Abstract
Abnormally altered precipitation patterns induced by climate change have profound global effects on crop production. However, the plant functional responses to various precipitation regimes remain unclear. Here, greenhouse and field experiments were conducted to determine how maize plant functional traits respond to drought, flooding and rewatering. Drought and flooding hampered photosynthetic capacity, particularly when severe and/or prolonged. Most photosynthetic traits recovered after rewatering, with few compensatory responses. Rewatering often elicited high photosynthetic resilience in plants exposed to severe drought at the end of plant development, with the response strongly depending on the drought severity/duration. The associations of chlorophyll concentrations with photosynthetically functional activities were stronger during post-tasseling than pre-tasseling, implying an involvement of leaf age/senescence in responses to episodic drought and subsequent rewatering. Coordinated changes in chlorophyll content, gas exchange, fluorescence parameters (PSII quantum efficiency and photochemical/non-photochemical radiative energy dissipation) possibly contributed to the enhanced drought resistance and resilience and suggested a possible regulative trade-off. These findings provide fundamental insights into how plants regulate their functional traits to deal with sporadic alterations in precipitation. Breeding and management of plants with high resistance and resilience traits could help crop production under future climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate change is now leading to an enhanced frequency and intensity of drought events (Dai 2012; Trenberth et al. 2014; Donat et al. 2016; Diffenbaugh et al. 2017), that are now placing staple crop production and food security at risk (Lobell et al. 2014; Myers et al. 2017; Leakey et al. 2019; Kimm et al. 2020). These changes, coupled with the acceleration of industrialisation and the rapid development of social economy, are now placing agricultural water resources in tighter supply across the globe. Water availability has now become a bottleneck for food production and even social and economic development, and lack of water has triggered a series of environmental and ecological problems that now threaten sustainable development of crop production and exacerbate global undernutrition (e.g. Daryanto et al. 2016; Myers et al. 2017; Rosa et al. 2020).
Drought is one of the most crucial environmental factors constraining crop plant productivity due to its deleterious effects on leaf photosynthetic capacity, plant growth and crop productivity at regional and global scales (Lobell et al. 2014; Daryanto et al. 2016; Myers et al. 2017). Plants that experience drought stress have their water balance destroyed and this leads to plant growth inhibition, stomatal closure and decreases in the photosynthetic rate (e.g. Chaves et al. 2003, 2009; Xu et al. 2009; Gupta et al. 2020). However, plants can invoke a number of regulative strategies to deal with water deficit. The responses to water deficit depend on the duration, severity and time of occurrence of the drought. For example, plants may not be affected, even favoured under mild or moderate drought, but it can be limited and even damaged by severe drought (e.g. Fereres and Soriano 2007; Xu et al. 2014b). Under a mild or moderate water deficit, an increased water use efficiency (WUE), improved nutritional content and stable grain yield often can be observed, which can improve sustainable development by allowing deficit irrigation and water-saving agricultural practices (Fereres and Soriano 2007; Geerts and Raes 2009; Du et al. 2015; Silveira et al. 2020). Further exploration of crop responses to various water conditions can therefore provide critical information for optimising crop management practices, particularly under future climate change (Lobell et al. 2014; Leakey et al. 2019; Kimm et al. 2020).
Effects of water stresses on plant growth and photosynthesis also strongly depend on leaf age/senescence and plant developmental stages (Otegui et al. 1995; Cakir 2004; Jiang et al. 2020). Most of the previous studies have indicated that severe drought could enhance senescence (e.g. David et al. 1998; Chaves et al. 2003; Xu et al. 2008; Jiang et al. 2020). Meanwhile, under water deficit, compared with mature/elder leaves, younger leaves often have higher growth rate and photosynthetic capacity, indicating that the former has high tolerance to drought stress (Hsiao 1973; Thiagarajah et al. 1981; David et al. 1998; Xu et al. 2008; Hofer et al. 2017). When maize plants were subjected to a prolonged water stress, huge grain yield losses of 66–93% were observed during tasseling and ear formation stages (Cakir 2004). The senescence rates of wheat leaf were rapider when water stress occurred at grain-filling than at greening and jointing stages (Jiang et al. 2020). This highlights the higher sensitivity to drought at the reproductive relative to vegetative growth stages, peculiarly at tasseling, flowering and early ear formation stages (e.g. Otegui et al. 1995; Farooq et al. 2014; Jiang et al. 2020). Flooding can affect the plant functional activities at different plant/leaf development stages. For instance, at early seedling stage, maize plants are highly susceptible to flooding relative to the later stages (Zaidi et al. 2004). As drought, flooding stress can also induce early leaf senescence in wheat at jointing or after anthesis (Araki et al. 2012). However, a comprehensive research on the responses of crops to drought, rewatering and flooding is still lacking.
Plants exposed to drought will frequently show a restoration of their normal physiological functions when rewatered, and to a certain degree, they can compensate for the damage caused by drought by accelerating their growth and enhancing their photosynthetic capacity (e.g. Xu and Zhou 2007; Xu et al. 2009, 2010; Hofer et al. 2017). An antecedent condition, such as soil water availability, may also drive the post-stress responses to other abiotic factors, indicating important complexities in plant responses to environmental factors (Xu et al. 2009; Guo and Ogle 2019). This ability to regain a normal original state after being disturbed is termed resilience (Holling 1973; Müller et al. 2016; Resilience Alliance 2020), and can be represented by the interference level, recovery time or recovery speed (Müller et al. 2016; Bhaskar et al. 2018; Harrison et al. 2018; Resilience Alliance 2020).
A recent report showed that a watering treatment following a drought can lead to a greater recovery of some key functional traits in plants (Harrison et al. 2018). For example, both full and partial recoveries of leaf pigment and nitrogen contents were observed in drought-stressed maize plants following rewatering (Sun et al. 2018). Similarly, Voronin et al. (2019) documented the physiological responses of maize plants to drought and rewatering. However, information is lacking regarding the changes in photosynthetic capacity and their associations with plant growth during drought and subsequent recovery upon rewatering. The increased frequency of drought due to global climate change emphasises the importance of understanding the mechanism underlying the plant responses to drought and rewatering for both theoretical and practical applications (e.g. Hofer et al. 2017; Abid et al. 2018; Guo and Ogle 2019).
Drought has been an important factor in the growth of maize, the most widely grown crop in the world. Water deficit causes unstable and low yields in many maize production areas in the world, seriously hampering plant growth and causing 25–30% reductions in grain yield in some vulnerable regions (Sharp et al. 2004; Ben-Ari et al. 2016; Beyene et al. 2016; Li et al. 2019; Kimm et al. 2020). For instance, the U.S. Corn Belt, the world's biggest maize production region, is recognised as being prone to drought and is therefore sensitive to climate change (Kimm et al. 2020). Similarly, the Corn Belt of Northeastern China (CBNC) is one of the major maize production regions in China and it too shows strong sensitivity to climate variations. Drought is a particularly critical factor constraining maize production in the CBNC (e.g. Liu et al. 2012; Li and Sun 2016).
Future climate change scenarios envisage an increase in the occurrences of both drought and flooding during the growth period in maize-growing regions (Roudier et al. 2016; Kimm et al. 2020). Thus, elucidating the maize plant responses to drought, rewatering and flooding is crucial for the development of technology for monitoring, evaluating and minimising the damage caused by drought and flood disasters. This knowledge can also provide insight to the factors that enhance resilience in maize plants, while also serving as a feasible reference for corn yield forecasting and field water management during the growing period.
The aim of the present study was to conduct greenhouse and field experiments to determine maize plant functional responses to drought, rewatering and flooding. The greenhouse experiments involved examination of these responses following different water treatments, including pre-drought, drought, rewatering and flooding. The field experiment was conducted in a large-sized rain shelter designed to grow maize plants under three irrigation regimes, including pre-drought and subsequent re-irrigation. Our focus was specifically on assessing the resilience of photosynthetic capacity in response to drought and rewatering. Three hypotheses were tested: (i) drought and flooding can constrain photosynthetic capacity in maize plants, particularly under severe, prolonged water stress; (2) rewatering can lead to a full recovery of photosynthetic capacity with a compensatory mechanism; (3) the resilience of photosynthetic capacity depends on the degree of drought stress. The findings may improve current knowledge and strengthen future quests to produce high-yield, drought-resistant and resilient crops (see also Gupta et al. 2020).
