Abstract
This study investigated the anti-inflammatory effect of hydrophilic and lipophilic extracts from juçara fruits (Euterpe edulis Martius) through measurement of nitric oxide (NOx) and cytokines (IL-12p70, TNF-α, INF-γ, MCP-1, IL-6, and IL-10). J774 macrophages were stimulated with lipopolysaccharides (1 µg/mL) and treated with various concentrations (1–100 µg/mL) of juçara fruits extracts from crude extracts, and hexane, dichloromethane, ethyl acetate, and butanol fractions. Potential relationships between the phenolic composition of the extracts determined by LC-ESI-MS/MS and their anti-inflammatory capacity were also evaluated. Hexane and dichloromethane fractions inhibited NOx and IL-12p70 while increased IL-10. Hexane fractions also decreased IL-6 and IFN-γ production. Hexane and dichloromethane fractions showed a higher number of phenolic compounds (32 and 34, respectively) than the other extracts tested and were also the only ones that presented benzoic acid and pinocembrin. These results suggest juçara fruits compounds as potential anti-inflammatory agents, especially those of a more apolar nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a complex response of cell- and molecular levels to biological, chemical, or physical stimuli, which is necessary for survival. In this response, there is recruit immune cells that modulate the secretion of inflammatory biomarkers that will guide the immune response to resolve inflammation [1]. However, when the initial inflammatory response is unresolved and a persistent inflammation occurs may generate a chronic condition, which has been associated with many diseases, such as arthritis, asthma, atherosclerosis, autoimmune diseases, diabetes, and cancer [1, 2].
Macrophages are among the main cells involved in the inflammatory process. They perform a key role as regulatory cells involved in the phagocytosis of pathogens, necrotic tissue, and secretion of pro- and anti-inflammatory cytokines [3]. Therefore, experimental models using these cells are commonly applied since they allow a fast and reliable initial screening, reducing laboratory animals experiments while seeking new anti-inflammatory agents [4]. Also, to discover pharmacologically active compounds from plants, appropriate extraction methods is necessary to obtain semi-pure extracts, fractions, and, finally, pure compounds. Generally, a solvent that allows the extraction of a greater number of compounds is used to reach the crude extract, and, subsequently, this extract is submitted to a liquid-liquid partition with solvents of increasing polarity, aiming at a semi-purification of the substances by their polarities [5, 6].
Foods rich in bioactive compounds, especially phenolic compounds, have been frequently associated with positive modulation of inflammation. This effect is mainly attributed to inhibition in the production of pro-inflammatory mediators, activation of transcription factors, and control of cell migration and proliferation [5, 7, 8].
Juçara fruits (Euterpe edulis Mart.) have been named superfruit due to their rich diversity and contents of phenolic compounds (especially anthocyanins, other flavonoids, and phenolic acids) as well as relevant health-promoting activities, including antioxidant, hypolipidemic, neuroprotective, and chemopreventive [9,10,11,12]. These black-violet fruits are native to Atlantic Forest distributed across the Brazilian coast and comparable to Amazonian açaí (Euterpe oleracea Mart.) [11].
In vitro [13] and in vivo [14,15,16] studies demonstrated that juçara fruits were able to modulate inflammation, mainly influencing the secretion of the cytokines TNF-α, IL-6, and IL-10. However, evidence for a possible relationship between the anti-inflammatory potential of the juçara fruits with the phenolic composition is still lacking. Those findings may provide a better understanding of the ability of the juçara extracts to modulate inflammatory mediators, potentially active compounds, besides possible synergistic and antagonistic effects that can influence the anti-inflammatory activity.
Thus, the aim of this study was to evaluate the phenolic composition and the effect of crude extracts and hexane, dichloromethane, ethyl acetate, and butanol fractions from juçara fruits harvested in Florianópolis and São Pedro de Alcântara cities (Santa Catarina state, Brazil) on the production of nitric oxide (NOx) and anti-(IL-10) and pro-(IL-12p70, TNF-α, INF-γ, MCP-1, IL-6) inflammatory cytokines by J774 macrophages stimulated with LPS.
