Abstract
Nonconvulsive electrotherapy (NET) defined as electrical brain stimulation administered like standard electroconvulsive therapy (ECT), but below seizure threshold, could be effective for patients with treatment-refractory depression (TRD) with fewer adverse neurocognitive outcomes. However, there is a lack of studies in Chinese patients with TRD. Thus, this study was conducted to examine the efficacy and safety of adjunctive NET for Chinese patients with TRD. Twenty TRD patients were enrolled and underwent six NET treatments. Depressive symptoms, response, and remission were assessed with the 17-item Hamilton Depression Rating Scale (HAMD-17) at baseline and after 1, 3, and 6 NET treatments. Neurocognitive function was assessed by the Wisconsin Card Sorting Test (WCST) at baseline and after the completion of six NET treatments. Mean HAMD-17 scores declined significantly from 26.2 to 10.4 (p < 0.001) after post-NET. The rates of response and remission were 60.0% (95% CI: 36.5–83.5) and 10.0% (95% CI: 0–24.4), respectively. Neurocognitive performance improved following a course of NET. No significant association was found between changes in depressive symptoms and baseline neurocognitive function. Adjunctive NET appeared to be effective for patients with TRD, without adverse neurocognitive effects. Randomized controlled studies were warranted to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is one severe mental disorder, accounting for 40.5% of disability-adjusted life years caused by all mental and substance use disorders [1]. Despite substantial improvements in treatment over the past decades, patients with treatment-refractory depression (TRD) are inadequately responsive to pharmaceutical and non-pharmaceutical interventions [2, 3]. Consequently, augmentation strategies, such as antidepressants co-treatment [4], and adjunctive antipsychotics [5], antiepileptic drugs [6], anxiolytics [7], esketamine [8], ketamine [9, 10], Chinese herbal medicine [11], repetitive transcranial magnetic stimulation (rTMS) [12], magnetic seizure therapy [13, 14], deep brain stimulation [15,16,17], electroconvulsive therapy (ECT) [18], and nonconvulsive electrotherapy (NET) [19, 20] had been used to enhance the therapeutic efficacy of antidepressants.
NET was performed by electrical brain stimulation undergoing the standard ECT technique [19]. Unlike ECT, electric stimulation of NET was insufficient strength to be convulsion-evoking, resulting in fewer adverse neurocognitive outcomes than ECT [19, 20]. Animal studies found that subconvulsive electrical stimulation could induce antidepressant-like effects similar to that of ECT, without inducing seizures [21]. Early studies from the 1940s and 1950s found that subconvulsive and nonconvulsive electric stimulation treatments appeared to be effective for major mental disorders including MDD, without adverse neurocognitive effects [22,23,24]. Similarly, Prudic et al. reported no adverse neurocognitive consequences of subconvulsive electrical stimulation [25].
In general, the antidepressant effects of ECT could attribute to inducing a bilateral generalized seizure [26], which has been challenged. For example, numerous clinical researchers had purported to establish the necessity of inducing a seizure [19, 20, 27]. The antidepressant response to rTMS further suggests that a seizure may not be necessary to achieve antidepressant response to ECT [28]. Importantly, a recent open-label study of adjunctive NET for TRD found that the antidepressant effects of NET were similar to that of ECT, without adverse neurocognitive outcomes induced by NET [19].
Regenold et al. that examined the role of NET in treating patients with TRD have several limitations [19]. For example, they recruited participants with small sample size (n = 13) and only focused on outpatients with treatment resistant unipolar and bipolar depression, limiting the generalizability of the findings [19]. Furthermore, a portion of depressed patients fail to respond to NET, but the reason is unclear [19]. Therefore, the objectives of this study were to (1) examine the antidepressant and neurocognitive effects of adjunctive NET in Chinese patients with TRD; and (2) evaluate the correlation of neurocognitive function at baseline and the change in severity of depressive symptoms.
Methods
Subjects
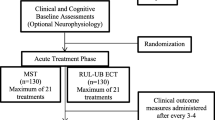
The study of adjunctive NET for TRD was approved by the Institutional Review Board of the Affiliated Brain Hospital of Guangzhou Medical University. Han Chinese patients with TRD who signed written informed consent before participation were recruited from the Inpatient Psychiatric Unit of the Affiliated Brain Hospital of Guangzhou Medical University during the period between January 2017 and December 2017. This study was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria were that the patients: (1) had written informed consent; (2) met the diagnostic criteria for MDD based on the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) [29] with a total score of 17 or more on the 17-item Hamilton Depression Rating Scale score (HAMD-17) [30]; (3) were aged 18 to 50 years with TRD failure to achieve a satisfactory response to at least two antidepressants administered at an adequate dose and duration during the current episode [9]; (4) had not undergone ECT within the past 3 months.