Materials and methods
Greenhouse experiment design
The first experimental site was located in a greenhouse (39° 48′ N, 116° 28′ E, 67 m a.s.l.), Institute of Botany, Chinese Academy of Sciences, Beijing, China. The soil was collected from field soil (0–30 cm soil profile) at Gucheng Ecological Environment and Agro-meteorology Test Station (39° 08′ N, 115° 40′ E, 15.2 m a.s.l.), Baoding city, Hebei province, North China. Plastic pots (diameter 21 cm, height 25 cm) were used. The maize cultivars is Zhengdan 958, which is currently planted extensively in North China. The seeds were sown on 28, June 2017. We filled 5.5 kg of soil per pot; and each pot was applied as 2.54 g of diammonium phosphate compound fertilizer (i.e. 750 kg ha−1). The three seeds were sown in each of the pot with a depth of 2.5 cm. Soon afterwards, only one healthy plant was left before the third leaf of seedlings emerged. The seedlings were grown in a naturally illuminated glasshouse with fluorescent lamp illumination addition with the maximum photosynthetic photon flux density (PPFD) of 1000 μmol m−2 s−1 and a day/night photoperiod of 12-h light/12-h darkness and a day/night mean temperature of c. 28.0/20.0 ºC.
The greenhouse experiment used four water treatments: (1) Control treatment: the soil relative water content (SRWC) was maintained at 65–75% throughout the whole experimental period. (2) Persistent drought stress: SRWC was reduced beginning at the three-leaf stage and extending to jointing stages (i.e. seven-leaf stage) to the SRWC of the permanent wilting point (PWP). (3) Flooding treatment: waterlogging stress was induced at the three-leaf stage and extended until the jointing stage. (4) Drought-rewatering treatment: SRWC was reduced initially at the three-leaf stage to 35% of SRWC at the jointing stage (the leaves wilted and the lowermost leaves began to turn yellow and withered); the plants were then rewatered to 65–75% of SRWC.
Field experimental design
The field experimental site was located at the Jinzhou Ecology and Agricultural Meteorology Center, Liaoning, Northeastern China (N 41° 49′, E 121° 12′, 27.4 m a.s.l.). The mean annual temperature and the mean annual precipitation over 40 years were 9.9 °C and 564 mm, respectively, with an average monthly temperature of 20.9 °C and a total precipitation of 468 mm during plant growing season. The soil is characterised as medium loam type, with pH 6.3, 1.8% organic matter and a soil bulk density of 1.61 g cm−3 at the 0–100 cm soil profile. The field capacity (FC) and PWP were 22.3% and 6.5% (gravimetric), respectively. The soil had an organic carbon content of 10.44 g kg−1, total nitrogen content of 0.69 g kg−1, phosphorus content of 0.50 g kg−1 and potassium content of 22.62 g kg−1. The planting date and maturity date were late April and late September, respectively (Mi et al. 2018; Song et al. 2018; Li et al. 2019).
The field experimental design was as detailed previously (Li et al. 2019). In brief, an electric-powered waterproof shelter (4 m in height) set up in the maize field was used to establish the various precipitation regimes that we desired. In total, 15 plots (15 m2, 5 m length, 3 m width) were covered to avoid rainfall falling with the rain shelter when it rained. The following three irrigation regimes were set up: a control (i.e. the normal irrigation every 7 day); moderate drought (water withholding for 20 days); and severe drought (water withholding for 27 days from the tasseling to milking stages). In this design, irrigation water was supplied at 296, 246 and 221 mm across the maize plant growing period (Table S1). The dates of measurements of chlorophyll content and photosynthetic traits were 1 July (V13, 62 days after sowing [DAS]), 12 July (VT, tasseling, 73 DAS), 20 July (R1, silking, 81 DAS), 5 August (R2, blistering, 97 DAS), 9 August (R3, milking, 101 DAS) and 2 September (R4, dough, 125 DAS). To measure SRWC, the method with soil auger was used to retrieve soil samples, and the samples were then dried at 105 °C to a constant dry weight. The SRWC at 0–50 cm soil depth was reduced progressively to reach severe drought levels at a range of 30–40% at the end of rainfall-withholding, whereas under normal irrigation, the SWRC was maintained at levels of 70–80% in the control and rewatering plots (Fig. S1). The maize cultivar was Danyu 39, with a seed sowing rate of 6.0 plants m−2 to ensure a planting density of 4.5 plants m−2. A compound fertiliser (accounting for 28%, 11% and 12% of N, P2O5 and K2O, respectively), applied at a c. 750 kg ha−1, was added before sowing (Song et al. 2018; Li et al. 2019).
Leaf chlorophyll content
We examined leaf chlorophyll concentrations with a SPAD 502 chlorophyll meter (Minolta Co. Ltd, Japan). In the greenhouse experiment, SPAD was measured only on the youngest fully expanded leaf (top leaf) at 20, 22, 26, 29, 32, 34 and 37 DAS. In field experiment, measurements were made in top (16–18th leaves), middle (12–14th leaves) and bottom leaves (8–10th leaves) along the plant position from bottom to top layers. Each position was measured thrice in the middle area of the selected leaf, avoiding the leaf main vein. Both measurements on SPAD and photosynthetic parameter were on the same leaves in the same days (below).
Leaf gas exchanges and chlorophyll a fluorescence
In the greenhouse experiment, the leaf gas exchange and chlorophyll a fluorescence were measured in both the top and mature leaves (the third leaf from the top of plant) with an open gas exchange system (LI-6400, LI-COR Inc., Lincoln, NE) equipped with a LI-6400-40 fluorometer. The measurements were made at an interval of 3–5-day with the treatment time. Leaves were acclimated in the chamber for at least 15 min at 28–30 °C with a CO2 concentration of 400 μmol mol−1 and a PPFD of 1200 μmol m−2 s−1. Chlorophyll a fluorescence was determined with the LI-6400-40 fluorometer. The steady-state fluorescence (Fs) was recorded at 1200 μmol m−2 s−1, and a second saturating pulse at ~ 8000 μmol photons m−2 s−1 was then given to obtain the maximal light-adapted fluorescence yield (Fm′). The actinic light was turned off, and the minimal fluorescence at the light-adapted state (Fo′) was obtained following a 3 s burst of far-red illumination.
In the field experiment, the leaf chlorophyll a fluorescence was determined with a miniaturised pulse-amplitude-modulated photosynthesis yield analyser (Mini-PAM, Walz, Effeltrich, Germany). After a 30-min dark adaptation, the minimal fluorescence yield (Fo) was determined with a modulated light at a sufficiently low intensity (< 0.1 μmol photon m−2 s−1). The maximal fluorescence yield (Fm) was made with a 0.8 s saturating pulse at a ~ 7000 μmol photons m−2 s−1. Leaves were continuously illuminated at 300 μmol photons m−2 s−1 for 15 min. After the Fs was recorded, the second saturating pulse at ~ 7000 μmol photons m−2 s−1 was then exposed to obtain Fm′. The actinic light was turned off and Fo′ was obtained following a 3 s far-red illumination. The maximal quantum efficiency of photosystem II (PSII, Fv/Fm), the efficiency of excitation captured by open PSII centres in the light-adapted leaves (Fv′/Fm′) and the yield of PSII photochemistry (ΦPSII) were obtained (Schreiber et al. 1994; Maxwell and Johnson 2000). Other fluorescence parameters were calculated with the following formulas by Kramer et al. (2004):
where qp and qL are photochemical quenching based on puddle and lake models, respectively. NPQ or qN is non-photochemical quenching, and both ΦNPQ and ΦNO are light-induced regulated non-photochemical quenching and quantum yield of non-regulated energy loss in PSII, respectively (Kramer et al. 2004).
Soil relative water content
Soil was placed in an experimental pot with holes at the bottom and weighed 48 h after excessive watering to reach a saturated weight (SW) point. The soil was then dried at 110 °C for at least 72 h to a constant weight (DW). The FC can be expressed as FC = (SW–DW)/DW × 100. The SRWC = Current soil water content/FC × 100.
