Abstract
Neuroinflammation appears to be associated with the neurobiology of depression, and treatments targeting inflammation have shown promising results in depression. This meta-analysis examined the efficacy and safety of minocycline, an anti-inflammatory drug, for the treatment of depressive symptoms. A systematic electronic literature search was independently conducted by two investigators. Standardized mean differences (SMDs) and risk ratio (RR) with their 95% confidence interval (CI) were calculated using a random-effect model. Four RCTs (n = 211) were identified for meta-analysis. Minocycline showed a significant trend of improvement in depressive symptoms compared to placebo [4 RCTs, n = 190, SMD: -0.54 (95%CI:-1.12, 0.04), P = 0.07; I2 = 73%]. Subgroup analyses showed that minocycline was superior to placebo in improving depressive symptoms in studies of unipolar depression (3 RCTs, n = 151, SMD: -0.77 (95%CI:-1.32, −0.22), P = 0.006; I2 = 60%) and in studies using minocycline monotherapy [SMD: -1.06 (95%CI:-1.68, −0.44), P = 0.0008]. The rates of discontinuation due to any reasons [RR: 1.48 (95%CI: 0.79, 2.77), P = 0.22, I2 = 0%] and adverse drug reactions [RR: 0.32 to 1.98 (95%CI: 0.03, 14.74), P = 0.19 to 0.84, I2 = 0% to 31%] were similar between minocycline and placebo. Minocycline appears to be effective and well-tolerated in ameliorating depressive symptoms in unipolar depression. Future large RCTs with sufficient duration is needed to confirm the positive effects of minocycline in treating depressive symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depressive symptoms are common and a major challenge in clinical practice [1]. The Sequenced Treatment Alternatives for the Relief of Depression (STAR*D) trial involving 3,671 outpatients found that the remission rate for major depressive disorder (MDD) was only 36.8% following the first treatment step, and the rate decreased to 13.0% over three subsequent treatment steps [1]. A recent network meta-analysis of 21 antidepressant drugs in acute treatment of MDD only found a small effect size compared to placebo [2], which is similar to the findings of another meta-analysis [3]. Hence, novel treatment agents for depression are urgently needed to improve the outcomes for patients.
Neuroinflammation appears to be associated with the neurobiology of depression and treatments targeting inflammation has shown promising results in depression [4,5,6]. Studies have found elevated circulating pro-inflammatory cytokines in patients with depressive symptoms [7], while patients with chronic inflammatory and autoimmune disorders frequently have comorbid depressive symptoms [8]. Moreover, inflammatory system dysregulation is associated with more severe course of depressive symptoms [9] and treatment-resistant depression [10].
The tetracycline antibiotic minocycline shows potent anti-inflammatory, anti-apoptotic and anti-oxidant properties, and is associated with the modulation of glutamate and monoamine neurotransmission [11, 12], and thus could have antidepressant effects [12]. The potential neuroprotective and antidepressant-like effects of minocycline have been confirmed in animal studies [13]. Preliminary data from open-label trials of adjunctive minocycline in patients with unipolar [14] and bipolar depression [15,16,17] also showed clinical efficacy and safety. However, the findings of randomized controlled trials (RCTs) [18,19,20,21] of the antidepressant effects of minocycline have been mixed.
A recent meta-analysis [22] of 3 RCTs (n = 158) [19,20,21] of the efficacy and safety of minocycline in the treatment of depressive symptoms found superiority of minocycline over placebo. However, the meta-analysis [22] was limited by the small number of RCTs and sample size, and since the meta-analysis did not include any studies from the Chinese databases.
This updated meta-analysis of RCTs in both English and Chinese databases examined the efficacy and safety of adjunctive minocycline for depressive symptoms, regardless of their primary diagnoses.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [23] (CRD42018102481; http://www.crd.york.ac.uk/prospero/).