Materials and Methods
The Materials and methods section is showed in supplementary material 1.
Results and Discussion
Phenolic Compounds by LC-ESI-MS/MS
The concentrations of phenolic compounds in the crude extracts and hexane, dichloromethane, ethyl acetate, and butanol fractions from juçara fruits are shown in supplementary material 2. Out of.
phenolic compounds analyzed, only 4-methylumbelliferone was not detected in any of the extracts. The results were in line with other studies that also evaluated the phenolic composition of these fruits [17,18,19,20,21]. However, it is important to note that of the 34 phenolic compounds found, we showed, for the first time, the presence of isorhamnetin, 2,5-dihydroxybenzoic acid, p-aminobenzoic acid, chrysin, hesperidin, naringin, pinobanksin, and coumarin.
The dichloromethane and hexane fractions showed the highest number of compounds, 34 and 32, respectively. Benzoic acid and pinocembrin were found only in these two fractions, while p-coumaric acid and coniferaldehyde were found only in the dichloromethane fraction.
Isoquercetrin and isorhamnetin were found in higher concentrations. Other prominent flavonoids in the crude extracts and in the hexane, ethyl acetate, and butanol fractions were rutin and quercetin, while in the dichloromethane fractions, the flavonoids isoquercetrin, isorhamnetin, and rutin, and the phenolic acids protocatechuic acid, ferulic acid, and 2,5 dihydroxybenzoic acid were the major.
The growing location did not influence the concentrations of p-aminobenzoic and 2,5-dihydroxybenzoic acids, apigenin, chrysin, galangin, isorhamnetin, naringin, and coumarin, which was not statistically different between the mean values for crude extracts and fractions (supplementary material 2). However, for most of the phenolic compounds, there was a statistically significant interaction between the type of extract and the growing location of the juçara fruits (supplementary material 3). The dichloromethane fraction B showed higher concentrations of phenolic acids and phenolic aldehydes than the dichloromethane fraction A. This phenolic composition variation in fruits grown in different locations is expected since the differences in edaphoclimatic conditions, affect the biosynthesis of these compounds [22, 23].
Cell Viability Assay and NOx Levels
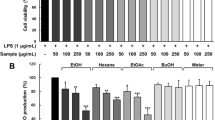
Crude extracts and fractions from juçara fruits were evaluated for their cytotoxic potential against the J774 macrophages. Figure 1 shows that the cell viability was unaffected by crude extracts, hexane, and ethyl acetate fractions, regardless of the concentration tested (1, 3, 10, 30, and 100 µg/mL). In contrast, dichloromethane fraction (2 A) (Fig. 1E) and butanol fractions (both samples) (Fig. 1I and J) decreased the cell viability at 100 µg/mL. Butanol fractions showed the highest cytotoxicity, with CC10 (concentration that kills 10% of the cell line) of 58.2 µg/mL for sample A (Fig. 1I) and 66.4 µg/mL for sample B (Fig. 1J). The dichloromethane fraction demonstrated a CC10 of 97.5 µg/mL (Fig. 1E).
