Abstract
Two environmentally friendly innovative extraction techniques - subcritical water (SWE) and microwave-assisted extraction (MAE) were applied for the extraction of phenolics from pomegranate peel. The impact of process conditions (SWE: temperature 100–220 °C, extraction time 5–30 min; MAE: solvent water and 50% ethanol, irradiation power 470 and 800 W) on the quality of extracts in terms of the content of total phenolics, total flavonoids, major phenolic constituents (gallic acid, ellagic acid, punicalin, punicalagin), as well as 5-hydroxymethylfurfural(HMF) amount was investigated. For SWE, temperature of 130 °C and 20 min extraction time were found optimal for obtaining high content of bioactive compounds and minimizing the yield of HMF. During MAE, phenolic compounds were effectively extracted by using lower microwave power and 50% ethanol. Comparing two techniques, MAE is more efficient than SWE for the extraction of phenolics from pomegranate peel while obtaining a HMF-free extracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pomegranate fruit (Punica granatum L., Punicaceae) has been used in the traditional medicine of many cultures, in particular in the Middle East and Mediterranean. Pomegranate fruit peel, commonly treated as the waste of the food industry is now considered as a valuable source of bioactive compounds [1, 2]. It is characterized by the presence of considerable levels of bioactive phenolics such as ellagitannins and gallotannins, proanthocyanidins, anthocyanins, phenolic acids, and flavonoids [1, 3, 4]. The intake of these compounds is associated with reduced risks of chronic diseases such as cancer, cardiovascular diseases, and diabetes due to their high antioxidant activity [5]. It has been reported that the content of antioxidant compounds in pomegranate peel was higher than the content in seeds and pulp. Among polyphenols present in pomegranate, special attention is put on peculiar metabolites punicalagin, punicalin, and granatin B for which high antioxidant activity has been reported [1]. Pomegranate peel also contains complex polysaccharides, mainly pectin, cellulose, and hemicellulose [6, 7].
Selection of compatible extraction technique and setup of optimal extraction parameters are the key factors for the extraction and recovery of valuable bioactive metabolites and production of high-quality extracts. Although conventional solid-liquid extraction is commonly used for the production of extracts from medicinal plants, it has been recently overcome by the application of modern extraction techniques, i.e., ultrasonic-assisted (UAE), microwave-assisted (MAE), pressurized liquid extraction, or extraction with green solvents such as aqueous solutions of cyclodextrins [8, 9]. As a type of pressurized fluid extraction technique, subcritical water extraction (SWE) has been widely used for the isolation of different polar to low-polar compounds such as phenolic compounds, flavonoids, anthocyanins, and essential oils [8, 10]. Water is extremely polar solvent at ambient conditions and it is not suitable for the extraction of non-polar or moderately polar compounds. This problem can be overcome by increasing temperature and pressure, causing decrease of dielectric constant which makes water more suitable for the extraction of such compounds. SWE is carried out using water in subcritical state above boiling temperature, but below critical temperature (100–374.15 °C), and at the pressure high enough (below 22 MPa) to keep it in the liquid state. Moreover, subcritical water is characterized by lower viscosity but higher diffusivity, enabling better diffusion into plant matrix and release of metabolites to liquid phase [11]. By applying different temperature and pressure, physical properties of water are changed, leading to different extraction selectivity toward various classes of metabolites. Extraction time of SWE technology is much shorter in comparison with classical extraction techniques, maintaining extract of high quality with lower number of operation units and higher work safety due to usage of non-toxic solvent, i.e., water.
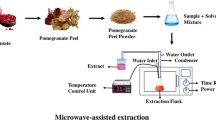
One more technology that fits in the concept of modern, fast, reproducible, and clean process technologies is the microwave-assisted extraction (MAE). MAE has proven to be an efficient method for extraction of polyphenols because of its advantages such as short extraction time, suitability for thermolabile compounds, and lower solvent consumption. Another advantage of applying microwave energy is non-contact heat source, faster energy transfer, reduced thermal gradients, selective heating, faster response to process heating control, and faster startup which altogether impacts the increase in production [12]. During MAE, the sample material becomes heated because of localized interaction with microwaves which further leads to a higher diffusion of polyphenols out of the plant matrix and into the solvent. Therefore, both the extraction time and microwave power are significant factors [13, 14].