Resistance, recovery and resilience
Resistance is expressed as a ratio of functional parameters (e.g. net photosynthetic rate, Anet) between drought stress and ample water status as control treatment (e.g. van Ruijven and Berendse 2010; Mariotte et al. 2013; Xu et al. 2014a). Recovery can be indicated by the ratio of functional parameters between drought/pre-drought and post-rewatering treatment (van Ruijven and Berendse 2010; Mariotte et al. 2013; Xu et al. 2014a). Sometime, the recovery is also named as resilience (Ruppert et al. 2015; Bhaskar et al. 2018); however, the resilience is calculated as the ratio of functional parameters between ample watering (control) and rewatering treatments (e.g. van Ruijven and Berendse 2010; Mariotte et al. 2013; Xu et al. 2014a). These indices can be expressed in different proportional terms such as the difference (e.g. van Ruijven and Berendse 2010; ratio (Mariotte et al. 2013), log ratio (van Ruijven and Berendse 2010; Xu et al. 2014a) and percentage ratio (Xu et al. 2014a; Sakschewski et al. 2016). In the current study, we consistently used the relative percentage ratios to indicate their changes (e.g. Xu et al. 2014a; Sakschewski et al. 2016):
where Xd and Xck are functional parameter values (e.g. Anet) under drought stress and ample water status (control treatment); Xw is functional parameter value following rewatering.
Data statistics
The data were statistically analysed with statistical software package SPSS 20.0 (SPSS Inc., Chicago, Illinois, USA). A one-way analysis of variance (ANOVA) with post hoc tests of Duncan and LSD multiple comparisons was used to test the differences of the functional traits between leaf layer at each watering treatment and/or at each sampling time. The effects of watering treatment and leaf layer at each day of sampling or plant developmental stages, and their interaction on the functional traits of plants, were tested with two-way ANOVAs, with Duncan and LSD multiple comparisons. The correlations among the functional traits were tested with Pearson’s correlation analysis, and the relationships of photosynthetically functional traits with leaf relative chlorophyll content (SPAD readings) at different plant growth stages were tested by linear regression analysis. In particular, because the VT, a transitional stage from the vegetative stage to reproductive stage, is a key stage linking the vegetative stage and reproductive stage (e.g. Abendroth et al. 2009), we separated the data for all leaves into two subsets to conduct the relationships of photosynthetically functional traits with leaf relative chlorophyll content to test whether the differences occur between the two stages. The comprehensive relationships between leaf photosynthetic functional traits, and their responses to irrigation regimes and plant/leaf developmental stages were determined by principal component analysis (PCA). The significance levels were set at P < 0.05 and 0.01, unless otherwise stated.
Results
Photosynthetic traits in the greenhouse
The greenhouse experiment showed that drought stress led to a slight reduction in the relative chlorophyll content (SPAD readings) in the top leaf 4 days after withholding water (i.e. 22 days after sowing, DAS), followed by a rise of 8 days after the drought-stressed treatment (Fig. 1). However, the chlorophyll content showed a sharp linear decrease from 26 to 37 DAS when the relative soil water content (RSWC) fell sharply to the severe drought stress level of 35%. After rewatering, the chlorophyll content significantly increased, with recovery values of 14.2, 15.2 and 25.6% under consecutive drought at 32, 34 and 37 DAS, respectively, indicating that a greater recovery may occur at the end of the measurement period. The resilience values were − 4.3, − 14.0 and − 5.0% at 32, 34 and 37 DAS, respectively, showing that the resilience rose initially following rewatering, then decreased and then increased again (Table S2). Compared with control, flooding led to significant declines in SPAD after 26 DAS (P < 0.05), indicating that chlorophyll degradation occurred under the flooded condition.
The changes in leaf relative chlorophyll content (SPAD values) in the top leaves of maize plants subjected to various watering regimes (blue line, ample watering as the control; grey line, flooding; red line, drought; green line, rewatering; green open triangle, resilience). The red arrow indicates the rewatering date. The data are shown as means ± SE (n = 3–6)
As shown in Fig. 2a, drought only led to a slight reduction in the net light-saturated photosynthetic rate (Asat) in top leaf within one week after withholding water. However, this rate sharply decreased from 28.8 to 4.8 μmol m−2 s−1 by 85.2% at 29 DAS when RSWC dropped to 35%. After rewatering, Asat sharply and significantly increased (P < 0.05), with recovery values of 5.53, 1.18 and 5.98 times the values seen under consecutive drought at 32, 34 and 37 DAS, respectively. The rate approached and even exceeded the control level at 32, 34 and 37 DAS. The resilience values increased gradually from − 12.1 to 10.2 and 25.4%, indicating a possible escalation of resilience with time after rewatering. Compared to control, a significant stimulation of the Asat occurred during the initial 6 days under flooding (P < 0.05); thereafter, flooding induced a decrease compared with the control treatment after 32 DAS (P < 0.05). However, Asat under flooding ultimately reached the level of the control treatment. The responses were also observed in the mature leaves. Drought also reduced Asat in mature leaf with great recovery and a positive resilience noted at the end of the experiment (Fig. 2d; Table S2). A sharp rise appeared during the initial flooding, but Asat decreased thereafter.
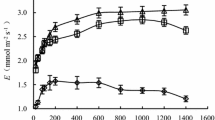
The changes in net light-saturated photosynthetic rate (Asat, a, d), stomatal conductance (gs, b, e) and transpiration rate (E, c, f) in both the top leaves (a–c) and mature leaves (d–f) of maize plants subjected to various watering regimes (blue line, ample watering as the control; grey line, flooding; red line, drought; green line, rewatering; green open triangle, resilience). Red arrows indicate the rewatering date; the data are shown as means ± SE (n = 3–6). Note different scales in the second y-axis
Compared with the control, stomatal conductance (gs) was significantly decreased (− 96.5%) at 29 DAS, just before rewatering (Fig. 2b). A greater recovery was observed, but only positive resilience was detected at 34 DAS. In mature leaves, a drastic gs resilience was also evident at 34 DAS (Fig. 2e). However, the low recovery was observed in mature leaves (Fig. 2b, e).
The transpiration rate (E) significantly decreased due to drought stress (P < 0.05), dropping to the lowest point at 29 DAS (a decrease of 94.5% relative to control, Fig. 2c). Rapid increases occurred following the rewatering, with recovery values of 9.3-fold, 8.9-fold and 4.6-fold the values under continuous drought at 3, 5 and 8 days following rewatering, respectively. However, the resilience values were − 10.4, 6.0 and − 15.7% at 3, 5 and 8 days following rewatering, respectively. A stimulation of E also appeared initially by flooding; thereafter, however, the similar E changes were similar to those of the control (Fig. 2c). In mature leaves, a rapid and drastic reduction in E was also observed by imposition of drought stress, with great recovery; however, the resilience remained negative (Fig. 2f).
The quantum yield of PSII electron transport (ΦPSII) decreased with drought-treatment time, dropping to its lowest value (by 79.3%) at 29 DAS (Fig. 3a). The recovery values were 3.05, 3.38 and 4.22 times relative to continuous drought at 3, 5 and 8 days after rewatering, respectively. Flooding also led to an initial stimulation in ΦPSII; thereafter, the value decreased below the control level. The photochemical quenching (qP) showed a substantial fluctuation even under the control treatment (Fig. 3b). However, a dramatic decline of 51.9% was observed after 9 days of water withholding. We also found recoveries of 1.3-fold, 16.6-fold and 14.8-fold at 3, 5 and 8 days following rewatering, respectively. However, the increases still did not reach the control level, so the resilience values were negative (− 5.74, − 26.22 and − 15.22 at 3, 5 and 8 days following rewatering). A stimulation of qP was also observed initially at 2 days after flooding exposure, but this disappeared thereafter and the value dropped to levels lower than the control levels. For mature leaves, a great recovery occurred for ΦPSII and qp; however, the negative resilience was still maintained (Fig. 3d, e).