Cytotoxicity and calculated CC10 (concentration that kills 10% of the cell line) of crude extracts and fractions from juçara fruits (Euterpe edulis Mart.). Blank (B) represents cells pretreated with vehicle and stimulated with PBS. Groups ‘A’ represent the extracts from the São Pedro de Alcântara region and groups ‘B’ represent the extracts from Florianópolis region, Santa Catarina, Brazil. Cells were pre-treated with crude extracts (A) (B), hexane fractions (C) (D), dichloromethane fractions (E) (F), ethyl acetate fractions (G) (H), and butanol fractions (I) (J) at 1–100 µg/mL. Each bar represents the average survival of the macrophages in independent experiments ± S.D. (n = 3)
Due to the cytotoxicity demonstrated by dichloromethane (3A) and butanol (5A and 5B) fractions, NOx was measured at concentrations of 1, 3, 10, and 30 µg/mL for crude extract and fractions. The ability of the crude extracts and hexane, dichloromethane, ethyl acetate, and butanol fractions to inhibit the production of NOx is shown in Fig. 2. No significant inhibition was observed for crude extracts, ethyl acetate, and butanol fractions. However, hexane (both samples) and dichloromethane (3B) fractions inhibited NOx production. This inhibition occurred at all tested concentrations (1, 3, 10, and 30 µg/mL): inhibition (%) of the hexane fraction 2A: 80.3 ± 3.5; 74.9 ± 5.5; 84.3 ± 11.0; 66.8 ± 4.8 (p < 0.001) (Fig. 2C); inhibition (%) of the hexane fraction 2B: 72.9 ± 0.8; 81.3 ± 3.6; 81.8 ± 5.1; 53.3 ± 11.8) (p < 0.001) (Fig. 3D); and inhibition (%) of the dichloromethane fraction 3B: 53.5 ± 6.3; 62.1 ± 7.7; 60.4 ± 12.8; 74.4 ± 3.2) (p < 0.001) (Fig. 2F).
Effects of crude extracts and fractions from juçara fruits (Euterpe edulis Mart.) on NOx metabolites levels. Blank (B) represents cells treated only with the vehicles (DMSO and sterile PBS). LPS represents cells treated only with LPS (1 µg/mL). Dexa represents the group pre-treated with dexamethasone (10 µM) before LPS. Groups ‘A’ represent the extracts from the São Pedro de Alcântara region and groups ‘B’ represent the extracts from Florianópolis region, Santa Catarina, Brazil. Cells were pre-treated with crude extracts (A) (B), hexane fractions (C) (D), dichloromethane fractions (E) (F), ethyl acetate fractions (G) (H), and butanol fractions (I) (J) at 1, 3, 10 and 30 µg/mL before LPS. The graph bars expressed as mean ± S.D. n = 3 (sample size in each group). *p < 0.05, ** p < 0.01, *** p < 0.001 vs LPS group (###)
Effects of the hexane and dichloromethane fractions from juçara fruits (Euterpe edulis Mart.) on cytokine levels: IL-10 (A) (a), IL-12p70 (B) (b), IL-6 (C) (c), INF-γ (D) (d), TNF-α (E) (e), and MCP-1 (F) (f). Blank (B) represents cells treated only with the vehicles (DMSO and PBS). LPS represents cells treated only with LPS (1 µg/mL). Dexa represents the group pre-treated with dexamethasone (10 µM) before LPS. Groups ‘A’ represent the extracts from the São Pedro de Alcântara region and groups ‘B’ represent the extracts from Florianópolis region, Santa Catarina, Brazil. Cells were pre-treated with hexane fractions (2 A, 2B) at 1 µg/mL and with dichloromethane fraction (3B) at 3 µg/mL before LPS. The graph bars expressed as mean ± standard deviation (S.D.); n = 3 (sample size in each group); *p < 0.05, ** p < 0.01, *** p < 0.001 vs LPS group (###)
To the best of our knowledge, only one study was published on the anti-inflammatory activity of juçara fruits using cell culture. Barroso et al. [13] evaluated the effects of juçara aqueous extracts (10–100 µg/mL) from different genotypes on LPS-induced RAW 264.7 macrophages. The percentages of inhibition of nitric oxide metabolites were lower than in the present study, ranging from 0 to 65% [13]. NOx is an important inflammatory mediator since iNOS expression is induced by pro-inflammatory cytokines TNF-α, IL-1, and IFN-γ, as well as by LPS [24]. Nitric oxide is the main responsible to increase the vasodilatation necessary to leukocyte migrate to the inflammatory side, as well as one of the most important sources for production of reactive species of nitrogen, like peroxynitrite, nitroxyl anion and nitrogen dioxide among others that are very dangerous to the cells [25, 26].