However, there are some drawbacks in the application of these extraction technologies. Major factors that are critical for their efficiency are the polarity of solvent and analytes of interest, and the extraction temperature [10, 15]. The use of high temperatures may affect heat sensitive compounds, such as polyphenols, causing their degradation. Furthermore, thermal processing may also induce chemical changes and formation of contaminants in foodstuff. 5-hydroxy-2-methylfurfural(HMF) is example of undesirable compound that is generated during heating process or long-term storage [16, 17]. HMF is not naturally present in fresh or untreated foods, but is formed from dehydration of monosaccharides when carbohydrate-rich foods are subjected to heat treatment. The concentration of HMF depends on the applied temperature and the type of sugar. It has been shown that fructose generates higher rates of HMF than glucose or mannose, and concentration of produced HMF increased with the rise of temperature [18]. In addition to that HMF is an indicator of the food freshness, there are also reports of its carcinogenic activity in rats and mice [17]. However, toxicological relevance on human health is not clearly documented. It is more likely that HMF show indirect toxic effect through its easily conversion to sulfoxymethylfurfural (SMF), compound which has been reported to exhibit direct mutagenicity [19]. Therefore, it is important to evaluate the presence of HMF compound from a food safety perspective. No article related to the HPLC determination of this compound in pomegranate peel have been published previously.
The aim of the present study was to investigate the effect of SWE and MAE on the extraction of polyphenols and the HMF formation in pomegranate peel. By varying different extraction parameters, the impact of these extraction technologies on the content of punicalin, punicalagin, ellagic and gallic acids, total phenolics and total flavonoids, as well as HMF was evaluated.
Materials and Methods
The detailed “Material and Methods” is presented as a Supplementary Material file.
Results and Discussion
Subcritical water extraction (SWE)
The contents of total phenolics (TP), total flavonoids (TF), and extraction yield (EY) in pomegranate peel extracts obtained by SWE at different temperatures (100, 130, 160, 190, and 220 °C) and two extraction times (10 and 20 min) are presented in Table 1. Total phenolics content in different extracts ranged from 4.69 to 14.16 g GAE/100 g DW. A significant rise in the content of phenolics was noted with the increase in temperature from 100 to 160 °C, and 160 °C at 10 min being the point at which the highest content of TP was obtained. With further increase in temperature the content of phenolics decreased, and the extract obtained at the highest temperature of 220 °C had a three times lower content of phenolics in comparison with the highest one. The same pattern was noticed for TF, their content increase with the temperature, reaching maximum (2.13 g CE/100 g) also at 160 °C. The increase of total phenolics and flavonoids content was caused by the increased solubility of these compounds in subcritical water due to the increase in temperature. Water at room temperature has a relatively high dielectric constant, however, at high temperatures it decreases and thus increases water’s extraction power. Moreover, high temperature favors the mass-transfer kinetics by disrupting the analyte-matrix interaction, especially by hydrogen bonding and dipole-dipole forces, thereby facilitating initial desorption of the analytes from the sample matrix. Also, it accelerates diffusivity and alters solubility [20]. However, higher temperatures, in this case higher than 160 °C, lead to degradation of phenolics. Our results are in accordance with previous report where temperature affected the content of TP and TF in a way that the two contents increased with the increase in temperature up to 130 °C, and after this temperature point they rapidly decreased [21].
Extraction time also affected TP and TF, but with no statistically significant effect. At lower temperatures, longer time (20 min) gave extracts with higher TP and TF content compared with shorter time (10 min). However, at temperature of 160 °C and higher, prolonged time of extraction decreased the level of observed responses and the lowest yields were obtained at temperature of 220 °C and 20 min time. Similar trend was recorded in previous reports, where longer period of time (after 20 min) was not favorable for the extraction of TP and TF [21, 22]. With a combined influence of extended exposure (20 min) of the material to high temperatures, the most effective temperature for extraction of total phenolics and flavonoids decreases. Therefore, the temperature at which the highest content of these compounds achieved during 20 min of extraction time was 130 °C, while the further increase in temperature decreases the content of phenols in extracts.