Exclusion criteria were that the patients: (1) satisfied the diagnostic criteria for any other serious mental disorder according to DSM-IV, such as organic mental disorders, schizophrenia, bipolar disorder, or substance use disorder; (2) had any neurological illnesses or serious medical conditions; (3) had a previous history of seizures; (4) were breast feeding or pregnant; (5) had a foreign body, such as pacemaker or intracranial electrode.
Treatment
Prior to undergoing NET, blood and urine tests, chest x-ray, electrocardiogram (ECG), electroencephalography (EEG), and physical and neurological examinations were evaluated for all participants. Bilateral NET treatment was given 3 times a week for two weeks with a total of 6 sessions using the Thymatron ® IV device (Somatics LLC, Lake Bluff, Illinois, USA) (pulse width, 0.5 ms; frequency, 20 Hz; current, 0.9A). All participants continued taking psychotropic agents during the NET course.
All patients first received atropine sulfate at a dose of 1 mg. Then, they received propofol at a dose of 1 mg/kg intravenous injection for NET anesthesia. Succinylcholine at a dose of 1 mg/kg was intravenously administered as a muscle relaxant. Following the reported methodology of Regenold et al. [19], the stimulus intensity of NET was set at 1/8 of the standard ECT dose as estimated by the “half age” stimulation strategy for ECT dosing. Seizure or lack of seizure was verified per usual ECT protocol by using EEG and observing tonic-clonic movements. Systolic and diastolic blood pressures, pulse frequency and respiratory rate were recorded just before anesthesia and approximately 10 min following the NET procedure.
Assessment of Depressive Symptoms and Neurocognitive Function
The severity of depressive symptoms was evaluated by the HAMD-17 [30] at baseline, and 1 day after the first, third, and sixth NET treatments. Participants were classified as responders and remitters based on a reduction of 60% or more in the HAMD-17 scores from the baseline [31, 32] and HAMD-17 scores ≤7 [33], respectively.
Neurocognitive performance was evaluated by a trained neuropsychological technician using the Wisconsin Card Sorting Test (WCST) [34] at baseline and 1 day after the sixth NET treatment. The WCST has been demonstrated to have satisfactory reliability and validity for the evaluation of cognition and are commonly used in clinical practice.
Statistical Analysis
All outcomes were analyzed using SPSS version 23.0 for windows. Statistical significance was set at p < 0.05. The comparison of demographic and clinical variables between responders and non-responders was performed by the Student’s t test or Mann–Whitney U test for continuous data, and by the Chi-square test or Fisher’s exact test for categorical data, as appropriate. Baseline and post-NET HAMD-17 and WCST scores were compared by paired-samples t-test, if necessary. HAMD-17 scores at each assessment time point during a course of NET were compared by linear mixed model analysis. Correlation analyses were conducted to examine the association of baseline neurocognitive function and the antidepressant effects of NET in patients with TRD.
Results
Twenty patients with TRD contributed to the analyses, including 9 females and 11 males. The mean age was 29.2 ± 8.7 years, and the mean baseline HAMD-17 scores were 26.2 ± 2.4. The demographic and clinical characteristics are presented in Table 1, finding no significant differences between responders and non-responders.
Antidepressant Outcomes
After post-NET, the rates of response and remission were 60.0% (95% CI: 36.5–83.5) and 10.0% (95% CI: 0–24.4), respectively. The linear mixed model with HAMD-17 scores showed significant main effects of time (F = 308.0, p < 0.001) and group-by-time interactions (F = 5.1, p = 0.005) (Table 2).
The mean HAMD-17 scores declined significantly from baseline (26.2) to the end of a course of NET (10.4) (Fig. 1 and Supplemental Table 1). The significant difference in HAMD-17 scores between responders and non-responders was found at the assessment of completing six NET treatments (effect size = 1.9, Supplemental Table 1).
Neurocognitive Function
Neurocognitive performance as measured by WCST obtained a significant improvement after post-NET (Fig. 2). As shown in Table 2, the linear mixed model analysis found a significant time main effects in each of the four domains of WCST, including completing classification number, total error number, persistent error number, and random error number. No significant differences between responders and non-responders were found in terms of baseline and post-NET neurocognitive function (Supplemental Table 1).