Cytokines
The quantification of pro-(TNF-α, IL-6, IL-12p70, MCP-1, and IFN-γ) and anti-(IL-10) inflammatory cytokines was performed for the fractions that inhibited NOx production (hexane fraction 2 A, hexane fraction 2B, and dichloromethane fraction 3B), using the best concentrations of each fraction (hexane 2 A: 1 µg/mL; hexane 2B: 1 µg/mL; dichloromethane 3B: 3 µg/mL).
The fractions tested were able to increase the IL-10 production by 107.1 ± 20.2% for hexane fraction 2 A (p < 0.05) (Fig. 3A), 96.6 ± 10.3% for hexane fraction 2B (p < 0.05) (Fig. 3A), and 133.6 ± 11.4% for dichloromethane fraction 3B (p < 0.01) (Fig. 3A). An increase in IL-10 levels was also observed in monocytes isolated from peripheral blood cells of obese humans who received freeze-dried juçara fruit pulp (5 g) for 6 weeks [14]. IL-10, as an anti-inflammatory cytokine, is an inhibitor of pro-inflammatory cytokines, such as IL-2, IL-8, IFN-γ, and TNF-α, promoting inflammation resolution [27, 28].
For pro-inflammatory cytokine IL-12p70, hexane fraction 2 A and dichloromethane fraction 3B significantly reduced the secretion by 51.1 ± 2.1% (p < 0.05) (Fig. 3B) and 47.7 ± 6.1% (p < 0.05) (Fig. 3b), respectively. This pro-inflammatory interleukin acts as an inductor of the synthesis of IFN-γ and TNF-α and as a differentiation factor on CD4 + T cells [29]. To our knowledge, no studies have reported the action of juçara fruits extracts on IL-12p70 cytokine modulation.
IL-6 production was also inhibited by hexane fraction 2 A (% of inhibition: 48.2 ± 10.2) (p < 0.01) (Fig. 3C). In contrast, hexane fraction 2B decreased IFN-γ release (% of inhibition: 68.3 ± 3.4) (p < 0.01) (Fig. 3D). IL-6 is also a cytokine with pro-inflammatory function since it stimulates the synthesis of acute-phase proteins [29]. Studies with freeze-dried juçara fruit powder (0.5%) have also demonstrated a down-regulation of IL-6 cytokine. The ingestion of this dose by Wistar rats during pregnancy and lactation promoted a decrease of IL-6 in the retroperitoneal white adipose tissue and liver [16] of 21-day-old offspring in a pro-inflammatory state.
IFN-γ is widely recognized for its pro-inflammatory capability, activating macrophages and natural killers and promoting lymphocyte differentiation and adhesion of CD4 + T lymphocytes [30]. A decrease in IFN-γ levels was also observed in Wistar rats fed defatted lyophilized juçara fruit pulp (10%) for 50 days [15].
In the present study, the differences observed for the effects on inflammatory mediators of the different extracts/fractions studied are possibly related to the phenolic composition of each one of them. The positive effects of the phenolic compounds on nitric oxide production and other multiple inflammatory components are already well established in the literature [7, 8].
The anti-inflammatory effects observed only for the hexane and dichloromethane fractions may be related to the higher number of phenolic compounds found in these fractions than the others tested. As previously mentioned, these two fractions were also the only ones that presented benzoic acid and pinocembrin, known for their anti-inflammatory potential [31, 32]. It is also important to highlight the possibility of a synergistic effect between the compounds in these two fractions, conferring a higher biological effect [33]. Still, a possible action of other phenolic compounds that were not evaluated in this study and other classes of compounds also needs to be considered. For instance, juçara fruits are rich in fatty acids, such as oleic and linolenic acids [19], nonpolar compounds that are present in the hexane fraction, and that may have anti-inflammatory effects [34, 35]. As we observed in this study, higher bioactivity of the hexane and dichloromethane fractions was also reported for HT22 cells when the neuroprotective effect of the crude extract and the hexane, dichloromethane, ethyl acetate, and butanol fractions from juçara fruits was studied [21].