The values of TP and TF in our study were lower compared with those published by Yan et al. [21], whereas the content of TF was higher than those obtained in the study of Çam and Hışıl [22]. The difference in polyphenols content might be caused by the origin of the raw material, the way it was stored and processed. Particle size is another factor that may influence the extraction efficiency. Extraction from smaller particles is more efficient than the extraction from larger particles due to increased contact surface and shorter diffusion path [22]. In the study from Yan et al. [21], they used raw material that was grounded to 250 μm size, which was smaller than particles used in our study (750–2000 μm).
Observing the situation from the economic point of view, the influence of temperature on the extraction yield is desirable because lower temperatures provide higher extraction yields. Using 100 °C temperature for 10 min resulted in the highest extraction yield of 47.48%, followed by yields obtained at 160 and 130 °C (40.98 and 40.55%, respectively) with no statistical difference between them. The lowest extraction yield takes place at temperature of 220 °C, as it was shown for TP and TF content.
The results of the effect of temperature and time on the amounts of individual compounds are shown in Table 1, and representative HPLC chromatogram is presented in Fig. 1A. The results indicated that lower temperatures (100 and 130 °C) favor higher yield of punicalin and punicalagin, while gallic and ellagic acid contents reached maximum values at 160 and 130 °C, respectively. At temperature of 190 °C, the concentrations of gallic acid, ellagic acid and punicalin sharply decreased, whereas punicalagin was not detected already at 160 °C. Furthermore, time also affected the contents of observed compounds at higher temperatures. Longer exposure time negatively affected the yields of gallic acid and punicalin at temperature of 190 °C, and punicalagin at 160 °C. It has been previously shown that the contents of ellagic acid, punicalin and punicalagin increased with temperature up to 130 °C, after which their yields decreased [21]. Regarding HMF, its content increased slowly as the temperature increased from 100 to 130 °C, after which increased rapidly reaching the highest level at 220 °C. As the temperature rose from 100 to 130 °C, HMF concentration increased 1.4 times whereas its content at 220 °C was up to 16-fold higher than at 100 °C. Time also influenced the rate of HMF formation, but this effect was not statistically significant. Higher amounts were detected with extended heating time at all temperatures up to 220 °C, after which it decreased from 10 to 20 min time.
HPLC analysis of HMF in pomegranate peel processed under subcritical water extraction has not been studied so far. However, our results are in agreement with previous findings for other foodstuffs. Kanmaz [23] reported noticeable rise of HMF in lemon peel when temperature increased from 50 to 180 °C. It has been also shown that higher temperatures (150–210 °C) led to higher level of HMF in coffee silverskin and pistachio hulls [24, 25]. According to the results obtained in our study as well in the cited reports, it seems that temperatures of 180–190 °C were critical for significant HMF increase. Formation of HMF at high temperatures might be due to sugar decomposition. Lignocellulose (cellulose, hemicellulose and lignin), pectin, and soluble sugars (glucose, arabinose, xylose, galactose, mannose and rhamnose) are the constituents of pomegranate peels, and their content vary with cultivars and extraction conditions [6, 7, 26, 27]. During thermal processing, cellulose and hemicellulose were hydrolysed to monomer units which were then converted to HMF. The increase of HMF at high extraction temperature could be explained by decomposition of these hexoses liberated from hemicellulose and cellulose.
In general, conditions of subcritical water extraction of pomegranate peel should be selected and optimized in order to produce high quality products. Taking into account the overall results, temperature of 130 °C and 20 min time were found optimal in terms of obtaining high yield of bioactive compounds and minimizing the yield of HMF.
Microwave-Assisted Extraction (MAE)
The effects of solvent type and microwave power on the EY, TP, TF and individual compounds in pomegranate peels were evaluated, and the results are shown in Table 2. The highest contents of all observed components were obtained when 50% ethanol was used, and for TP, TF, EY and punicalagin this effect was statistically significant. Our observations are in alignment with previously published studies for the MAE of pomegranate peel, where 50 and 60% aqueous ethanol were found optimal for the extraction of TP and TF, respectively, and further increase in ethanol percentage decreased their yield [28, 29]. It has been also shown that phenolic compounds from blueberry powder, chokeberries, and sea buckthorn were effectively extracted under MAE with 50% ethanol [30,31,32]. The choice of solvent represents a very important parameter that impacts the efficacy of the microwave extraction. Selectivity towards the analyte and dielectric properties of the solvent are significant factors for obtaining extracts with high quality. Water has high dielectric constant, followed by methanol and ethanol, which makes them good absorbers of microwave energy. On the other hand, methanol and ethanol have higher dielectric loss than water. This further implies that these organic solvents convert electromagnetic energy into heat more effectively than water. Therefore, combined solvents with high dielectric constant (e.g., water) and high dissipation factor (e.g., ethanol) shows synergistic effect which lead to better extraction efficiency of phenolic compounds.
