Abstract
High-sucrose high-fat diets are one of the causes of malnutrition, and may induce metabolic alterations such as dyslipidemia, insulin resistance, and adipogenesis. The objective of this work was to investigate the possible protective effect of traditionally edible avocado creole peel (Persea americana Mill var. drymifolia) when consuming a high-sucrose and fat diet (HSFD). The experimental animal model included 21 male Wistar rats divided in three groups: the control group received a standard diet of purina®, the HSFD group received a high fat diet plus 30% sucrose in drinking water, and finally the HSFD + AP group received the HSFD diet supplemented with 200 mg/kg of avocado peel for 14 weeks. It was observed that alterations included higher cholesterol, glucose, insulin, fatty acids and TNF-α levels as well as lower HDL, and adiponectin. The addition of avocado peel reverted some of these effects, resulting in normal values of triglicerides, insulin and adiponectin, while attenuated the levels of total cholesterol. Liver weight of the group added with avocado peel was similar to the control group. The neuronal density in the hippocampal areas CA1 and dentate gyrus DC were lower in the high glucose fat group, while the ingestion of the avocado peel showed a neuroprotective effect. The avocado creole ingestion reverted or attenuated most of the metabolic effects caused by a high-sucrose fat diet which was attributed to the compounds detected by HPLC-MS and GC-MS that included bioactive polyphenols such as flavanol quercetin, flavanone naringenin, flavan 3-ol catechin, cyanidin 3-glucoside, pelargonidin 3-glucoside, pelargonidin 3-rhamnoside, hydroxydelphinidin, eugenol and estragole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excess intake of fat and/or carbohydrates increases plasma triglycerides, induces metabolic alterations and is a risk factor for chronic diseases as obesity, diabetes and cardiovascular disorders provoking metabolic abnormalities that may include insulin resistance, hyperglicemia, and hyperlipidemia [1]. In addition, alterations in the hippocampus may be provoked by an insulin signalling dysfuntion associated with cognitive impairment and reduction of adult hippocampal neurogenesis [2]. Avocado consumption has been associated with the prevention and treatment of some ailments such as inflammatory, diabetes and cardiovascular diseases. These effects are attributed to bioactive compounds both lipophilic and hydrophilic present in pulp, but also has been found in extracts of leaves, peels, and stones. The following classes of bioactive compounds of avocado include phenolic compounds, phytosterols, fatty alcohols, furan derivatives, diterpenoids, carotenoids, tocopherols, particularly the phenolic acids, flavonoids and anthocyanidins which have been associated to antioxidant, cardioprotective and neuroprotective activities. On the other hand, extracts of leafs, peels and stones have shown greater antioxidant capacity compared to that of the pulp and more studies are needed to investigate the bioavailability of these bioactive compounds and their potential toxicity [3]. Avocado (Persea americana Mill) var. drymifolia is characterized for its smooth and thin peel which is traditionally consumed by the Mexican population, which supports the idea that does not contain toxic compounds and may be used in preclinical and clinical studies. Additionally, thin-skinned varieties like Drymifolia contain lower amounts of stone-cell masses than thick-skinned varieties [4], which cause their acceptable texture. The methanolic extracts of this avocado peel showed high antioxidant values that may be attributed to phenolic acids and anthocyanins [5]. In consequence, the probable beneficial effect of consuming the peel is worth investigating. Therefore, the aim of this study was to analyze the chemical composition and the effect of avocado peel (Persea americana Mill var. drymifolia) in metabolic disorders and hippocampus morphology in Wistar rats, caused by a high-sucrose fat diet.