Search Strategy
An electronic literature search was independently conducted in both English (PubMed, PsycINFO, EMBASE and the Cochrane Library) and Chinese (Chinese Journal Net and WanFang) databases from their inception date up to June 26, 2018 by two investigators (DBC and WZ), using the following search terms: (“minocycline”[Mesh] OR “minocyline”[Mesh] OR minocyline OR minocycline) AND (“depression”[Mesh] OR depressive OR depressed OR melancholia). Reference lists of included RCTs [18,19,20,21], meta-analyses [22] and reviews [24] were also hand-searched for additional studies.
Study Selection
The eligibility of studies was independently determined by two investigators (WZ and DBC) according to the following PICOS acronym: (i) Participants: subjects with depressive symptoms (age ≥ 18 years), regardless of their primary diagnoses [25]. (ii) Intervention: minocycline monotherapy or minocycline plus treatment as usual (TAU). (iii) Comparison: TAU or TAU plus placebo. (iv) Outcomes: the primary outcome was depressive symptoms measured by standardized rating scales [such as the Hamilton Depression Rating Scale (HAMD), Montgomery-Asberg Depression Rating Scale (MADRS) or Beck Depression Inventory (BDI)]. If one study concurrently used the HAMD and other rating scales on depressive symptoms, the HAMD assessment was preferred. The key secondary outcomes included treatment response, discontinuation due to any reasons and adverse drug reactions (ADRs). (v) Study design: double-blinded RCTs with meta-analyzable data.
Data Extraction
Data were independently extracted from the included RCTs, entered into a standardized Microsoft Excel spreadsheet, checked, and analyzed by two investigators (DBC and WZ). Any disagreement was resolved by consensus or by involving a third reviewer (YTX). Whenever essential data were not reported, the first/correspondence authors were contacted for more information.
Statistical Methods
All outcomes were meta-analyzed using the Review Manager (Version 5.3) (http://www.cochrane.org) in case when at least 2 studies provided data for a given outcome as per other meta-analysis [26]. Due to the heterogeneity across studies, the random effects model was used to synthesize outcomes [27]. For continuous and dichotomous data, we calculated standardized mean differences (SMDs) and risk ratios (RRs) with their 95% confidence intervals (CIs).
Study heterogeneity was assessed using the chi-squared and I-squared statistics, with chi-squared P < 0.10 and I-squared≥50% suggesting high heterogeneity. When heterogeneity for depressive symptoms (primary outcome) was high, a sensitivity analysis [i.e., removing an outlying study [21] with comorbid Human Immunodeficiency Virus (HIV) infection] was conducted to explore the heterogeneity source. Furthermore, the following two subgroup analyses were conducted: (1) studies with minocycline as monotherapy vs. studies with minocycline as adjunctive therapy; (2) studies on MDD vs. studies on bipolar disorder. Funnel plots and Egger’s test [28] for primary outcomes were conducted to evaluate publication bias. All meta-analytic outcomes were 2 tailed, with alpha set at 0.05.
Quality Assessment
Evaluation of methodological quality of each study was independently conducted by two investigators (WZ and DBC) using the Cochrane risk of bias [29] and Jadad scale [30]. The Jadad total score of ≥ 3 was considered as “high quality” [30]. The grading of recommendations, assessment, development, and evaluation (GRADE) system [31, 32] was administered to estimate the recommendation level for adjunctive minocycline in treating depressive symptoms.
Results
Results of the Search
Figure 1 shows the PRISMA flow diagram. Out of 855 initial hits from English (n = 839) and Chinese (n = 16) databases, 851 were excluded: duplicates (n = 53), after reading title/abstract (n = 787), and full text review (n = 11). Finally, 4 RCTs [18,19,20,21] in English databases were included.