In the case of power level, lower irradiation power (470 W) had a more positive effect on the quality of extracts, only TF and ellagic acid contents were higher at increased power (800 W)(Table 2). This is in accordance with the study of Zheng et al. [33] who found that TP content in pomegranate peel increased with increasing the power from 150 to 600 W, after which declined at 750 W. Kaderides et al. [29] also reported that extraction rate of TP was highest by applying 600 W microwave power. In general, the yield of polyphenol compounds increases with an increase of microwave power. At higher power the solution is heated, promoting the transfer of polyphenols from cell to extraction system. However, exposure to higher power with prolonged time can cause overheating of the plant samples and loss of thermally sensitive components. In that sense, it is important to choose the right power to avoid such effects. The optimal microwave power depends of the properties of phenolic compounds present in plant matrix. For phenolics from pomegranate peel as well as from mango leaves and chokeberries [29, 32,33,34], lower microwave power was found suitable for their extraction. Moreover, taking energy costs into consideration, using lower energy for the process is desirable.
Fig. 1B shows HPLC chromatogram of pomegranate peel extract obtained by microwave-assisted extraction. It should be noted that HMF was not detected in any of analyzed sample, which is another advantage of applying this type of extraction.
Comparison between SWE and MAE
Both SWE and MAE fit in the concept of green extractions which overcome the shortcomings of classical extractions. The two techniques are fast, convenient, ensure low solvent consumption, and apart from filtration, do not require additional purification of extracts. Water and ethanol are solvents recommended by the US Food and Drug Administration as eco-friendly and non-toxic food grade solvents [35]. However, the nature of the plant material and compounds of interest significantly affect the choice and adequacy of the technology. Principal component analysis (PCA) was applied to identify potential clasterizations of extraction techniques and separate them according to the polyphenols content and extraction yield. PCA is graphically presented by PC1 and PC2, which together explaining nearly 90% of total variance (Fig. 2). All MAE loadings were negatively related with PC1 and positively related to PC2, while majority of SWE loadings were indifferent to PC1 and slightly negatively related to PC2. It is obvious that cluster of MAE favor accumulation of TP, TF, ellagic acid, and punicalagin in the final extract. On the other hand, centralized cluster of SWE extraction is closely correlated with gallic acid, punicalin, and extraction yield. The PCA also indicated that distant SWE loadings (6–10) were extremely poor in observed determinants, showing that temperature above 160 °C was not favorable for their extraction.
Principal component analysis of bioactive constituents of extracts obtained by using different extraction techniques and conditions (1: SWE 100 °C, 10 min; 2: SWE 100 °C, 20 min; 3: SWE 130 °C, 10 min; 4: SWE 130 °C, 20 min; 5: SWE 160 °C, 10 min; 6: SWE 160 °C, 20 min; 7: SWE 190 °C, 10 min; 8: SWE 190 °C, 20 min; 9: SWE 220 °C, 10 min; 10: SWE 220 °C, 20 min; 11: MWE 800 W, water; 12: MWE 800 W, 50% ethanol; 13: MWE 470 W, water; 14: MWE 470 W, 50% ethanol). SWE - subcritical water extraction; MWE - microwave-assisted extraction; TP – total phenolics; TF – total flavonoids; EY – extraction yield; PC- punicalin; PCG – punicalagin; GAL – gallic acid; EA – ellagic acid
In general, considering the quality of extracts it is evident that MAE is more efficient technique for the extraction of phenolics from pomegranate peel, at the same time obtaining a HMF-free extracts. In MAE, the herbal material becomes heated as a result of a localized interaction with microwaves, which further leads to an increased diffusion of polyphenolic compounds from the herbal material to the surrounding solvent. It was recorded that higher irradiation power causes lower efficacy of the extraction of polyphenols probably due to the degradation of thermolabile components. This also represents a potential explanation for the better efficiency of MAE compared to SWE considering that the temperatures used in SWE are the possible cause of thermal degradation resulting in a lower extraction rate of polyphenols. Furthermore, having in mind that the SWE equipment is more sophisticated and applies very high temperatures, it can be concluded that, apart from the positive influence on the quality of extracts, MAE is also more suitable in terms of process safety and cost.