Materials and Methods
Mature avocados (Persea americana Mill var. drymifolia) were purchased at a local market in Mexico City. The ripeness of the avocados was confirmed by using a colorimeter Konica Minolta CR-10, Konica Minolta Sensing (New Jersey, USA). The media of CIELAB parameters were L = 17.6, a*=33.4 and b*= -7.2 and corresponded to purplish-black [4]. The peel was then separated, washed, and dried for 3 h at 55 ° C in a laboratory oven. The dehydrated peel was grounded to obtain the powder to be used in all the experiments. Total phenols were determined in this avocado peel powder by extracting the phenols with 70% methanol:water according to a method previously described [6]. Results were expressed as mg gallic acid equivalents (GAE/g).
Chemical Composition of the Avocado Peel and Obtention of the Extracts
Five grams of avocado peel (dried powder) were treated with 200 mL of n-hexane at 70 °C for 4 h, to obtain a lipophilic extract, to be analyzed by gas chromatography-MS. The residue was then extracted with a 70% methanol:water solution to obtain the hydrophilic extract to be analyzed by Direct Ionization-MS.
DIESI-MS and GC-MS Analysis
The methanolic extract of the avocado peel was analyzed by Direct Ionization analysis (DIESI-MS) in a Bruker MicrOTOF-QII system, using an electrospray ionization (ESI) interface (Bruker Daltonics, Biellerica, MA, USA). The spectrometer was calibrated with an ESI-TOF tuning mix calibrant (Sigma-Aldrich, Toluca, Estado de México, México). MS/MS analysis was performed by using positive and negative electrospray ionization (ESI+/-), and the obtained fragments were analyzed by a Bruker Compass Data Analysis 4.0 (Bruker Daltonics, Technical Note 008, 2004). An accuracy threshold of 5 ppm was established to confirm the elemental compositions. The lipophilic extract of avocado peel obtained with hexane, was analyzed by gas chromatography in a Bruker SCION 456 gas chromatograph (Biellerica, MA, USA) coupled to a TQ Bruker mass spectrometer. A RESTEK column (Rtx-20, 30 m × 0.32 mm × 0.20 µm) was used. Full scan data was acquired in the mass range of m/z 30–500 amu and the EI voltage adjusted at 70 V. The temperature of the transfer line was 250 °C.
Animal Model and Experimental Design
For the animal model, 21 male Wistar rats (Harlan Teklab) 21 days old and newly weaned were selected. Animal maintenance was in accordance to the laboratory animal specifications provided by the National Institute of Health “Guide for the Care and Use of Laboratory Animals” [7]. Rats were housed in individual cages at room temperature, with 12-h light/12-h dark cycle. The animals were divided in three groups: (1) Control group (CG) (n = 7) that received a standard diet of Roden Lab Chow 5001 purina (St. Louis, MO, USA) and water ad libitum, (2) High-sucrose fat diet group (HSFG) (n = 7) was fed with a high-sucrose and high-fat pellet diet (containing 68% standard feed, 20% sugar, 0.5% cholesterol from Sigma Aldrich) and (3) a group feed with HSFG + avocado peel containing 11.5% vegetable shortening plus 30% sucrose solution ad libitum, and high-sucrose fat Diet + avocado peel (n = 7) were fed with a high-sucrose and high-fat pellet diet plus 30% sucrose solution ad libitum supplemented with 200 mg/kg of body weight of avocado peel (Persea americana Mill var. Drymifolia) for 14 weeks. Administration of avocado peel was carried out with an orogastric tube. After 14 weeks of administering the experimental diets, the adipose tissues, brains, and livers were collected, weighed and frozen until further analysis. Glucose, triglicerides, total cholesterol, and HDL cholesterol levels in the blood were determined as described [8]. The concentration of insulin was measured by the kit Rat Ins1/insulin ELISA kit, adiponectin kit ELISA adiponectin RAB1136-1KT from and RAT tumor necrosis factor alfa-ELISA kit RAB0479-1KT from Sigma-Aldrich (St. Louis, USA).