The changes in quantum yield of PSII electron transport (ΦPSII, a, d), photochemical quenching (qP, b, e) and water use efficiency (WUE, c, f) in both the top leaves (a–c) and mature leaves (d–f) of maize plants subjected to various watering regimes (blue line, ample watering as the control; grey line, flooding; red line, drought; green line, rewatering; green open triangle, resilience). Red arrows indicate the rewatering dates; the data are shown as means ± SE (n = 3–6). Note different scales in the second y-axis
Leaf water use efficiency (WUE) was significantly increased by drought during 26–32 DAS (P < 0.001), but subsequently decreased with drought-exposure time, indicating that the enhancement of WUE may be attenuated by the water deficit intensity and its persistent duration. Rewatering led to a decline in WUE at the earlier stage, but thereafter WUE remained stable relative to both the control and continuous drought plants. WUE was not affected significantly by flooding during the experimental periods (Fig. 3c). Drought always also elevated the WUE in the mature leaves, whereas flooding did not substantially affect it. Only a small positive resilience was observed at the end of the experiment (Fig. 3f).
Based on one-way ANOVA, there were significant effects on photosynthetic traits except for WUE before watering treatment at 20 DAS (Table S3). The two-way ANOVAs indicated that the effects on photosynthetic parameters including the leaf gas exchange and chlorophyll fluorescence traits were significant from watering treatment, and leaf layer as a single factor alone (except leaf layer for WUE at 26, 29, 32 DAS). However, significant interactions between water and leaf layer occurred only for some photosynthetic traits (Table S4).
Photosynthetic traits in the field
In the field experiment, the top leaves in the control treatment showed gradual increases in the relative chlorophyll content (SPAD values) from 1 July (62 DAS, V13), 12 July (73 DAS, VT, tasseling), July 20 (81 DAS, R1, silking), to August 5 (97 DAS, R2, blistering), until reaching a maximum on 97 DAS; the relative chlorophyll content then decreased as plant development progressed (Fig. 4a). Episodic severe drought led to significant dramatic declines (P < 0.05), whereas rewatering led to more increases (i.e. a positive recovery) under moderate drought (MD) than under severe drought (SD). Negative resilience values were observed under both drought treatments at 101 and 125 DAS. Similar responses were also observed in both middle and bottom leaves: SPAD values significantly decreased under SD (P < 0.05), particularly on 97 DAS; and no positive resilience was observed (Fig. 4d, g; Table S5).
SPAD and chlorophyll a fluorescence in top (a–c), middle (d–f) and bottom (g–i) leaves under drought and rewatering. Green and red open triangles represent the resilience of moderate and severe drought, respectively, at 101 and 125 days after sowing (DAS). Red open arrows indicate the DAS of water withholding; while grey and red close arrows indicate the rewatering DASs of moderate and severe drought treatments, respectively. SPAD, leaf relative chlorophyll content; Fv/Fm maximal quantum efficiency of photosystem II (PSII); ΦPSII the yield of PSII photochemistry. The data are shown as means ± SE (n = 3–6)
The maximum quantum efficiency of PSII (Fv/Fm) in top leaves showed a similar pattern to that seen for the relationship of SPAD with DAS; i.e. a unimodal curve (Fig. 4b). A decline seemed to appear under SD; however, recovery was greater following rewatering. In the middle leaves, positive resilience was noted for Fv/Fm at 125 DAS (Fig. 4e). In the bottom leaves, a severe drought episode resulted in a marked reduction in Fv/Fm at 97 DAS, but a rapid recovery occurred at 4 d after rewatering. Rewatering resulted in high Fv/Fm resilience in the plants exposed to previous MD and SD at the end of plant development (Fig. 4h).
In top leaves, ΦPSII values under control decreased with plant growth (Fig. 4c). An increase occurred under MD, but SD led to a marked decline with greater resilience at both 101 and 125 DAS. In middle leaves, greater resilience appeared under MD at 101 and 125 DAS and under SD only at 125 DAS (Fig. 4f). In bottom leaves, a decline in ΦPSII was observed from 62 to 81 DAS, but a stable ΦPSII change remained thereafter during the later plant developmental periods. Marked resilience appeared for both pre-drought treatments at the two final developmental stages (Fig. 4i).
The changes in qp and its resilience in top leaves were similar to those of ΦPSII (Fig. 5a). In middle leaves, the qp values decreased with plant development under the control treatment, but greater resilience appeared under MD at 101 DAS (Fig. 5d). In bottom leaves, the changes in qp showed a similar pattern to that in top leaves. However, a great recovery and marked resilience appeared at 101 DAS (Fig. 5g).
Chlorophyll a fluorescence in in top (a–c), middle (d–f) and bottom (g–i) leaves under drought and rewatering. Green and red open triangles represent the resilience of moderate and severe drought, respectively, at 101 and 125 days after sowing (DAS). Red open arrows indicate the DAS of water withholding; while grey and red close arrows indicate the rewatering DASs of moderate and severe drought treatments, respectively. qp photochemical quenching based on puddle model; qN non-photochemical quenching; ΦNPQ light-induced regulated non-photochemical quenching. The data are shown as means ± SE (n = 3–6)
In top leaves, the changes in non-photochemical quenching (qN) and the yield of light-induced regulated non-photochemical quenching (ΦNPQ) and their resilience showed the same changing trends (Fig. 5b, c): they increased with DAS, and MD led to a drastic decline with a high resilience under SD at 101 DAS. In middle leaves, both qN and ΦNPQ also increased with DAS, with negative resilience under MD at both 101 and 125 DAS (Fig. 5e, f). In bottom leaves, under ample irrigation, both qN and ΦNPQ increased until 81 DAS and then remained stable. Drought led to declines in qN and ΦNPQ with considerable recovery at 125 DAS in the plants exposed to the SD episode; however, the resilience still remained negative (Fig. 5h, i).
Across all leaf types, SPAD decreased under both MS and SD, and no positive resilience was observed (Fig. S2a). A decline Fv/Fm occurred at 97 DAS, and a positive resilience appeared at 125 DAS (Fig. S2b). Both ΦPII and qp values decreased with plant development under control treatment, but greater resilience was observed under MD at 101 and 125 DAS (Fig. S2c, d). Both qN and ΦNPQ increased under control treatment, but decreased under MD and SD with DAS, with the negative resilience values (Fig. S2e, f).
One-way ANOVAs indicated the effects on photosynthetic parameters of leaf layer before watering treatment (Table S6): There were significant effects of leaf layer on SPAD, Fv/Fm and ΦPSII at 62 DAS; only on SPAD at 73 DAS; on Fv/Fm, ΦPSII and qp at 81 DAS. According to the two-way ANOVAs (Table S7), the effects on SPAD, Fv/Fm, ΦPSII and ΦNPQ were all significant from watering treatment alone at each DAS following rewatering (P < 0.05). Significant effects of water on qp and qN appeared only at 101 DAS. All photosynthetic traits measured were affected significantly from leaf layer alone at 101 DAS, but significant effects on SPAD and ΦPSII appeared only at 97 DAS. The interactive effects of two factors occurred on SPAD only at 97 DAS; ΦPSII at 62 DAS; ΦPSII, qp, qN and ΦNPQ at 101 DAS.
Relationships between the functional traits
The relationships between fluorescence parameters and chlorophyll contents (SPAD values) in the maize field are shown in Fig. S3. Before/at previous tasseling stages (i.e. VT, a transitional stage from the vegetative stage to reproductive stage), only strong relationship was observed between Fv/Fm and chlorophyll content (R2 = 0.39, P < 0.001; Fig. S3a). Using the data after VT revealed significant and positive relationships of SPAD values with fluorescence parameters, especially for Fv/Fm (R2 = 0.61, P < 0.001, Fig. S3a). However, others with low R2 may imply that SPAD does not explain much of the existing variation in fluorescence parameters, possibly due to excessive random variation, due to other parameters or (to a lower extend) to a substantial non-linearity.