Study Characteristics
All 4 RCTs (n = 211) were double-blind and the weighted mean study duration was 9.2 (range = 6–12 weeks; Table 1). The weighted mean age of the 170 patients in 3 RCTs with available data was 43.3 (range = 35.5–49.4) years; males accounted for 42.9% (range = 28.6%–65.2%) of the 211 patients. The weighted mean illness duration was 9.8 years (range = 3.9–14.0) in 2 RCTs with available data. The given dose of minocycline was 200 mg/day in all RCTs.
Quality Assessment
All four RCTs were rated as low risk in terms of selective bias, attrition bias, and reporting bias (Supplemental Fig. 1). The weighted mean score of the Jaded scale was 5 and all included RCTs were considered as high quality (Table 1). Using the GRADE approach, the overall evidence levels for 12 outcomes ranged from “low” (8.3%; 1/12) to “moderate” (91.7%; 11/12) (Supplemental Table 1).
Depressive Symptoms
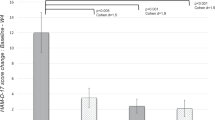
Meta-analysis of depressive symptoms measured by HAMD (2 RCTs) and MADRS (2 RCTs) showed that minocycline had a significant trend of improving depressive symptoms compared to placebo [4 RCTs, n = 190, SMD: -0.54 (95%CI:-1.12, 0.04), P = 0.07; I2 = 73%, Fig. 2]. The marginal significance disappeared [SMD: -0.37 (95%CI:-1.03, 0.29), P = 0.27; I2 = 71%] after removing one study [21] with comorbid HIV infection. Subgroup analyses showed that minocycline was significantly superior to placebo in studies on unipolar depression (3 RCTs, n = 151, SMD: -0.77 (95%CI:-1.32, −0.22), P = 0.006; I2 = 60%) and in studies using minocycline monotherapy [SMD: -1.06 (95%CI:-1.68, −0.44), P = 0.0008]. Meta-analysis of response (≥50% reduction in HAMD or MADRS score) did not find any group differences [3 RCTs, n = 133, RR: 1.94 (95%CI: 0.93, 4.04), P = 0.08, I2 = 12%; Table 2].
Discontinuation Rate and ADRs
Meta-analysis of all-cause discontinuations did not find any group differences [RR: 1.48 (95%CI: 0.79, 2.77), P = 0.22, I2 = 0%; Table 2]. Meta-analysis of ADRs including abdominal pain, diarrhea, dizziness, headache, fast/irregular heartbeat, insomnia, myalgia, nausea, and rash [RR: 0.32 to 1.98 (95%CI: 0.03, 14.74), P = 0.19 to 0.84, I2 = 0% to 31%; Table 2] also did not show any group differences.
Publication Bias
Four RCTs were meta-analyzed, which is less than the minimum 10 RCTs to perform funnel plot or Egger’s test. Thus, publication bias was not assessed for depressive symptoms (n = 4 RCTs).
Discussion
This updated meta-analysis of 4 RCTs with 211 subjects with depressive symptoms found that minocycline was superior to placebo in studies on unipolar depression and studies using minocycline monotherapy. The efficacy and safety of minocycline in depression appears to be consistent with effect of minocycline in the treatment of schizophrenia [26, 33].
The potential antidepressant mechanism of minocycline could be attributed to its anti-inflammatory and neuroprotective effects [34]. Biological mediators of stress (such as glucocorticoids) and peripheral inflammation are associated with the activation of neuroinflammatory processes [35], in which microglia associated with the release of inflammatory mediators, such as pro-inflammatory cytokines, exert an important role [36]. Minocycline could modulate microglial activation [12, 37] and reduce pro-inflammatory cytokines (i.e. tumour necrosis factor (TNF)-α, interleukin-1β and glucocorticoids), which is associated with the improvement of depressive and anxiety symptoms [38].
The recent meta-analysis [22] with 3 RCTs (n = 158) [19,20,21] found a significant antidepressant effect of minocycline (SMD = -0.78), which is inconsistent with our findings which included one additional study with 53 patients [18]. This study [18] focused on patients with bipolar depression, which could increase the study heterogeneity. Further, unlike the prior meta-analysis [22], study quality assessment using Jadad scale and the grading of recommendations of meta-analytic outcomes using GRADE analyses were included in this meta-analysis.