Histology of Hippocampus and Liver
The collected brains were fixed by using formaldehyde (10%) for one month. The brains were dehydrated with ethanol as described in [9]. The brain blocks were cutted using a microtome (Leica model 820) to a thickness of 10 µm and attached to gelatin-coated slides and Nissl’s staining was used to determine the pyramidal neurons. The hippocampus DG; dentate gyrus, CA1, CA2, CA3: cornu ammonis were observed using a light microscope (Leica model DM750) at a magnification of 40x. Using the rat brain atlas, coronal sections from interaural 5.70 mm to bregma − 3.30 were selected for analysis [10]. A total of five sections were assessed in each brain for the histological study. The quantification of the number of cells in the hippocampus was calculated by using the software ImageJ v.1.5f (National Institutes of Health, Bethesda, USA). The liver was fixed in 10% (v/v) formaldehyde, by successive dehydration by ethanol inmersion and embedded in paraffin. Serial sections were cut in slices 10 µm thick and stained with hematoxylin-eosin (HE) and observed in a light microscope (Leica model DM750) at a magnification of 40x.
Statistical Analysis
A one-way ANOVA followed by a Tukey test for multiple mean comparison were carried out to analyze all experimental results which were expressed as means ± standard error. The significance was set at p ≤ 0.05. Data were analyzed using MINITAB 17 statistical software.
Results and Discussion
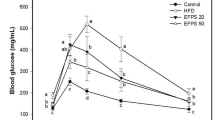
The high-sucrose fat diet was designed to provoke metabolic disorders in rats. The control group was fed with a diet containing 3% fat, 55% carbohydrates, 23% of proteins with water ad libitum. The model groups were provided with a diet having 17% fat, 62% carbohydrates, 15% proteins and 30% sucrose in water as the only source of liquid. The third group ingested this last diet added with avocado peel (200 mg/kg animal weight). The chemical characterization of the avocado peel included the total phenolics content for avocado peel and was 155 mg/g. The DIESI-MS analysis revealed the presence of compounds recognized by its biologically activity such as flavanon naringenin, flavan 3-ol catechin, phenolic acid chlorogenic acid, flavonols quercetin, anthocyanins: cyanidin 3-glucoside, pelargonidin 3-glucoside, hydroxydelphinidin 3-glucoside as well as the volatile compounds eugenol and estragole, determined by GC-MS. Given the above, it was concluded that avocado creolle peel represents a good source of polyphenols. The benefical effects of the consumption of diets rich in polyphenols as well as their benefits against certain chronic diseases such as cardiovascular disorders, type 2 diabetes, osteoporosis, pancreatitis, lung damage, and neurodegenerative diseases have been reported [11].
Food and Liquid Intake; Body and Adipose Tissues Weights
It was observed that the average of the food intake was lower for the HSFD diet group (14 ± 2.9 g/day), and HSFD added with avocado peel group (12 ± 2.4 g/day), showing a significant decrease (p ≤ 0.05) as compared to the control group (CG) (20 ± 2.06 g/day) throughout the experiment. Liquid intake of the HSFD and HSFD + AP groups (30 ± 8.0 and 30 ± 9.8 mL/day, respectively) administered with a solution of 30% sucrose was significantly (p ≤ 0.05) lower as compared to the control group (38 ± 5.4 mL/day). These results may be attributed to the high concentration of sucrose 30% in the liquid given to the rats in the model groups. On the other hand, no significant differences were found in body weights between the control and the hypercaloric feed groups which may be attributed to a reduced ingestion of liquid and solid food by the rats and explained by the fact that an excess of sugars in the drinking water is less acceptable and induces satiety [12] but also, to the fact that adipose tissue density is smaller than the corresponding to other tissues. It has been reported [13] that the administration of high-sucrose diet induced increased visceral adipose tissue without increasing body weight in Sprague-Dawley rats, which coincided with our results.
