Abstract
Light-harvesting complex II (LHCII) absorbs light energy and transfers it primarily to photosystem II in green algae and land plants. Although the trimeric structure of LHCII is conserved between the two lineages, its subunit composition and function are believed to differ significantly. In this study, we purified four LHCII trimers from the green alga Chlamydomonas reinhardtii and analyzed their biochemical properties. We used several preparation methods to obtain four distinct fractions (fractions 1–4), each of which contained an LHCII trimer with different contents of Type I, III, and IV proteins. The pigment compositions of the LHCIIs in the four fractions were similar. The absorption and fluorescence spectra were also similar, although the peak positions differed slightly. These results indicate that this green alga contains four types of LHCII trimer with different biochemical and spectroscopic features. Based on these findings, we discuss the function and structural organization of green algal LHCII antennae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light-harvesting complex II (LHCII) absorbs solar energy in green algae and land plants and transfers it primarily to photosystem II (PSII) to produce chemical energy. LHCIIs form three transmembrane and two short α-helices (Janik et al. 2017) with chlorophyll (Chl) a and b and carotenoids (violaxanthin or zeaxanthin, neoxanthin, and lutein) (Liu et al. 2004; Standfuss et al. 2005). Oligomerization of LHCIIs into either trimers or monomers has been demonstrated by structural biological studies.
LHCII trimers are composed of three trimeric LHCII proteins, including Lhcb1, Lhcb2, and Lhcb3, where Lhcb1 is coded for by multiple genes and Lhcb2 and Lhcb3 are coded for by smaller number genes (Jansson 1999). LHCII trimers are thought to form homotrimers of Lhcb1 or Lhcb2 and heterotrimers of Lhcb3 and Lhcb1 and/or Lhcb2 (Jackowski et al. 2001; Caffarri et al. 2004), whereas the minor monomeric LHCIIs consist of CP24 (Lhcb6), CP26 (Lhcb5), and CP29 (Lhcb4) (Jackowski et al. 2001; Caffarri et al. 2004; Wei et al. 2016). LHCII trimers can be categorized as strongly (S) or moderately (M) bound LHCII trimers based on the strength of their interaction with the PSII core (C). Recent cryo-electron microscopy analyses of the structure of the C2S2M2-type PSII–LHCII complex showed that Lhcb3 is the LHCII monomer in the M-trimer in contact with CP24 (Su et al. 2017).
In contrast to plant LHCII, LHCII subunits in the green alga Chlamydomonas reinhardtii consist of the products of nine genes (LhcbM1–9): Type I (LhcbM3, LhcbM4, LhcbM6, LhcbM8, and LhcbM9), Type II (LhcbM5), Type III (LhcbM2 and LhcbM7), and Type IV (LhcbM1) (Minagawa et al. 2004; Drop et al. 2014; Natali and Croce 2015). Previous studies proposed the following functions for each Chlamydomonas LHCII: Type I (LhcbM9) acts as a quencher under conditions of sulfur deficiency (Nguyen et al. 2008; Grewe et al. 2014); Type II functions as a monomer and is involved in state transition in Chlamydomonas (Takahashi et al. 2006); Type III (LhcbM2 and M7) is also thought to be involved in state transition in Chlamydomonas, similar to Type II (Ferrante et al. 2012); and Type IV (LhcbM1) plays a role in thermal dissipation but not state transition (Elrad et al. 2002; Natali et al. 2015).
A prominent feature of green algal LHCII is that the PSII-LHCII complex includes a third LHCII trimer known as loosely (L)-bound LHCII trimers, in addition to the S- and M-trimers. L-trimers are associated with the PSII core instead of CP24, thereby forming a C2S2M2L2-type PSII-LHCII complex (Tokutsu et al. 2012). However, there is no biochemical evidence clearly indicating whether the LHCII trimers of green algae are composed of a single type or multiple types of LHCII subunit (Minagawa et al. 2004; Drop et al. 2014; Natali and Croce 2015).
To elucidate the characteristics of Chlamydomonas LHCII, we performed biochemical analyses of LHCII trimers. Four distinct trimeric forms of LHCII with different subunit compositions but similar molecular weights were purified by ion-exchange chromatography via sucrose density gradient (SDG) ultracentrifugation. The pigment composition and spectroscopic properties of all four trimeric forms were similar, although the peak positions differed slightly. These findings suggest that the four LHCII trimers have similar light-harvesting capability, whereas the structural and interaction properties of LHCII monomers vary.
Materials and methods
Strain and culture
The PsaA-His strain (JVD1-1B[pGG1-46]) was used in this study (Gulis et al. 2008). The mutant was grown in Tris–acetate–phosphate medium (Gorman and Levine 1965) under low light (< 20 μmol photons m−2 s−1) with ambient air bubbling at 23 °C until reaching mid-log growth phase (3–6 × 106 cells/mL). Freshly harvested cells were used to prepare thylakoid membranes.