Conclusion
In this study, different process parameters of green extraction methods, microwave-assisted extraction and subcritical water extraction, were investigated in order to determine the most adequate ones which provide high quality of pomegranate peel extracts and maximal exploitation of the raw material without the negative impact on the environment. It was concluded that lower temperatures of subcritical water extraction are more convenient for the valorization of pomegranate peel because they provide a higher content of phenols and minimize the presence of HMF. However, microwave-assisted extraction proved to be the more suitable method in terms of quality and product safety – high content of polyphenols without the presence of HMF, as well as significant reduction of costs of the process and equipment. The results of the study provide a significant contribution to the concept of circular economy considering that they suggest sustainable solutions which include reduction of food waste/by-products, exploitation of pomegranate peel through efficient separation of nutrients, and at the same time, maximal exploitation of natural resources.
References
Akhtar S, Ismail T, Fraternale D, Sestili P (2015) Pomegranate peel and peel extracts: chemistry and food features. Food Chem 174:417–425. https://doi.org/10.1016/j.foodchem.2014.11.035
Sood A, Gupta M (2015) Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci 12:100–106. https://doi.org/10.1016/j.fbio.2015.09.004
Fischer UA, Carle R, Kammerer DR (2011) Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem 127:807–821. https://doi.org/10.1016/j.foodchem.2010.12.156
Li J, He X, Li M, Zhao W, Liu L, Kong X (2015) Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem 176:7–11. https://doi.org/10.1016/j.foodchem.2014.12.040
Salgado JM, Ferreira TRB, de Oliveira BF, dos Santos Dias CT (2012) Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum Nutr 67(1):39–43. https://doi.org/10.1007/s11130-011-0264-y
Pocan P, Bahcegul E, Oztop MH, Hamamci H (2018) Enzymatic hydrolysis of fruit peels and other lignocellulosic biomass as a source of sugar. Waste Biomass Valori 9:929–937. https://doi.org/10.1007/s12649-017-9875-3
Hasnaoui N, Wathelet B, Jiménez-Araujo A (2014) Valorization of pomegranate peel from 12 cultivars: dietary fibre composition, antioxidant capacity and functional properties. Food Chem 160:196–203. https://doi.org/10.1016/j.foodchem.2014.03.089
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375. https://doi.org/10.3390/molecules18022328
Diamanti AC, Igoumenidis PE, Mourtzinos I, Yannakopoulou K, Karathanos VT (2017) Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins. Food Chem 214:61–66. https://doi.org/10.1016/j.foodchem.2016.07.072
Herrero M, Castro-Puyana M, Mendiola JA, Ibañez E (2013) Compressed fluids for the extraction of bioactive compounds. TrAC Trend Anal Chem 43:67–83. https://doi.org/10.1016/j.trac.2012.12.008
Teo CC, Tan SN, Yong JWH, Hew CS, Ong ES (2010) Pressurized hot water extraction (PHWE). J Chromatogr A 1217:2484–2494. https://doi.org/10.1016/j.chroma.2009.12.050
Metaxas AA, Meredith RJ (1983) Industrial microwave heating (no. 4). IET
Ameer K, Shahbaz HM, Kwon JH (2017) Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr Rev Food Sci Food Saf 16:295–315. https://doi.org/10.1111/1541-4337.12253
Delazar A, Nahar L, Hamedeyazdan S, Sarker SD (2012)Microwave-assisted extraction in natural products isolation. In: Natural products isolation, Humana Press, pp 89–115
Wang L, Weller CL (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 17(6):300–312. https://doi.org/10.1016/j.tifs.2005.12.004
Ameur LA, Trystram G, Birlouez-Aragon I (2006) Accumulation of 5-hydroxymethyl-2-furfural in cookies during the backing process: validation of an extraction method. Food Chem 98:790–796. https://doi.org/10.1016/j.foodchem.2005.07.038
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci Technol 44:793–810. https://doi.org/10.1016/j.lwt.2010.11.002