Regarding the adipose tissue weight (Table 1), it could be observed that high-sucrose-fat diet caused a significant increase (p ≤ 0.05) in the weigth of abdominal (121%), epidymal (77%), and pericardial (97%) adiposse tissues, when compared to those values of the control group. Meanwhile, in the rats supplemented with the avocado peel, the increase in the weigth of the adipose tissue was less pronounced: abdominal (82%), epidymal (57%), and pericardial (71%). These effects may be attributed to the polyphenols present in the avocado peel such as quercetin, anthocyanins and other phenolic compounds which have been reported to inhibit the effects on adipocyte differentiation. In this respect, quercetin is involved in the down-regulation of adipogenesis-related enzymes including on CCAAT/enhancer binding protein (C/EBPα), peroxisome proliferator-activated receptors (PPAR), and sterol regulatory element-binding proteins (SREBP)-1. Additionally, cyanidin-glucoside and peonidin-glucoside have effects on lipid accumulation via down-regulation of PPARγ expression as reported in a study with purple corn silk [14].
Weigth and Histology of the Liver
The weigth of the liver of rats in the control group was in average 10.1 ± 0.4 g and significantly increased (p ≤ 0.05) to 12.0 ± 0.2 g in the high-sucrose fat group, while in the group administered with avocado peel it was observed an intermediate value of 10.9 ± 0.5 g. Histological examination of the liver sections revealed normal morphology and architecture in the control group (Fig. 1 CG). It was observed that a high-sucrose fat diet produced an increase of fat accumulation within hepatocytes and sinusoids of the rats (Fig. 1-HSFD). However, the avocado peel intake decreased the accumulation of the hepatocytes in the liver (Fig. 1-HSFD + AP) which supports the idea of the potential use of avocado peel to prevent accumulation of lipids in the liver (Fig. 1) which may be attributed to polyphenols. It has been reported that naringin may enhance hepatic AMP activated protein kinase, since it stimulates the expression of SREBP-1c and PPAR-α that regulates lipid metabolism through lipid synthesis and fatty acid oxidation [15].
Biochemical Lipid Markers and Effect of Avocado Peel Addition to Hypercaloric Diet
In Table 2 it is possible o observe that because of the ingestion of high-sucrose fat diet on the alteration of lipid metabolism biomarkers of the model group, significantly higher (p ≤ 0.05) levels of total cholesterol, triglycerides, and reduced values of high-density lipoproteins HDL-C were detected when compared to the control group. The ingestion of avocado peel as well as the high-sucrose fat diet were very efficient in lowering the levels of total cholesterol and triglycerides to the observed normal values of the control group for which the high-density lipoproteins remained lower than normal and close to the values of the high-sucrose fat diet group. Nevertheless, the administration of avocado peel reduced the atherogenic index in the HSFD + AP group when compared to the HSFD group. The obtained results suggested that avocado peel consumption could contribute to prevent some lipid metabolic alterations due to the content of polyphenols. Significant improvements in dyslipidemia by the addition of purple maize pericarp extract to a high fat diet, was attributed to the modulation of genes related to adipogenesis, which have been shown to enhance liver β-oxidation, increasing the utilization of fatty acids as an energy source by the phenolic acids, catechin, naringenin and anthocyanins present in the extract [16]. Catechin promotes the expression of proteins that play a key role in thermogenesis and β-oxidation and may inhibit pancreatic and gastric lipases, attenuating fat emulsification [16]. Additionally, a beneficial effect when blue maize was added to the diet has been reported [8] since anthocyanins bioactivity includes the regulation and expression of key enzymes such as lipoprotein lipase (LPL) fatty acid synthase and ATP-binding cassette transporter 1 (ABCA1), which are involved in TG and cholesterol metabolism [17]. Another compound identified in the peel was chlorogenic acid, to which a hypolipidemic effect has been attributed due to the activation of PPAR-α, which modulates the transcription of the proteins involved in the synthesis, transportation and enhancement of the ß-oxidation of free fatty acids (FFA). PPAR-α activation also elevates fatty acid combustion which leads to a reduction of hepatic triglyceride content, thus increasing insulin sensitivity [18]. Therefore, it is likely that the benefical effect of avocado peel on dyslipedemia is due to the presence of polyphenols.