The following limitations need to be acknowledged. First, although broad study entry criteria were used, the number of studies and sample sizes were still relatively small, which limits the capacity to assess publication bias. Second, heterogeneous diagnoses in the four studies (MDD, bipolar disorder and HIV infection) were included. Third, the treatment duration was relatively short (6–12 weeks), thus long-term effects of minocycline could not be examined.
Conclusions
Minocycline appears to be effective and safe in treating depressive symptoms in unipolar depression. Future large RCTs of sufficient duration is needed to confirm the positive effects of minocycline in treating depressive symptoms.
Availability of Data and Material
The data of this article are included within the article.
Abbreviations
- ADRs :
-
Adverse drug reactions
- BDI :
-
Beck Depression Inventory
- BP-I :
-
Bipolar I disorder
- BP-II:
-
Bipolar II disorder
- BP-NOS :
-
Bipolar disorder not otherwise specified
- CI :
-
Confidence interval
- DB :
-
Double blind
- DSM-IV :
-
Diagnostic and Statistical Manual of Mental Disorders 4th edition
- DSM-IV-TR :
-
Diagnostic and Statistical Manual of Mental Disorders 4th edition, Text Revision
- DSM-5 :
-
Diagnostic and Statistical Manual of Mental Disorders 5th edition
- FD :
-
Fixed dosage
- GRADE :
-
grading of recommendations, assessment, development, and evaluation
- HAART :
-
Highly Active Antiroviral Therapy
- HAMD :
-
Hamilton Depression Rating Scale
- HAMD-17 :
-
Hamilton Depression scores 17-item
- HIV :
-
Human Immunodeficiency Virus
- ITT :
-
intent to treat
- MADRS :
-
Montgomery-Asberg Depression Rating Scale
- MDD :
-
Major depressive disorder
- MINO :
-
Minocycline
- NR :
-
Not report
- OC :
-
Observed cases
- Pbo :
-
Placebo
- PRISMA :
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCTs :
-
randomized controlled trials
- RR :
-
Risk ratio
- SMDs :
-
Standardized mean differences
- STAR*D :
-
The Sequenced Treatment Alternatives for the Relief of Depression
- T :
-
Total
- TAU :
-
Treatment as usual
- TRD :
-
Treatment-resistant depressive symptoms
- wks :
-
Weeks
- yrs :
-
Years
References
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–66.
Linde K, Kriston L, Rucker G, Jamil S, Schumann I, Meissner K, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015;13(1):69–79.
Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200.
Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, et al. Elevated Translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry. 2018;83(1):61–9.
Dean OM, Datafranco J, Giorlando F, Berk M. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391–401.
Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43(11):962–9.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–38.
Zalli A, Jovanova O, Hoogendijk WJ, Tiemeier H, Carvalho LA. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 2016;233(9):1669–78.
Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, et al. Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology. 2016;233(9):1679–88.
Hashimoto K, Ishima T. A novel target of action of minocycline in NGF-induced neurite outgrowth in PC12 cells: translation initiation [corrected] factor eIF4AI. PLoS One. 2010;5(11):e15430.
Soczynska JK, Mansur RB, Brietzke E, Swardfager W, Kennedy SH, Woldeyohannes HO, et al. Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav Brain Res. 2012;235(2):302–17.
Arakawa S, Shirayama Y, Fujita Y, Ishima T, Horio M, Muneoka K, et al. Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol Biochem Behav. 2012;100(3):601–6.
Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;37(2):222–6.
Murrough JW, Huryk KM, Mao X, Iacoviello B, Collins K, Nierenberg AA, et al. A pilot study of minocycline for the treatment of bipolar depression: effects on cortical glutathione and oxidative stress in vivo. J Affect Disord. 2018;230:56–64.