Khajavi SH, Kimura Y, Oomori T, Matsuno R, Adachi S (2005) Degradation kinetics of monosaccharides in subcritical water. J Food Eng 68:309–313. https://doi.org/10.1016/j.jfoodeng.2004.06.004
Lee CH, Chen KT, Lin JA, Chen YT, Chen YA, Wu JT, Hsieh CW (2019) Recent advances in processing technology to reduce 5-hydroxymethylfurfural in foods. Trends Food Sci Technol 93:271–280. https://doi.org/10.1016/j.tifs.2019.09.021
Plaza M, Turner C (2015) Pressurized hot water extraction of bioactives. TrAC Trend Anal Chem 71:39–54. https://doi.org/10.1016/j.trac.2015.02.022
Yan L, Cao Y, Zheng G (2017) Optimization of subcritical water extraction of phenolic antioxidants from pomegranate (Punica granatum L.) peel by response surface methodology. Anal Methods 9:4647–4656. https://doi.org/10.1039/C7AY01475A
Çam M, Hışıl Y (2010) Pressurised water extraction of polyphenols from pomegranate peels. Food Chem 123:878–885. https://doi.org/10.1016/j.foodchem.2010.05.011
Kanmaz EÖ (2018)5-Hydroxymethylfurfural(HMF) formation during subcritical water extraction. Food Sci Biotechnol 27:981–986. https://doi.org/10.1007/s10068-018-0328-y
Narita Y, Inouye K (2012) High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem 135:943–949. https://doi.org/10.1016/j.foodchem.2012.05.078
Erşan S, Üstündağ ÖG, Carle R, Schweiggert RM (2018) Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem 253:46–54. https://doi.org/10.1016/j.foodchem.2018.01.116
Abid M, Renard CM, Watrelot AA, Fendri I, Attia H, Ayadi MA (2016) Yield and composition of pectin extracted from Tunisian pomegranate peel. Int J Biol Macromol 93:186–194. https://doi.org/10.1016/j.ijbiomac.2016.08.033
Dafny-Yalin M, Glazer I, Bar-Ilan I, Kerem Z, Holland D, Amir R (2010) Color, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. J Agric Food Chem 58:4342–4352. https://doi.org/10.1021/jf904337t
Huang J, He W, Yan X, Sh X (2017) Microwave assisted extraction of flavonoids from pomegranate peel and its antioxidant activity. BIO Web of Conferences 8:03008. https://doi.org/10.1051/bioconf/20170803008
Kaderides K, Papaoikonomou L, Serafim M, Goula AM (2019)Microwave-assisted extraction of phenolics from pomegranate peels: optimization, kinetics, and comparison with ultrasounds extraction. Chem Eng Process 137:1–11. https://doi.org/10.1016/j.cep.2019.01.006
Galan AM, Calinescu I, Trifan A, Winkworth-Smith C, Calvo-Carrascal M, Dodds C, Binner E (2017) New insights into the role of selective and volumetric heating during microwave extraction: investigation of the extraction of polyphenolic compounds from Sea buckthorn leaves using microwave-assisted extraction and conventional solvent extraction. Chem Eng Process 116:29–39. https://doi.org/10.1016/j.cep.2017.03.006
Zheng X, Xu X, Liu C, Sun Y, Lin Z, Liu H (2013) Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Sep Purif Technol 104:17–25. https://doi.org/10.1016/j.seppur.2012.11.011
Simić VM, Rajković KM, Stojičević SS, Veličković DT, Nikolić NČ, Lazić ML, Karabegović IT (2016) Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep Purif Technol 160:89–97. https://doi.org/10.1016/j.seppur.2016.01.019
Zheng X, Liu B, Li L, Zhu X (2011)Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J Med Plant Res 5:1004–1011
Zou T, Wu H, Li H, Jia Q, Song G (2013) Comparison of microwave-assisted and conventional extraction of mangiferin from mango (Mangifera indica L.) leaves. J Sep Sci 36:3457–3462. https://doi.org/10.1002/jssc.201300518
Bartnik DD, Mohler CM, Houlihan M (2006) Methods for the production of food grade extracts. US Patent 20060088627, 27 April
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, contract number 451-03-68/2020-14/ 200003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Vladić, J., Janković, T., Živković, J. et al. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum Nutr 75, 553–560 (2020). https://doi.org/10.1007/s11130-020-00848-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00848-6