The model group, that was feed with the high-sucrose fat diet, showed alterations in serum glucose metabolism biomarkers, noting that glucose increased by 30%, and insulin by 40% and therefore, the homeostasis model assessment of insulin resistance (HOMA-IR) index showed a significant difference (p ≤ 0.05) with that of the control group. In addition, the levels of adiponectin decreased by 59% and levels of TNF-α where higher than the values obtained for the control group. Higher intake of sucrose and fat is related with proinnflamatory factors such as TNF-α. Table 3 shows a significant (p ≤ 0.05) increase of this factor in HSFD group as compared to the control group (12%). The addition of avocado peel to the HSFD + AP group did not change glucose levels, neither the proinnflamatory factors such as TNF-α. However, the addition of avocado peel was able to revert insulin levels in serum until they were statistically equal to those of the CG group (p ≤ 0.05). The HOMA-IR index was also calculated and in HSFD + AP group showed lower levels of HOMA-IR index (20%) as compared to HSFD group. It was observed that the levels of glucose and insulin may be attenuated by modifying the type of sugar and fatty acids in the diet [1]. In this regard, oleic and linoleic acids contained in the avocado peel may also help to reduce the damage produced by the consumption of the high-sucrose fat diet.
Concerning the effect of the administration of avocado peel on adiponectin, it was observed that resulted in a significant (p ≤ 0.05) increase of serum adiponectin as compared to HSFD group. It was also noted that the concentration of adiponectin in HSFD + AP group was statistical equal to the corresponding to the control group. Adiponectin is the most abundant plasma adipokine and is important in the regulation of glucose and lipid metabolism. In addition, low levels of serum adiponectin have been correlated with metabolic dysfunctions as well as with various central nervious system disorders such as Alzheimer, Parkinson, anxiety, and depression [19]. In this regard, a previous work showed that (HFD) HOMA-IR significantly reduced adiponectin levels in mice fed with a high-fat diet added with blueberry and mulberry juice, [20].
Neuronal Density on Different Regions of Hippocampus
The adult hippocampal neurogenesis is affected by aging and stress conditions [21] which include metabolic disorders due to malnutrition, high fat diets, and diabetes, among others [22]. In our study, a neuronal density (pyramidal cells) measurement was done in the DG, CA1, CA2 and CA3 regions of the hippocampus of experimental groups (Fig. 2). Regarding the effect of high-sucrose and high-fat diet on cell density, it was observed a significant (p ≤ 0.05) reduction of the number of cells of the DG and CA1 regions of the HSFD group as compared to the control group (Fig. 3). However, in CA2 and CA3 regions, there were not found any statistically significant changes. The avocado peel improved neuronal density in the DG to the normal values of the control group. The cell density in the CA1 region slightly increased in the group supplemented with avocado peel compared with control group (Fig. 3). The CA1 region is a hippocampal section, having the essential role in new memory construction and strength. The addition of Ocimum sanctum extract to the diet of rats reverted the effect of aging in the reduction of pyramidal cells in regions CA1 and CA3 [9]. On the other hand, dentate gyrus is a structure with a critical role in the consolidation of information from short-term memory to long-term memory, besides that most of adult neurogenesis occurs in the dentate gyrus [2]. It was observed that a high-fat diet caused a deficient execution in memory and learning tasks associated with structural and physiological neuronal changes such as a reduced number and complexity of dendritic spines in CA1 and long-term depression, as well as diminished DG neurogenesis [22].