We also performed a PCA to test the relationships between functional traits and the different patterns in both greenhouse (Fig. S4) and field experiments (Fig. 6). In the greenhouse experiment (Fig. S4), the first two principal components (PCs) explained 60.3% of the total photosynthetic variations. The loadings of Fm′, FO′, qP and qN were sorted in quadrant I, while that of Fv′/Fm′ was in quadrant IV. The loadings of the gas exchange traits and ΦPSII were placed between quadrant I and quadrant IV, with positive loadings of Asat, gs, E, ΦPSII and a negative loading of WUE. PC scores of control and rewatering were distributed in the right parts, whereas those of drought were placed in the left area (Fig. S4). In the field experiment, the first two principal components (PCs) accounted for 70.1% of the total variations. The loadings of SPAD, Fv/Fm, Fm, FO and FO′ were in quadrant I, while those of ΦPSII, Fm′, qP and qL were in quadrant II. The markers most representative of non-photochemical quenching traits (e.g. NPQ, qN and ΦNPQ) in relation to non-photochemical radiative energy dissipation capability were sorted into quadrant III. Projection on the treatment effects showed that the three irrigation regimes were sorted in the coordinate plane, with control treatment mostly in quadrant II and severe drought scattered in all four quadrants (Fig. 6).
Principal component analysis on plant functional traits under the three irrigation regimes [i.e. control, moderate drought (MD) and severe drought (SD)]. The traits’ loadings on the first two principal components (PCs) are shown, and their projections are sorted by the three irrigation regimes. Dotted blue, closed black and dotted red circles with glowing represent the PC scores of control, moderate and severe drought treatments, respectively. Fm maximal fluorescence yield; Fm′ maximal light-adapted fluorescence yield; Fo minimal fluorescence yield; Fo′ minimal fluorescence at light-adapted state; Fs steady-state fluorescence; Fv/Fm maximal quantum efficiency of photosystem II (PSII);ΦPSII yield of PSII photochemistry; qp photochemical quenching based on puddle model; qL photochemical quenching based on lake models, qN non-photochemical quenching; ΦNPQ light-induced regulated non-photochemical quenching; ΦNO quantum yield of non-regulated energy loss
Discussion
Water cycle changes could substantially impact plant growth, photosynthetic processes and many crucial physiological functions and nutrient status, thereby affecting plant productivity and crop yield (e.g. Izanloo et al. 2008; Lobell et al. 2014; Kimm et al. 2020). Drought and rewetting may often occur at intervals and are predicted to happen more frequently and severely under climatic change (Dai 2012; IPCC 2014; Donat et al. 2016; Diffenbaugh et al. 2017). Indeed, sporadic precipitation is a critical issue in maintaining ecosystem productivity and its structural stability, particularly in arid and semi-arid areas and/or in rain-fed planting regions (Reynolds et al. 2004; Cooper et al. 2008; Song et al. 2018; Guo and Ogle 2019).
Maize plays a critical role in meeting the global food demands and is one of the most widely planted staple crops worldwide (Haarhoff and Swanepoel 2018; FAO 2020). In this study, the greenhouse and field experiments demonstrated how maize photosynthetic functional traits respond to drought, flooding and rewatering, particularly at different growth stages, thereby providing key information for managing crop production. Our main findings were that (1) drought hampered photosynthetic capacity in both young and mature leaves, whereas flooding only limited them for mature leaves, in major support of the first hypothesis; (2) rewatering could result in partial recovery of some photosynthetic traits, with few compensatory responses, in partial support of our second hypothesis; and (3) the photosynthetic resilience to drought was dependent on the drought severity, largely supporting the third hypothesis. In particular, the associations of chlorophyll concentrations with photosynthetically functional activities were stronger during post-tasseling than pre-tasseling (Fig. S3), implying an involvement of leaf age/senescence in responses to episodic drought and subsequent rewatering. A PCA clearly illuminated the associations and trade-off among chlorophyll content, gas exchange and photosynthetic potentials, and the effect patterns of the watering treatments (Figs. S4, 6). These findings can shed light on ways to improve regulation of crop functional traits to deal with erratic precipitation regimes and may lead to better breeding and management practices for crops that have high drought resistance and drought-resilience traits at different growth stages (Kromdijk et al. 2016; Song et al. 2018; Gupta et al. 2020).
Drought and flooding
In agreement with the previous work (e.g. Chaves et al. 2003, 2009; Xu and Zhou 2006; Xu et al. 2009; Gupta et al. 2020), the results of the present study indicated that severe drought stress can substantially reduce photosynthetic capacity, as characterised by declines in chlorophyll content, net light-saturated photosynthetic rate (Asat), stomatal conductance (gs) and quantum yield of PSII electron transport (ΦPSII) in both the greenhouse and field experiments. However, the photosynthetic capacity attenuated more substantially and steeply as the drought stress persisted in our experiment, indicating a strong dependence on the duration, severity and timing of droughts. Thus, only mild or moderate or short drought stresses were conducive to the development of a regulative response of plants for resistance to water deficit. For instance, maintaining Asat at a stable level with a low gs can elevate WUE during moderate drought stress (e.g. Chaves et al. 2009; Xu and Zhou 2011). This observation may aid in implementations of deficit irrigation, water-saving agriculture and sustainable development (Fereres and Soriano 2007; Geerts and Raes 2009; Du et al. 2015; Silveira et al. 2020; Kimm et al. 2020).
The present findings demonstrated that flooding led to a decline in SPAD and Asat, but not gs. Our results also indicated a higher tolerance of maize to flooding stress in terms of ΦPSII and photochemical quenching (qP), relative to drought stress, highlighting the distinct effects of these two stresses (Mutava et al. 2015; Zhu et al. 2020). An antagonistic effect on gs has been reported (see also Zhu et al. 2020). Maintaining stomatal opening may promote water release to alleviate the stress due to excessive water, again highlighting the positive regulation in response to anoxic conditions (Zhu et al. 2020).
Recovery and resilience
As previously reported, a depression in photosynthesis potentials by a previous drought can be markedly stimulated by rewetting; however, whether or how much these potentials recover depends on drought intensity and/or the persistence period (e.g. Xu et al. 2009, 2010; Creek et al. 2018). In the current experiment, partial, full and over recovery of photosynthetic traits were all observed in terms of both recovery and resilience indices, specifically depending on the duration and persistence of the drought, the plant developmental stages and the different functional traits, as well as the crop species and cultivar (Figs. 1, 2, 3, 4 and 5; Xu et al. 2009; Creek et al. 2018). For instance, an over-compensatory recovery (i.e. a positive percentage of the resilience) in gs was observed in maize (Fig. 2b, e); however, gs only achieved a partial recovery in a grass species (Xu et al. 2009). Creek et al. (2018) reported that, after rewatering, the Anet of a semi-arid species can return to the pre-drought stress level within 2–4 weeks, whereas gs performs a slower recovery. A recent report by Johnson et al. (2018) indicated that photosynthesis was not fully recovered in wheat plants because of the photosynthetic damage due to hydraulic decline in the leaves subjected to drought. Increased embolism is tightly related to a complete lack of photosynthetic recovery. However, Creek et al. (2018) found that photosynthetic recovery can be decoupled from the recovery of plant hydraulics, indicating that the impaired hydraulic function throughout the recovery period perhaps does not influence the complete recovery of Anet from drought. Thus, the underlying mechanism needs to be investigated further.
The enhancement of plant functional activities (e.g. plant growth rate, grain yield and Asat) following rewatering has been found by many researchers (Reynolds et al. 2004; Siopongco et al. 2006; Xu et al. 2009; Song et al. 2018). As recently reported by Abid et al. (2018), tolerant wheat plants showing high photosynthetic capacity during drought and rapid recovery after re-irrigating did not show marked yield declines relative to the sensitive cultivars, indicating that the plant’s ability to maintain/restore growth and physiological functions during pre/post-drought in the vegetative period might play a crucial role in determining crop productivity. Upon rewatering, the rapid growth of new tissues, such as a new leaf, might accelerate plant growth, potentially enhancing CO2 assimilation (Pinheriro et al. 2004). This may be a result of positive source–sink interactions, as a strong sink requirement (e.g. new tissue) can enhance the carbon assimilation rate (Minchin and Lacointe 2005; White et al. 2015; Parvin et al. 2020). Higher resilience of Asat and gs occurred in the younger leaves relative to mature ones, implying a greater ability to recover in the new leaves that serve as both active source–sink organs (Fig. 2; Roitsch 1999). The maize plants were exposed to drought stress for only several days, so leaf length after rewatering was restored to a similar level to that of the control plants, indicating no occurrence of overcompensation (Acevedo et al. 1971; Xu et al. 2009; Hofer et al. 2017). Thus, the extent of compensation for drought by the triggering of new tissues following rewatering might determine the final plant/crop production and would depend strongly on the severity, duration and timing of the drought stress (Hsiao 1973; Xu et al. 2009; Hofer et al. 2017).