Association between Baseline Neurocognitive Function and Change in HAMD-17 Scores
As shown in Supplemental Table 2, correlation analysis shows no significant association of HAMD-17 change score with baseline neurocognitive function (all p > 0.05).
Discussion
To our knowledge, this is the first study to examine the antidepressant and neurocognitive effects of adjunctive NET for Chinese patients with TRD. The main finding was that adjunctive NET had antidepressant efficacy for Chinese patients with TRD and was not associated with short-term neurocognitive impairments typically seen with ECT. In contrast, significant improvements were found in each of the four domains of WCST after completing six consecutive NET treatments. Baseline neurocognitive performance was not a predictor of NET treatment outcome.
Consistent with the findings of Regenold et al. [19], the current study shown NET can have the dramatic antidepressant efficacy in patients with TRD. In this study, 60% of patients with TRD reached the criteria of response after the course of treatments. Similarly, Regenold et al. [19] found a response rate of 73.0% after a course of NET. In this study, mean HAMD-17 scores significantly declined from 26.2 to 10.4 by the end of treatment. Similarly, Regenold et al. [19] reported that mean HAMD-17 scores were significantly declined from 20.3 to 8.6 after a course of NET. Taken together, these findings indicate that a seizure may not be necessary for some individuals with TRD to achieve a therapeutic response.
ECT is considered the gold standard for the treatment of patients with TRD, but also has several limitations, particularly inducing neurocognitive deficits, mainly in memory and executive functions [35, 36]. Neurocognitive impairment was associated with higher treatment non-compliance, disease and disability burden, and suicide risk [37]. Fear of ECT and distressing adverse neurocognitive outcomes were commonly described among patients [6]. In this study, however, neurocognitive performance as measured by WCST had a significant improvement after a course of NET. Similarly, an early study reported that Mini-Mental State Exam (MMSE) scores increased by a mean of 0.9 points from baseline to the end of NET treatment [19]. Taken together, these findings suggest that NET did not appear to induce neurocognitive impairment.
Although Regenold et al. [19] had examined the neurocognitive effects of adjunctive NET for treatment resistant unipolar and bipolar depression, the association between baseline neurocognitive function and treatment outcomes of NET was not investigated. In this study, we found that baseline neurocognitive function does not predict the antidepressant effects of NET in TRD. Similarly, Bjolseth et al. found that neurocognitive function at baseline appeared to be not a predictor of ECT treatment outcome [38]. The depression-executive dysfunction (DED) model predicts that depressed patients suffering from impaired executive performance are likely to respond poorly to antidepressant therapy [39]. However, the DED model was not strongly supported by a recent meta-analysis including 17 antidepressant trials (n = 1269) [40]. Our finding did not necessarily refute the DED model because the severity and extension of white matter abnormalities were not evaluated in our sample.
Some limitations of this study need to be mentioned. First, similar to Regenold et al’s study [19], the sample size of this study was relatively small and the open-label study protocol limits the interpretation of efficacy. Second, NET was not associated with the usual adverse neurocognitive effects seen with ECT, while the evaluations of neurocognitive function in this study were not extensive. Thus, other neurocognitive assessment tool, such as magnetic resonance imaging (MRI) together with clinical and behavioral assessment [41], should be utilized to detect subtle neurocognitive deficits induced by NET that were undetected in this study. Finally, although this study firstly examined the association of baseline neurocognitive functioning and antidepressant effects of NET, the optimal biomarker assessments were related to the multi-modal data acquisition, peripheral blood markers [42], neuroimaging [43], and quantitative electroencephalography [44].
Conclusion
Adjunctive NET appeared to be effective for patients with TRD, without adverse neurocognitive effects. Randomized controlled studies were warranted to confirm these findings.
References
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382(9904):1575–86. https://doi.org/10.1016/s0140-6736(13)61611-6.
Voineskos D, Daskalakis ZJ, Blumberger DM. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat. 2020;16:221–34. https://doi.org/10.2147/ndt.s198774.
Strawn JR, Croarkin PE. Commentary: treatment failure and success: a commentary on defining and treating pediatric treatment-resistant depression - reflections on Dwyer et al. (2020). J Child Psychol Psychiatry. 2020;61:333–5. https://doi.org/10.1111/jcpp.13207.
Galling B, Calsina Ferrer A, Abi Zeid Daou M, Sangroula D, Hagi K, Correll CU. Safety and tolerability of antidepressant co-treatment in acute major depressive disorder: results from a systematic review and exploratory meta-analysis. Expert Opin Drug Saf. 2015;14(10):1587–608. https://doi.org/10.1517/14740338.2015.1085970.