Some studies have shown that the consumption of a diet rich in antioxidants can reduce the incidence of neurodegenerative disorders, and in this regard, the protective effect is most likely due to the presence of phenolic compounds as flavonoids, anthocyanins, and phenolic acids, as in the case of polyphenols of wine [23] which are potential agents in neuroprotection. Furthermore, it has been observed that most of red wine varieties induced neuroprotection through their antioxidant ability in astrocytes [24]. Anthocyanins exert neuroprotective actions through their potential to protect neurons against injury induced by neurotoxins as well as an ability to suppress neuroinflammation, and the potential to promote memory, learning, and cognitive function [25]. Additionally, the flavonoids catechin and epicatechin gallate have also shown an ability to suppress neuroinflammation and can attenuate and inhibit activation of microglia and/or astrocytes associated with the release of the mediators linked to the apoptotic death of neurons [26]. Another biomarker related to the regulation of neurogenesis and central nervous system disorders [2] is adiponectin which is a protein hormone involved in fatty acid breakdown and regulation of glucose levels. Adiponectin has shown neuroprotective properties regulating neurogenesis and synaptic plasticity. In this work reduced levels of adiponectin were found in rats that consumed high-sucrose fat diet, but this effect was attenuated in the group administrated with avocado peel.
According to our results and to the published evidence by other authors, it could be concluded that the ingestion of avocado creole peel showed a beneficial effect in malnutrition conditions such as high-sucrose and fat diets, probably due to its content of flavonoids, anthocyanins and phenolic acids.
References
Billingsley H, Carbone S, Lavie C (2018) Dietary fats and chronic noncommunicable diseases. Nutrients 10:1385–1401
Calvo-Ochoa E, Arias G (2014) Celular and metabolic alterations in the hippocampus caused by insulin signalling dysfunction and its association with cognitive impairment during aging and alzheimer´s disease: studies in animal models. Diabetes Metab Res Rev 31:1–13
Bhuyan DJ, Alsherbiny MA, Perera S, Low M, Basu A, Devi OA, Barrooah MS, Li CG, Papoutsis K (2019) The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antiox 8:426
Espinosa-Velázquez R, Dorantes-Alvarez L, Gutiérrez-López GF, García-Armenta E, Sánchez-Segura L, Perea-Flores MJ, Ceballos-Reyes GM, Ortíz-Moreno A (2016) Morpho-structural description of unripe and ripe avocado pericarp (Persea americana Mill var. drymifolia) description. Rev Mex Ing Quim 15(2):469–480. https://rmiq.org/ojs311/index.php/rmiq/article/view/1144
Corrales-García JE, García-Mateos MR, Martínez-Lopéz E, Barrientos-Priego AF, Ybarra-Moncada MC, Ibarra-Estrada E, Méndez-Zúñiga SM, Becerra-Morales D (2019) Anthocyanin and oil contents, fatty acids profiles and antioxidant activity of Mexican landrace avocado fruits. Plant Foods Hum Nutr 74:210–215. https://doi.org/10.1007/s11130-019-00721-1
Yoo KM, Lee CH, Lee H, Moon B, Lee CY (2008) Relative antioxidant and cytoprotective activities of common herbs. Food Chem 106:929–936
National Research Council (US) (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington, DC, pp 11–40
Guzmán-Gerónimo RI, Alarcón-Zavaleta TM, Oliart-Ros RM, Meza-Alvarado JE, Herrera-Meza S, Chávez-Servia JL (2016) Blue maize extract improves blood pressure, lipid profiles, and adipose tissue in high-sucrose diet-induced metabolic syndrome in rats. J Med Food 20:110–115
Kusindarta DL, Wihadmadyatami H, Haryanto A (2018) The analysis of hippocampus neuronal density (CA1 and CA3) after Ocimum sanctum ethanolic extract treatment on the young adulthood and middle age rat model. Vet World 11(2):135–140