Associations between functional traits
The distinct responses of the functional traits such as Asat and gs to drought, flooding and rewetting suggested that coordinated associations between the functional traits could reveal the underlying mechanism (see also Creek et al. 2018). For instance, the SPAD reading (e.g. Ciganda et al. 2009), as an indicator of relative chlorophyll concentration, has different associations with photosynthetic function activities at different plant development stages: stronger associations were observed post-VT (tasseling stage) than pre-VT (Fig. S3). This might indicate that a greater coupling relationship appears at later plant developmental stages and that leaf age/senescence could be involved in the responses to drought and rewatering (also see Valentinuz and Tollenaar 2004; Song et al. 2018; Jiang et al. 2020). This finding may further improve our understanding of how plants respond to water status changes at different developmental stages. For instance, many previous studies have indicated that drought damage increases, while tolerance decreases, with increasing senescence (e.g. David et al. 1998; Shah and Paulsen 2003; Chaves et al. 2003; Xu et al. 2008; Jiang et al. 2020). However, rewatering may lessen the senescence processes (Xu et al. 2010; Jiang et al. 2020), thereby leading to changes in associations between functional traits such as coupling and trade-off occurrences. As revealed by the PCA in greenhouse experiment (Fig. S4), the distinctive distribution of loadings of gas exchange (i.e. Asat, gs, E, WUE), PSII functional activities (i.e. Fv′/Fm′, ΦPSII) and photochemical quenching processes (i.e. qp and qN) (Schreiber et al. 1994; Maxwell and Johnson 2000), reflect their coordination and/or trade-off in response to watering regimes (Xu et al. 2014b). Moreover, from the PCA in field experiment (Fig. 6), a distinct pattern of loadings between ΦPSII, ΦNPQ and ΦNO highlights a feasible trade-off strategy by balancing the yields among photochemical processes for the energy absorbed by PSII, dissipation of non-photochemical responses and other non-photochemical losses, which would involve the xanthophyll cycle and PsbS protein expression (Murchie and Lawson 2013; Kromdijk et al. 2016; Sacharz et al. 2017). In addition, the contribution of the photosynthetic parameters for the discrimination between watering treatments could be demonstrated by the PCA plots. For instance, those representing photochemical quenching processes (qN, NPQ and ΦNPQ) may contribute to more variation of control treatment in the field experiment (Fig. 6). Together, the association between functional traits and their effects of environmental factors can be well illustrated with PCA (e.g. Vile et al. 2012; Xu et al. 2014b).
As noted, leaf ranks of maize plants could be each considered as an entity to indicate the time course of senescence (e.g. Escobar-Gutiérrez and Combe 2012). In the greenhouse, because the maize seedings were only used during this experiment, we measured the two types of leaf: the young and mature leaves. The changes in photosynthetic traits measured in the two types of leaf with DAS time would roughly reflect the effects of leaf age. In the field experiment, the maize plants were used at later stages of plant development; thus, we conveniently selected the three-leaf layers of plant canopy to measure the photosynthetic traits. The top, middle and bottom leaves along plant height may represent the leaf development ages/stages: relatively young leaf, mature leaf and elderly leaf, respectively (Xu et al. 2008; Song et al. 2018; Li et al. 2019; Feng et al. 2020). However, the more information on relations among the leaf age, leaf senescence and its position along plant height, and the response to climatic change may deserve further study with its specific design.
Conclusion
Quantifying and defining plant functional traits to assess and predict drought effects and post-drought recovery are relevant issues due to the pressing needs imposed by climate change (e.g. Creek et al. 2018; Gupta et al. 2020). In this study, we conducted greenhouse and field experiments to explore how maize photosynthetic functional traits respond to drought, flooding and rewatering at different growth stages. The main findings were the following: (1) drought and flooding constrains photosynthetic capacity, particularly under severe and/or long water stress; (2) rewatering results in a partial recovery of most of the photosynthetic traits, with few compensatory responses; (3) photosynthetic resilience to drought following rewetting strongly depends on the drought severity and its persistence and duration. The distinct responses of various functional traits to drought, flooding and rewetting can translate to a regulative strategy of trade-off. The coordinated changes in chlorophyll content, gas exchange, fluorescence parameters (quantum efficiency of PSII, and photochemical and non-photochemical radiative energy dissipation) may largely contribute to the enhancements of drought resistance and resilience of plants. The associations of chlorophyll concentrations with photosynthetic functional activities were also stronger post-VT than pre-VT, implying that leaf age/senescence may be involved in the responses to drought and rewatering. These findings may further improve our understanding of how plants respond to water status changes at different plant developmental stages. This knowledge can be helpful for breeding crops with high drought-resistant and drought-resilience traits and for establishing management practices when facing climate change (e.g. Kromdijk et al. 2016; Leakey et al. 2019; Kimm et al. 2020; Gupta et al. 2020).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abendroth L, Elmore R, Hartzler RG, McGrath C, Mueller DS, Munkvold GP, Pope R, Rice M, Robertson AE, Sawyer J, Schaefer KJP, Tollefson JJ, Tylka GL (2009) "Corn Field Guide". Extension and Outreach Publications. 26. https://lib.dr.iastate.edu/extension_pubs/26. Accessed 6 Nov 2020
Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T (2018) Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep 8:4615. https://doi.org/10.1038/s41598-018-21441-7
Acevedo E, Hsiao TC, Henderson DW (1971) Immediate and subsequent growth responses of maize leaves to changes in water statues. Plant Physiol 48:631–636. https://doi.org/10.1104/pp.48.5.631
Araki H, Hamada A, Hossain MA, Takahashi T (2012) Waterlogging at jointing and/or after anthesis in wheat induces early leaf senescence and impairs grain filling. Field Crops Res 137:27–36. https://doi.org/10.1016/j.fcr.2012.09.006
Ben-Ari T, Adrian J, Klein T, Calanca P, Van der Velde M, Makowski D (2016) Identifying indicators for extreme wheat and maize yield losses. Agric For Meteorol 220:130–140. https://doi.org/10.1016/j.agrformet.2016.01.009
Beyene Y, Semagn K, Crossa J, Mugo S, Atlin GN, Tarekegne A et al (2016) Improving maize grain yield under drought stress and non‐stress environments in sub‐Saharan Africa using marker‐assisted recurrent selection. Crop Sci 56:344–353. https://doi.org/10.2135/cropsci2015.02.0135
Bhaskar R, Arreola F, Mora F, Martinez-Yrizar A, Martinez-Ramos M, Balvanera P (2018) Response diversity and resilience to extreme events in tropical dry secondary forests. For Ecol Manage 426:61–71. https://doi.org/10.1016/j.foreco.2017.09.028
Cakir R (2004) Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crops Res 89:1–16. https://doi.org/10.1016/j.fcr.2004.01.005
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264. https://doi.org/10.1071/FP02076
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Ciganda V, Gitelson A, Schepers J (2009) Non-destructive determination of maize leaf and canopy chlorophyll content. J Plant Physiol 166:157–167. https://doi.org/10.1016/j.jplph.2008.03.004