Conclusions
Our results showed that juçara fruits extracts have anti-inflammatory potential on inflammation induced by LPS in the J774 macrophages. The hexane and dichloromethane fractions showed an increase in anti-inflammatory cytokine and a decrease in pro-inflammatory mediators, with differences between the two regions studied. These effects can be associated with the phenolic composition of these samples and their fractions, mainly phenolic compounds of a more apolar nature, as well as by the interaction between the compounds, which can act in synergy in the protection against inflammation.
This was the first work that evaluated the protective effects of juçara fruits on J774 macrophages and sequential extracts to better understand the potential relationships between the juçara fruit phenolic composition and its anti-inflammatory capacity. This knowledge furthers the understanding of the juçara fruits health benefits and may lead to novel therapeutic agents to inhibit inflammatory conditions. Additional studies should target which are the active components of the hexane and dichloromethane fractions as well as the signaling pathways involved in the modulation of inflammatory mediators.
Data Availability
No datasets were generated or analysed during the current study.
References
Germolec DR, Shipkowski KA, Frawley RP, Evans E (2018) Markers of inflammation. In: DeWitt J, Rockwell C, Bowman C (eds) Immunotoxicity Testing. Methods in Molecular Biology. Humana, New York, pp 57–79
Sears B, Saha AK (2021) Dietary Control of inflammation and resolution. Front Nutr 8:1–19
McKallip RJ, Lombard C, Martin BR et al (2002) 2–2.Delta(9)-tetrahydrocannabinol-Induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther 302:451–465
Facchin BM, dos Reis GO, Vieira GN et al (2022) Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: a systematic review and meta-analysis. Inflamm Res 71:741–758
Azab A, Nassar A, Azab A (2016) Anti-inflammatory activity of natural products. Molecules 21:1321
Cechinel Filho V, Yunes RA (1998) Estrategies for obtaining pharmacologically active compounds from medicinal plants: concepts about structural modification for improve the activity. Quim Nova 21:99–105
Rahman MM, Rahaman MS, Islam MR et al (2021) Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules 27:233
Yahfoufi N, Alsadi N, Jambi M, Matar C (2018) The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 10:1618
Cardoso AL, de Liz S, Rieger DK et al (2018) An update on the biological activities of Euterpe edulis (juçara). Planta Med 84:487–499
Schulz M, Seraglio SKT, Brugnerotto P et al (2020) Composition and potential health effects of dark-colored underutilized Brazilian fruits – A review. Food Res Int 137:109744
Pereira DC, de Gomes S, Tonon F dos RV, et al (2022) Towards chemical characterization and possible applications of juçara fruit: an approach to remove Euterpe Edulis Martius from the extinction list. J Food Sci Technol 60(2):429–440
Schulz M, Tischer Seraglio SK, Gonzaga LV et al (2021) Phenolic compounds in Euterpe fruits: composition, digestibility, and stability – a review. Food Reviews Int 1–28
Barroso MES, Oliveira BG, Pimentel EF et al (2019) Phytochemical profile of genotypes of Euterpe Edulis Martius – Juçara palm fruits. Food Res Int 116:985–993
Santamarina AB, Jamar G, Mennitti LV et al (2020) Obesity-related inflammatory modulation by juçara berry (Euterpe edulis Mart.) Supplementation in Brazilian adults: a double-blind randomized controlled trial. Eur J Nutr 59:1693–1705
Freitas RB, Rômulo DN, Bianca GM et al (2018) Euterpe edulis extracts positively modulates the redox status and expression of inflammatory mediators. Food Agric Immunol 29:95–108
Morais CA, Oyama LM, de Moura Conrado R et al (2015) Polyphenols-rich fruit in maternal diet modulates inflammatory markers and the gut microbiota and improves colonic expression of ZO-1 in offspring. Food Res Int 77:186–193
Bicudo MOP, Ribani RH, Beta T (2014) Anthocyanins, phenolic acids and antioxidant properties of juçara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Foods Hum Nutr 69:142–147