Gerhard T, Stroup TS, Correll CU, Huang C, Tan Z, Crystal S, et al. Antipsychotic medication treatment patterns in adult depression. J Clin Psychiatry. 2018;79(2). https://doi.org/10.4088/JCP.16m10971.
Solmi M, Veronese N, Zaninotto L, van der Loos ML, Gao K, Schaffer A, et al. Lamotrigine compared to placebo and other agents with antidepressant activity in patients with unipolar and bipolar depression: a comprehensive meta-analysis of efficacy and safety outcomes in short-term trials. CNS Spectr. 2016;21(5):403–18.
Davies P, Ijaz S, Williams CJ, Kessler D, Lewis G, Wiles N. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:Cd010557. https://doi.org/10.1002/14651858.CD010557.pub2.
Zheng W, Cai DB, Xiang YQ, Zheng W, Jiang WL, Sim K, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63–70. https://doi.org/10.1016/j.jad.2020.01.002.
Zheng W, Zhou YL, Liu WJ, Wang CY, Zhan YN, Li HQ, et al. Investigation of medical effect of multiple ketamine infusions on patients with major depressive disorder. J Psychopharmacol (Oxford, England). 2019;33(4):494–501. https://doi.org/10.1177/0269881119827811.
Zheng W, Zhou YL, Liu WJ, Wang CY, Zhan YN, Li HQ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res. 2018;106:61–8. https://doi.org/10.1016/j.jpsychires.2018.09.013.
Wang Y, Shi YH, Xu Z, Fu H, Zeng H, Zheng GQ. Efficacy and safety of Chinese herbal medicine for depression: a systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2019;117:74–91. https://doi.org/10.1016/j.jpsychires.2019.07.003.
Gellersen HM, Kedzior KK. Antidepressant outcomes of high-frequency repetitive transcranial magnetic stimulation (rTMS) with F8-coil and deep transcranial magnetic stimulation (DTMS) with H1-coil in major depression: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):139. https://doi.org/10.1186/s12888-019-2106-7.
Wang J, Vila-Rodriguez F, Ge R, Gao S, Gregory E, Jiang W, et al. Accelerated magnetic seizure therapy (aMST) for treatment of major depressive disorder: a pilot study. J Affect Disord. 2020;264:215–20. https://doi.org/10.1016/j.jad.2019.12.022.
Kayser S, Bewernick BH, Wagner S, Schlaepfer TE. Effects of magnetic seizure therapy on anterograde and retrograde amnesia in treatment-resistant depression. Depress Anxiety. 2020;37(2):125–33. https://doi.org/10.1002/da.22958.
Kisely S, Li A, Warren N, Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468–80. https://doi.org/10.1002/da.22746.
Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, Quevedo J. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. 2018;23(5):1094–112. https://doi.org/10.1038/mp.2018.2.
Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;82:224–32. https://doi.org/10.1016/j.pnpbp.2017.11.012.
Bahji A, Hawken ER, Sepehry AA, Cabrera CA, Vazquez G. ECT beyond unipolar major depression: systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand. 2019;139(3):214–26. https://doi.org/10.1111/acps.12994.
Regenold WT, Noorani RJ, Piez D, Patel P. Nonconvulsive electrotherapy for treatment resistant unipolar and bipolar major depressive disorder: a proof-of-concept trial. Brain Stimul. 2015;8(5):855–61. https://doi.org/10.1016/j.brs.2015.06.011.
Perciaccante A, Coralli A, Cambioli L, Riva MA. Nonconvulsive electrotherapy in psychiatry: the treatment of the mental disorders of the Norwegian painter Edvard munch. Bipolar Disord. 2017;19(2):72–3. https://doi.org/10.1111/bdi.12483.
Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol Psychiatry. 2010;67(2):125–32. https://doi.org/10.1016/j.biopsych.2009.09.015.
Beran M, Perkins JC, Scollon RW. Psychological studies on patients undergoing nonconvulsive electric-stimulation treatment. Am J Psychiatry. 1952;109(5):367–74. https://doi.org/10.1176/ajp.109.5.367.
Alexander L. Nonconvulsive electric stimulation therapy; its place in the treatment of affective disorders, with notes on the reciprocal relationship of anxiety and depression. Am J Psychiatry. 1950;107(4):241–50. https://doi.org/10.1176/ajp.107.4.241.
Olkon DM. Subconvulsive electrotherapy in mental disorders. Dis Nerv Syst. 1957;18(7 Part 1):271.