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier Ltd., London
Cory H, Passarello S, Szeto J, Tamez M, Mattei J (2018) The role of polyphenols in human health and food systems: a mini-review. Front Nutr 5:87. https://doi.org/10.3389/fnut.2018.00087
Drewnowski A, Almiron-Roig E (2010) Human perceptions and preferences for fat-rich foods. In: Montmayeur JP, le Coutre J (eds) Fat detection: taste, texture, and post ingestive effects, 1st edn. CRC Press/Taylor & Francis, Boca Raton, FL, pp 243–268
Cao L, Liu X, Cao H, Lv Q, Tong N (2012) Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid Med Cel Longev 2012:374346
Chaiittianan R, Sutthannut K, Rattanathongkom A (2017) Purple corn silk: a potential anti-obesity agent with inhibition on adipogenesis and induction on lipolysis and apoptosis in adipocytes. J Ethnopharmacol 201:9–16
Pu P, Gao D, Mohamed S, Chen J, Zhang J, Zhou X, Zhou N, Xie J, Jiang H (2012) Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high- fat diet. Arch Biochem Biophys 518:61–70
Luna-Vital D, Luzardo-Ocampo I, Cuellar-Nuñez L, Loarca-Pina G, Gonzalez de Mejia E (2019) Maize extract rich in ferulic acid and anthocyanins prevents high-fat induced obesity in mice by modulationg SIRT1, AMPK, and IL-6 associated metabolic and inflammatory pathways. J Nutr Biochem 79:108343. https://doi.org/10.1016/j.jnutbio.2020.108343
Tsuda T, Ueno Y, Kojo H, Yoshikawa T, Osawa T (2005) Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim Biophys Acta 1733:137–147
Huang K, Liang X, Zhong Y, He W, Wang Z (2014) 5-Caffeoylquinic acid decreases diet-induced obesity in rats bymodulating PPARα and LXRα transcription. J Sci Food Agric 95:1903–1910
Bloemer J, Pinky P, Govindarajulu M, Hong H, Judd R, Amin R, Moore T, Dhanasekaran M, Reed M, Suppiramaniam V (2018) Role of adiponectin in central nervous system disorders. Neural Plast 2018:4593530. https://doi.org/10.1155/2018/4593530
Wu T, Tang Q, Gao Z, Yu Z, Song H, Zheng X, Chen W (2013) Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS One 8:10
Bracke A, Domanska G, Bracke K, Harzsch S, Brandt JV, Bröker B, Halbach O (2019) Obesity impairs and adult hippocampal neurogenesis. J Exp Neurosci 13:1–10
Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP, Gracía A, Del Olmo N, Ruiz-Gayo M, Cano V (2012) High- fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab 302:396–402
Basil A, Soulet S, Chaher N, Mérillon JM, Chibane M, Monti JP, Richard T (2012) Wine polyphenols: potential agents in neuroprotection. Oxid Med Cell Longev 2012:805762. https://doi.org/10.1155/2012/805762
Gómez-Serranillos MP, Martín S, Ortega T, Palomina OM, Palomina OM, Prodanov M, Vacas V, Hernández T, Estrella I, Carretero ME (2009) Study of red wine neuroprotection on astrocytes. Plant Foods Hum Nutr 64:238–243
Tsuda T (2012) Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res 56:159–170
Pereira GD, Cavegn N, Nix A, Do Nascimento MC, Stangl D, Anwar MS, Nardi AE, Franca PG, Thuret S (2012) The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid Med Cell Longev 2012:541971. https://doi.org/10.1155/2012/541971
Acknowledgements
This work was supported by the Instituto Politécnico Nacional (Mexico) projects SIP 20201014 and 20195428.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest in the study.
Electronic Supplementary Material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Miñón-Hernández, D., Dorantes-Alvarez, L., Guzmán-Gerónimo, R.I. et al. Avocado Creole Peel Ameliorates Metabolic Alterations Caused by a High Sucrose Fat Diet in a Wistar Rats Model. Plant Foods Hum Nutr 76, 12–19 (2021). https://doi.org/10.1007/s11130-020-00867-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00867-3