Inada KOP, Oliveira AA, Revorêdo TB et al (2015) Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J Funct Foods 17:422–433
Schulz M, Borges G, da SC, Gonzaga LV et al (2015) Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res Int 77:125–131
Schulz M, Biluca FC, Gonzaga LV et al (2017) Bioaccessibility of bioactive compounds and antioxidant potential of juçara fruits (Euterpe edulis Martius) subjected to in vitro gastrointestinal digestion. Food Chem 228:447–454
Schulz M, Gonzaga LV, de Souza V et al (2019) Neuroprotective effect of juçara (Euterpe edulis Martius) fruits extracts against glutamate-induced oxytosis in HT22 hippocampal cells. Food Res Int 120:114–123
Nehring P, Katia Tischer Seraglio S, Schulz M et al (2022) Grumixama (Eugenia brasiliensis Lamarck) functional phytochemicals: Effect of environmental conditions and ripening process. Food Res Int 157:111460
Pinto AA, Fuentealba-Sandoval V, López MD et al (2022) Accumulation of delphinidin derivatives and other bioactive compound in wild maqui under different environmental conditions and fruit ripening stages. Ind Crops Prod 184:115064
Cinelli MA, Do HT, Miley GP, Silverman RB (2020) Inducible nitric oxide synthase: regulation, structure, and inhibition. Med Res Rev 40:158–189
Piacenza L, Zeida A, Trujillo M, Radi R (2022) The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol Rev 102:1881–1906
Lancaster JR (2015) Nitric oxide: a brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci OA 1(1):FSO59
Sugimoto MA, Vago JP, Perretti M, Teixeira MM (2019) Mediators of the resolution of the inflammatory response. Trends Immunol 40:212–227
Kany S, Vollrath JT, Relja B (2019) Cytokines in inflammatory disease. Int J Mol Sci 20:6008
Liu C, Chu D, Kalantar-Zadeh K et al (2021) Cytokines: from clinical significance to quantification. Adv Sci 8:2004433
Abbas AK, Lichtman AH, Pillai S (2015) Cellular and Molecular Immunology, 8th edn. Elsevier, Philadelphia
Lan X, Wang W, Li Q, Wang J (2016) The natural favonoid pinocembrin: molecular targets and potential therapeutic applications. Mol Neurobiol 53:1794–1801
Franck T, Mouithys-Mickalad A, Robert T et al (2013) Differentiation between stoichiometric and anticatalytic antioxidant properties of benzoic acid analogues: a structure/redox potential relationship study. Chem Biol Interact 206:194–203
Qin X, Lu Y, Peng Z et al (2018) Systematic chemical analysis approach reveals superior antioxidant capacity via the synergistic effect of flavonoid compounds in red vegetative tissues. Front Chem 6:1–13
Farag MA, Gad MZ (2022) Omega-9 fatty acids: potential roles in inflammation and cancer management. J Genetic Eng Biotechnol 20:48
Tortosa-Caparrós E, Navas-Carrillo D, Marín F, Orenes-Piñero E (2017) Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit Rev Food Sci Nutr 57:3421–3429
Acknowledgements
The authors thank Júlio Carvalho Machado and Jânio Lohn for donating the juçara fruit samples used in this study.
Funding
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (Finance code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brazil (No. 165753/2020-0) for financial support.
Author information
Authors and Affiliations
Contributions
MS: Conceptualization, Investigation, Writing- original draft, Writing – review and editing. LVG: Conceptualization, Supervising, Writing – Review & Editing. ACNA: Investigation, Writing – review and editing. TL: Investigation, Writing- original draft. ETBM: Investigation. EMD: Supervising, Writing – Review & Editing. CTPD: Investigation. RBH: Investigation, Supervising. FMZ: Sample collection, Writing – Review & Editing. ACOC: Supervising, Writing – Review & Editing. RF: Conceptualization, Supervising, Writing – Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schulz, M., Gonzaga, L.V., Antunes, A.C.N. et al. The Protective Effect of Juçara Fruit (Euterpe edulis Martius) Extracts on LPS-Activated J774 Macrophages. Plant Foods Hum Nutr 79, 677–684 (2024). https://doi.org/10.1007/s11130-024-01204-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-024-01204-8