Cooper PJM, Dimes J, Rao KPC, Shapiro B, Shiferaw B, Twomlow S (2008) Coping better with current climatic variability in the rain-fed farming systems of sub-Saharan Africa: an essential first step in adapting to future climate change? Agric Ecosyst Environ 126:24–35. https://doi.org/10.1016/j.agee.2008.01.007
Creek D, Blackman C, Brodribb TJ, Choat B, Tissue DT (2018) Coordination between leaf, stem and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell Environ 41:2869–2881. https://doi.org/10.1111/pce.13418
Dai A (2012) Increasing drought under global warming in observations and models. Nat Clim Change 3:52–58. https://doi.org/10.1038/nclimate1633
Daryanto S, Wang L, Jacinthe PA (2016) Global synthesis of drought effects on maize and wheat production. PLoS ONE 11:5. https://doi.org/10.1371/journal.pone.0156362
David MM, Coelho D, Barrote I, Correia MJ (1998) Leaf age effects on photosynthetic activity and sugar accumulation in droughted and rewatered Lupinus albus plants. Aust J Plant Physiol 25:299–306. https://doi.org/10.1071/PP97142
Diffenbaugh NS, Singh D, Mankin JS, Horton DE, Swain DL, Touma D, Charland A, Liu Y, Haugen M, Tsiang M, Rajaratnam B (2017) Quantifying the influence of global warming on unprecedented extreme climate events. Proc Natl Acad Sci USA 114:4881–4886. https://doi.org/10.1073/pnas.1618082114
Donat MG, Lowry AL, Alexander LV, Gorman PAO, Maher N (2016) More extreme precipitation in the world's dry and wet regions. Nature Clim Change 6:508–513. https://doi.org/10.1038/nclimate2941
Du T, Kang S, Zhang J, Davies WJ (2015) Deficit irrigation and sustainable water-resource strategies in agriculture for China’s food security. J Exp Bot 66:2253–2269. https://doi.org/10.1093/jxb/erv034
Escobar-Gutiérrez AJ, Combe L (2012) Senescence in field-grown maize: from flowering to harvest. Field Crops Res 134:47–58. https://doi.org/10.1016/j.fcr.2012.04.013
FAO (2020) FAOSTAT database. FAO, Rome. http://www.fao.org/faostat/en/#data/QC. Accessed 19 June 2020
Farooq M, Hussain M, Siddique KH (2014) Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci 33:331–349. https://doi.org/10.1080/07352689.2014.875291
Feng L, Raza MA, Shi J, Ansar M, Titriku JK, Meraj TA, Shah GA, Ahmed Z, Saleem A, Liu W, Wang X (2020) Delayed maize leaf senescence increases the land equivalent ratio of maize soybean relay intercropping system. Eur J Agron 118:126092. https://doi.org/10.1016/j.eja.2020.126092
Fereres E, Soriano MA (2007) Deficit irrigation for reducing agricultural water use. J Exp Bot 58:147–259. https://doi.org/10.1093/jxb/erl165
Geerts S, Raes D (2009) Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric Water Manage 96:1275–1284. https://doi.org/10.1016/j.agwat.2009.04.009
Guo JS, Ogle K (2019) Antecedent soil water content and vapor pressure deficit interactively control water potential in Larrea tridentate. New Phytol 221:218–232. https://doi.org/10.1111/nph.15374
Gupta A, Rico-Medina A, Caño-Delgado AI (2020) The physiology of plant responses to drought. Science 368:266–269. https://doi.org/10.1126/science.aaz7614
Haarhoff SJ, Swanepoel PA (2018) Plant population and maize grain yield: a global systematic review of rainfed trials. Crop Sci 58:1–11. https://doi.org/10.2135/cropsci2018.01.0003
Harrison SP, LaForgia ML, Latimer AM (2018) Climate-driven diversity change in annual grasslands: drought plus deluge does not equal normal. Glob Change Biol 24:1782–1792. https://doi.org/10.1111/gcb.14018
Hofer D, Suter M, Buchmann N, Lüscher A (2017) Nitrogen status of functionally different forage species explains resistance to severe drought and post-drought overcompensation. Agric Ecosyst Environ 236:312–322. https://doi.org/10.1016/j.agee.2016.11.022
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23. https://doi.org/10.1146/annurev.es.04.110173.000245
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570. https://doi.org/10.1146/annurev.pp.24.060173.002511
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change (Core Writing Team, Pachauri RK, Meyer LA, eds). IPCC, Geneva, Switzerland, 151 pp
Izanloo A, Condon AG, Langridge P, Tester M, Schnurbusch T (2008) Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J Exp Bot 59:3327–3346. https://doi.org/10.1093/jxb/ern199
Jiang T, Dou Z, Liu J, Gao Y, Malone RW, Chen S, Feng H, Yu Q, Xue G, He J (2020) Simulating the influences of soil water stress on leaf expansion and senescence of winter wheat. Agric For Meteorol 291:108061. https://doi.org/10.1016/j.agrformet.2020.108061
Johnson KM, Jordan GJ, Brodribb TJ (2018) Wheat leaves embolised by water stress do not recover function upon rewatering. Plant Cell Environ 41:2704–2714. https://doi.org/10.1111/pce.13397
Kimm H, Guan K, Gentine P, Wu J, Bernacchi CJ, Sulman BN, Griffis TJ, Lin C (2020) Redefining droughts for the US Corn Belt: the dominant role of atmospheric vapor pressure deficit over soil moisture in regulating stomatal behavior of Maize and Soybean. Agric For Meteorol 287:107930. https://doi.org/10.1016/j.agrformet.2020.107930
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218. https://doi.org/10.1023/B:PRES.0000015391.99477.0d
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354:857–861. https://doi.org/10.1126/science.aai8878
Leakey AD, Ferguson JN, Pignon CP, Wu A, Jin Z, Hammer GL, Lobell DB (2019) Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annu Rev Plant Biol 70:781–808. https://doi.org/10.1146/annurev-arplant-042817-040305
Li Z, Sun Z (2016) Optimized single irrigation can achieve high corn yield and water use efficiency in the Corn Belt of Northeast China. Eur J Agron 75:12–24. https://doi.org/10.1016/j.eja.2015.12.015
Li Y, Song H, Zhou L, Xu Z, Zhou G (2019) Vertical distributions of chlorophyll and nitrogen and their associations with photosynthesis under drought and rewatering regimes in a maize field. Agric For Meteorol 272:40–54. https://doi.org/10.1016/j.agrformet.2019.03.026
Liu Z, Yang X, Hubbard KG, Lin X (2012) Maize potential yields and yield gaps in the changing climate of northeast China. Glob Change Biol 18:3441–3454. https://doi.org/10.1111/j.1365-2486.2012.02774.x
Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL (2014) Greater sensitivity to drought accompanies maize yield increase in the US Midwest. Science 344:516–519. https://doi.org/10.1126/science.1251423
Mariotte P, Vandenberghe C, Kardol P, Hagedorn F, Buttler A (2013) Subordinate plant species enhance community resistance against drought in semi-natural grasslands. J Ecol 101:763–773. https://doi.org/10.1111/1365-2745.12064
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Mi N, Cai F, Zhang Y, Ji R, Zhang S, Wang Y (2018) Differential responses of maize yield to drought at vegetative and reproductive stages. Plant Soil Environ 64:260–267. https://doi.org/10.17221/141/2018-PSE
Minchin PEH, Lacointe A (2005) New understanding on phloem physiology and possible consequences for modelling long‐distance carbon transport. New Phytol 166:771–779. https://doi.org/10.1111/j.1469-8137.2005.01323.x
Müller F, Bergmann M, Dannowski R, Dippner JW, Gnauck A, Haase P, Jochimsen M, Kasprzak P, Kröncke I, Kümmerlin R, Küster M (2016) Assessing resilience in long-term ecological data sets. Ecol Indic 65:10–43. https://doi.org/10.1016/j.ecolind.2015.10.066
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998. https://doi.org/10.1093/jxb/ert208
Mutava RN, Prince SJK, Syed NH, Song L, Valliyodan B, Chen W, Nguyen HT (2015) Understanding abiotic stress tolerance mechanisms in soybean: a comparative evaluation of soybean response to drought and flooding stress. Plant Physiol Biochem 86:109–120. https://doi.org/10.1016/j.plaphy.2014.11.010