Soczynska JK, Kennedy SH, Alsuwaidan M, Mansur RB, Li M, McAndrews MP, et al. A pilot, open-label, 8-week study evaluating the efficacy, safety and tolerability of adjunctive minocycline for the treatment of bipolar I/II depression. Bipolar Disord. 2017;19(3):198–213.
Iosifescu DV. The antidepressant effects of minocycline, a glutamatergic and antioxidant agent, in bipolar disorder. Biol Psychiatry. 2013;73(9):158S.
Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, et al. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry. 2018;8(1):27.
Husain MI, Chaudhry IB, Husain N, Khoso AB, Rahman RR, Hamirani MM, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: a pilot randomised placebo-controlled trial. J Psychopharmacol. 2017;31(9):1166–75.
Dean OM, Kanchanatawan B, Ashton M, Mohebbi M, Ng CH, Maes M, et al. Adjunctive minocycline treatment for major depressive disorder: a proof of concept trial. Aust N Z J Psychiatry. 2017;51(8):829–40.
Emadi-Kouchak H, Mohammadinejad P, Asadollahi-Amin A, Rasoulinejad M, Zeinoddini A, Yalda A, et al. Therapeutic effects of minocycline on mild-to-moderate depression in HIV patients: a double-blind, placebo-controlled, randomized trial. Int Clin Psychopharmacol. 2016;31(1):20–6.
Rosenblat JD, McIntyre RS. Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. J Affect Disord. 2018;227:219–25.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9 w264.
Fond G, Hamdani N, Kapczinski F, Boukouaci W, Drancourt N, Dargel A, et al. Effectiveness and tolerance of anti-inflammatory drugs' add-on therapy in major mental disorders: a systematic qualitative review. Acta Psychiatr Scand. 2014;129(3):163–79.
Fernandes BS, Dean OM, Dodd S, Malhi GS, Berk M. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457–66.
Solmi M, Veronese N, Thapa N, Facchini S, Stubbs B, Fornaro M, et al. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr. 2017;22(5):415–26.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Higgins J, Higgins J. Cochrane handbook for systematic reviews of interventions. Ltd: Chichester: John Wiley & Sons; 2008.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490.
Xiang YQ, Zheng W, Wang SB, Yang XH, Cai DB, Ng CH, et al. Adjunctive minocycline for schizophrenia: a meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol. 2017;27(1):8–18.
Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196(2):168–79.
Frank MG, Weber MD, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming: a liability factor in the etiology of psychiatric disorders. Neurobiol Stress. 2016;4:62–70.
Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45.
Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun. 2014;42:147–56.
Majidi J, Kosari-Nasab M, Salari AA. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res Bull. 2016;120:1–13.
Funding
This study was supported by the University of Macau (SRG2014–00019-FHS; MYRG2015–00230-FHS; MYRG2016–00005-FHS), the Affiliated Brain Hospital of Guangzhou Medical University (2016YFC0906302; 2014Y2–00105; 2015BAI13B02), and the Scienceand Technology Department of Guangdong Province Major Science and Technology (2016B010108003). The University of Macau and the Affiliated Brain Hospital of Guangzhou Medical University had no role in the study design, generating or interpreting the results and publication of the study.
Author information
Authors and Affiliations
Contributions
Study design: YTX; Data extraction: DBC and WZ; Data analysis: WZ and QEZ; Drafting of the manuscript: DBC, WZ and YTX. Critical revision of the manuscript: CHN and GSU; Approval of the final version for publication: All the author.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no conflicts of interest concerning this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 83 kb)
Rights and permissions
About this article
Cite this article
Cai, DB., Zheng, W., Zhang, QE. et al. Minocycline for Depressive Symptoms: a Meta-Analysis of Randomized, Double-Blinded, Placebo-Controlled Trials. Psychiatr Q 91, 451–461 (2020). https://doi.org/10.1007/s11126-019-09707-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11126-019-09707-3