohol Quality of Life Scale for use in Japan, China, and Korea. In International Society For Pharmacoeconomics and Outcomes Research, 17th Annual European Congress. Amsterdam.
Acknowledgments
The study was funded by Lundbeck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HJA has received sponsorship to attend scientific meetings, speaker honoraria and consultancy fees from Bioprojet, D&A Pharma, Ethypharm, Lundbeck, Merck-Serono, Novartis and Pfizer. BF has received sponsorship to attend scientific meetings, speaker honoraria and consultancy fees from Sanofi, Servier, Pierre-Fabre, MSD, Lilly, Janssen, Otsuka, Lundbeck, Genzyme, Roche, BMS, Novartis, GSK, Pfizer, Celgene, Gilead and Astrazeneka. PL is an employee of Lundbeck. AL has received sponsorship to attend scientific meetings from Lundbeck. JM declares no potential conflict of interest. FP has received sponsorship to attend scientific meetings, speaker honoraria and consultancy fees from D&A Pharma, Ethypharm, Lundbeck and Merck-Serono. JR: reports grants from GWT-TUD and Lundbeck, personal fees and being board member (Nalmefene) for Lundbeck, all outside the submitted work DW is an employee of RTI Health Solutions. RTI Health Solutions received funding from Lundbeck to support this study.
Research involving human participants
Prior to initiation of the study, the study protocol was subjected to human ethics and research review by the relevant authorities in each country.
Informed consent
Each patient signed a consent form to enter the study, after having been given an information notice on the study protocol.
Rights and permissions
About this article
Cite this article
Luquiens, A., Whalley, D., Laramée, P. et al. Validation of a new patient-reported outcome instrument of health-related quality of life specific to patients with alcohol use disorder: the Alcohol Quality of Life Scale (AQoLS). Qual Life Res 25, 1549–1560 (2016). https://doi.org/10.1007/s11136-015-1190-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-1190-5