Myers SS, Smith MR, Guth S, Golden CD, Vaitla B, Mueller ND, Dangour AD, Huybers P (2017) Climate change and global food systems: potential impacts on food security and undernutrition. Annu Rev Publ Health 38:259–277. https://doi.org/10.1146/annurev-publhealth-031816-044356
Otegui ME, Andrade FH, Suero EE (1995) Growth, water use, and kernel abortion of maize subjected to drought at silking. Field Crops Res 40:87–94. https://doi.org/10.1016/0378-4290(94)00093-R
Parvin S, Uddin S, Tausz-Posch S, Armstrong R, Tausz M (2020) Carbon sink strength of nodules but not other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol 227:132–145. https://doi.org/10.1111/nph.16520
Pinheriro C, Passarinho JA, Ricardo CP (2004) Effect of drought and rewatering on the metabolism of Lupinus albus organs. J Plant Physiol 161:1203–1210. https://doi.org/10.1016/j.jplph.2004.01.016
Resilience Alliance (2020) http://www.resalliance.org/about. Accessed 6 Nov 2020
Reynolds JF, Kemp PR, Ogle K, Fernández RJ (2004) Modifying the ‘pulse-reserve’ paradigm for deserts of North America: precipitation pulses, soil water and plant responses. Oecologia 141:194–210. https://doi.org/10.1007/s00442-004-1524-4
Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2:198–206. https://doi.org/10.1016/S1369-5266(99)80036-3
Rosa L, Chiarelli DD, Rulli MC, Dell’Angelo J, D’Odorico P (2020) Global agricultural economic water scarcity. Sci Adv 6:eaaz6031. https://doi.org/10.1126/sciadv.aaz6031
Roudier P, Andersson JC, Donnelly C, Feyen L, Greuell W, Ludwig F (2016) Projections of future floods and hydrological droughts in Europe under a +2°C global warming. Clim Change 135:341–355. https://doi.org/10.1007/s10584-015-1570-4
Ruppert JC, Harmoney K, Henkin Z, Snyman HA, Sternberg M, Willms W, Linstädter A (2015) Quantifying drylands’ drought resistance and recovery: the importance of drought intensity, dominant life history and grazing regime. Glob Change Biol 21:1258–1270. https://doi.org/10.1111/gcb.12777
Sacharz J, Giovagnetti V, Ungerer P, Mastroianni G, Ruban A (2017) The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat Plants 3:16225. https://doi.org/10.1038/nplants.2016.225
Sakschewski B, Von Bloh W, Boit A et al (2016) Resilience of Amazon forests emerges from plant trait diversity. Nat Clim Change 6:1032–1036. https://doi.org/10.1038/nclimate3109
Schreiber UBWN, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. Ecophysiol Photosynth 100:49–70. https://doi.org/10.1007/978-3-642-79354-7_3
Shah NH, Paulsen GM (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226. https://doi.org/10.1023/A:1026237816578
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55:2343–2351. https://doi.org/10.1093/jxb/erh276
Silveira LK, Pavão GC, dos Santos Dias CT, Quaggio JA, de Matos Pires RC (2020) Deficit irrigation effect on fruit yield, quality and water use efficiency: a long-term study on Pêra-IAC sweet orange. Agric Water Manag 231:106019. https://doi.org/10.1016/j.agwat.2020.106019
Siopongco JDLC, Yamauchi A, Salekdeh H, Bennett J, Wade LJ (2006) Growth and water use response of doubled-haploid rice lines to drought and rewatering during the vegetative stage. Plant Prod Sci 9:141–151. https://doi.org/10.1626/pps.9.141
Song H, Li Y, Zhou L, Xu Z, Zhou G (2018) Maize leaf functional responses to drought episode and rewatering. Agric For Meteorol 249:57–70. https://doi.org/10.1016/j.agrformet.2017.11.023
Sun CX, Li CC, Zhang CY, Hao LY, Song M, Liu W, Zhang YL (2018) Reflectance and biochemical responses of maize plants to drought and re-watering cycles. Ann Appl Biol 172:332–345. https://doi.org/10.1111/aab.12423
Thiagarajah MR, Hunt LA, Mahon JD (1981) Effects of position and age on leaf photosynthesis in corn (Zey mays). Can J Bot 59:28–33. https://doi.org/10.1139/b81-005
Trenberth KE, Dai A, Van Der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J (2014) Global warming and changes in drought. Nat Clim Change 4:17–22. https://doi.org/10.1038/nclimate2067
Valentinuz OR, Tollenaar M (2004) Vertical profile of leaf senescence during the grain-filling period in older and newer maize hybrids. Crop Sci 44:827–834. https://doi.org/10.2135/cropsci2004.8270
Van Ruijven J, Berendse F (2010) Diversity enhances community recovery, but not resistance, after drought. J Ecol 98:81–86. https://doi.org/10.1111/j.1365-2745.2009.01603.x
Vile D, Pervent M, Belluau M, Vasseur F, Bresson J, Muller B, Granier C, Simonneau T (2012) Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant Cell Environ 35:702–718. https://doi.org/10.1111/j.1365-3040.2011.02445.x
Voronin PY, Maevskaya SN, Nikolaeva MK (2019) Physiological and molecular responses of maize (Zea mays L.) plants to drought and rehydration. Photosynthetica 57:850–856. https://doi.org/10.32615/ps.2019.101
White AC, Rogers A, Rees M, Osborne CP (2015) How can we make plants grow faster? A source–sink perspective on growth rate. J Exp Bot 67:31–45. https://doi.org/10.1093/jxb/erv447
Xu ZZ, Zhou GS (2006) Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224:1080–1090. https://doi.org/10.1007/s00425-006-0281-5
Xu ZZ, Zhou GS (2007) Photosynthetic recovery of a perennial grass Leymus chinensis after different periods of soil drought. Plant Prod Sci 10:277–285. https://doi.org/10.1626/pps.10.277
Xu Z, Zhou G (2011) Responses of photosynthetic capacity to soil moisture gradient in perennial rhizome grass and perennial bunchgrass. BMC Plant Biol 11:21. https://doi.org/10.1186/1471-2229-11-21
Xu ZZ, Zhou GS, Wang YL, Han GX, Li YJ (2008) Changes in chlorophyll fluorescence in maize plants with imposed rapid dehydration at different leaf ages. J Plant Growth Regul 27:83–92. https://doi.org/10.1007/s00344-007-9035-2
Xu Z, Zhou G, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60:3737–3749. https://doi.org/10.1093/jxb/erp216
Xu ZZ, Zhou GS, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654. https://doi.org/10.4161/psb.5.6.11398
Xu Z, Ren H, Cai J, Wang R, Li MH, Wan S, Han X, Lewis BJ, Jiang Y (2014a) Effects of experimentally-enhanced precipitation and nitrogen on resistance, recovery and resilience of a semi-arid grassland after drought. Oecologia 176:1187–1197. https://doi.org/10.1007/s00442-014-3081-9
Xu Z, Shimizu H, Ito S, Yagasaki Y, Zou C, Zhou G, Zheng Y (2014b) Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239:421–435. https://doi.org/10.1007/s00425-013-1987-9
Zaidi PH, Rafique S, Rai PK, Singh NN, Srinivasan G (2004) Tolerance to excess moisture in maize (Zea mays L.): susceptible crop stages and identification of tolerant genotypes. Field Crops Res 90:189–202. https://doi.org/10.1016/j.fcr.2004.03.002
Zhu R, Wu F, Zhou S, Hu T, Huang J, Gao Y (2020) Cumulative effects of drought–flood abrupt alternation on the photosynthetic characteristics of rice. Environ Exp Bot 169:103901. https://doi.org/10.1016/j.envexpbot.2019.103901
Acknowledgements
The authors are grateful to Yuhui Wang, Bingrui Jia, Yanling Jiang and Jian Song for their help during the study. The authors are also grateful to Hongying Yu, Quanhui Ma and Lang Li for their loyal assistances during the two experiments.
Funding
This study was jointly funded by National Key Research and Development Program of China (2016YFD0300106), China Special Fund for Meteorological Research in the Public Interest (GYHY201506001-3) and National Natural Science Foundation of China (31661143028).
Author information
Authors and Affiliations
Contributions
ZX and GZ deceived and designed the study; MQ, XL, YL, HS and FZ conducted the experiment works. MQ, XL, ZY, YL, QH and ZX performed the data analyses. All authors wrote and proofread the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qi, M., Liu, X., Li, Y. et al. Photosynthetic resistance and resilience under drought, flooding and rewatering in maize plants. Photosynth Res 148, 1–15 (2021). https://doi.org/10.1007/s11120-021-00825-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-021-00825-3