Prudic J, Sackeim HA, Devanand DP, Krueger RB, Settembrino JM. Acute cognitive effects of subconvulsive electrical stimulation. Convuls Ther. 1994;10(1):4–24.
Krystal AD, Weiner RD. ECT seizure therapeutic adequacy. Convuls Ther. 1994;10(2):153–64.
Crow TJ. The scientific status of electro-convulsive therapy. Psychol Med. 1979;9(3):401–8. https://doi.org/10.1017/s0033291700031937.
Post RM, Kimbrell TA, McCann U, Dunn RT, George MS, Weiss SR. Are convulsions necessary for the antidepressive effect of electroconvulsive therapy: outcome of repeated transcranial magnetic stimulation. L'Encephale. 1997;23(Spec No 3):27–35.
APA. American Psychiatric Association: structured clinical interview for DSM-IV. Washington, DC: American Psychiatric Press; 1994.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. https://doi.org/10.1136/jnnp.23.1.56.
Heijnen WT, Birkenhager TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616–9. https://doi.org/10.1097/JCP.0b013e3181ee0f5f.
Lin CH, Chen MC, Yang WC, Lane HY. Early improvement predicts outcome of major depressive patients treated with electroconvulsive therapy. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2016;26(2):225–33. https://doi.org/10.1016/j.euroneuro.2015.12.019.
Lin HS, Lin CH. Early improvement in HAMD-17 and HAMD-6 scores predicts ultimate response and remission for depressed patients treated with fluoxetine or ECT. J Affect Disord. 2019;245:91–7. https://doi.org/10.1016/j.jad.2018.10.105.
Channon S. Executive dysfunction in depression: the Wisconsin card sorting test. J Affect Disord. 1996;39(2):107–14. https://doi.org/10.1016/0165-0327(96)00027-4.
Berman RM, Prudic J, Brakemeier EL, Olfson M, Sackeim HA. Subjective evaluation of the therapeutic and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(1):16–26. https://doi.org/10.1016/j.brs.2007.08.005.
Wang G, Zheng W, Li XB, Wang SB, Cai DB, Yang XH, et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res. 2018;105:23–32. https://doi.org/10.1016/j.jpsychires.2018.08.002.
Lara E, Olaya B, Garin N, Ayuso-Mateos JL, Miret M, Moneta V, et al. Is cognitive impairment associated with suicidality? A population-based study. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015;25(2):203–13. https://doi.org/10.1016/j.euroneuro.2014.08.010.
Bjolseth TM, Engedal K, Benth JS, Dybedal GS, Gaarden TL, Tanum L. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67–75. https://doi.org/10.1016/j.jad.2015.06.021.
Alexopoulos GS. "The depression-executive dysfunction syndrome of late life": a specific target for D3 agonists? The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2001;9(1):22–9.
McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25(10):933–44. https://doi.org/10.1002/gps.2431.
Basagni B, Errante A, Pinardi C, De Gaetano K, Crisi G, De Tanti A, et al. Rehabilitation of unilateral spatial neglect: a combined behavioral and fMRI single-case study. Neuropsychology. 2019;33(3):343–57. https://doi.org/10.1037/neu0000523.
Uddin M. Blood-based biomarkers in depression: emerging themes in clinical research. Mol Diagn Ther. 2014;18(5):469–82. https://doi.org/10.1007/s40291-014-0108-1.
Kambeitz J, Cabral C, Sacchet MD, Gotlib IH, Zahn R, Serpa MH, et al. Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biol Psychiatry. 2017;82(5):330–8. https://doi.org/10.1016/j.biopsych.2016.10.028.
Cook IA, Hunter AM, Gilmer WS, Iosifescu DV, Zisook S, Burgoyne KS, et al. Quantitative electroencephalogram biomarkers for predicting likelihood and speed of achieving sustained remission in major depression: a report from the biomarkers for rapid identification of treatment effectiveness in major depression (BRITE-MD) trial. J Clin Psychiatry. 2013;74(1):51–6. https://doi.org/10.4088/JCP.10m06813.
Acknowledgements
This study was funded by the Science and Technology Planning Project of Guangdong Province (B2016109), the Science and Technology Plan Project of Guangdong Province (2019B030316001), and Guangzhou Clinical Characteristic Technology Project (2019TS67).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest concerning this article.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
NA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Zheng, W., Jiang, ML., He, HB. et al. A Preliminary Study of Adjunctive Nonconvulsive Electrotherapy for Treatment-Refractory Depression. Psychiatr Q 92, 311–320 (2021). https://doi.org/10.1007/s11126-020-09798-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11126-020-09798-3