Isolation of thylakoid membranes and purification of LHCII trimers
Cells were disrupted twice using a BioNeb® cell disruption system (BioNeb Limited, London, England) at 7.5 kg/cm2, and thylakoid membranes were isolated as described previously (Tokutsu et al. 2012). Thylakoid membranes were solubilized with 1.4% (w/v) dodecyl-α-D-maltoside (α-DDM) at a Chl concentration of 0.5 mg/mL for 30 min at 4 °C in the dark. In this study, we used α-DDM instead of dodecyl-β-D-maltoside (β-DDM) for solubilization because the detergent type affects the oligomerization of LHCII and homogenous LHCII trimers cannot be obtained by solubilization with β-DDM (Tokutsu et al. 2012). The resultant supernatant was subjected to nickel-affinity column chromatography to remove photosystem I (PSI)-LHCII complexes. The fraction that was passed through the Ni column was subjected to SDG ultracentrifugation (Tokutsu et al. 2012). The LHCII trimer fraction in the upper region of the SDG (Fig. 1a, inset photographs) was then subjected to chromatography on an ÄKTApure system (GE Healthcare Life Sciences, Chicago, IL, USA) equipped with an OMNIFIT column (50 mm diameter× 300 mm length; International Scientific Instrument Supply Co. Ltd., New Delhi, India) packed with Q-Sepharose high-performance resin (GE Healthcare Life Sciences) and equilibrated with a buffer containing 25 mM 2-(N-morpholino) ethanesulfonic acid (MES)–NaOH (pH 6.5), 0.02% (w/v) α-DDM, and 1.0 M betaine (buffer A). The column was washed with buffer A until the eluate became colorless. Four LHCII fractions were separated by a 0–500 mM NaCl linear gradient in three column volumes (CVs) (1 CV = 10 mL) at a flow rate of 1.0 mL/min. Each of the four fractions was subjected to further SDG ultracentrifugation to obtain pure LHCII fractions.
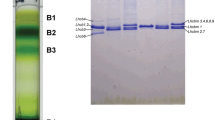
Purification of the four types of LHCII trimers. a LHCII trimers isolated by the first SDG ultracentrifugation (inset photograph) were separated into four fractions of LHCII by anion-exchange chromatography (fractions 1–4). b LHCII trimers isolated by the second SDG ultracentrifugation. c Blue native PAGE analysis of the four LHCII fractions. Fractions 1–4 were purified by the second SDG ultracentrifugation after chromatography. d SDS-PAGE analysis of the four LHCII fractions obtained by the second SDG ultracentrifugation after chromatography. Lane 1, mixture of LHCII isolated by the first SDG ultracentrifugation; lanes 2–5, fractions 1–4 purified after the second SDG ultracentrifugation
Sodium dodecyl sulfate and blue native polyacrylamide gel electrophoresis
Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) was performed according to the method described by Ikeuchi et al. (Ikeuchi and Inoue 1988) and carried out at room temperature with a 16–22% polyacrylamide gradient gel and stacking gel containing 7.5 M urea. The reservoir buffer described by (Laemmli 1970) was used for SDS-PAGE. Samples (3 µg of Chl) were solubilized in a buffer containing 2% lithium dodecyl sulfate, 60 mM dithiothreitol, and 60 mM Tris–HCl (pH 8.5) on ice for 5 min before loading onto the gel.
Blue native PAGE was performed according to the method described by Wittig et al. (2006), with slight modifications. The LHCII samples (3–4 μg of Chl) were buffer exchanged with a buffer containing 25 mM MES-NaOH (pH 6.5), 25% (w/v) glycerol, and 10 mM MgCl2 to a final Chl concentration of 0.5 mg Chl a and b/mL, and then solubilized with 1.0% (w/v) α-DDM followed by incubation on ice for 5 min. LHCII samples were mixed with a Coomassie brilliant blue (CBB) solution containing 5% (w/v) Serva brilliant blue G-250 and 750 mM ε-amino-n-caproic acid at a ratio of 10:1 (v/v) and immediately loaded onto the gel. Electrophoresis was performed at a constant voltage of 100 V until the samples entered the stacking gel, and then carried out at a constant current of 5 mA.
The gels were stained with CBB solution consisting of CBB R-250, 40% (v/v) methanol, and 10% (v/v) acetic acid for 3 h, and then destained overnight at room temperature in a destaining solution consisting of 25% (v/v) methanol and 10% (v/v) acetic acid. The stoichiometries of each LHCII type in the four LHCII trimers were determined by densitometric analysis of a CBB-stained gel (Fig. 1d) using a gel imaging system (ChemiDoc XRS + system; Bio-Rad, Hercules, CA).
Absorption and fluorescence spectroscopy
Absorption spectra were acquired at room temperature using an ultraviolet–visible spectrophotometer (UV-2600; Shimadzu Co., Kyoto, Japan). Fluorescence spectra were measured at 77 K using a fluorescence spectrophotometer (RF-5300; Shimadzu Co.) equipped with a red sensitive photomultiplier (R928-08, Hamamatsu Photonics, Hamamatsu City, Japan) using an excitation and emission bandwidth of 1.5 nm. The excitation wavelength was set at 440 nm.
Pigment analysis
Pigments were extracted from purified LHCII with methanol–acetone (50:50, v/v). In brief, the methanol–acetone treated samples were centrifuged at 20,000×g in the dark at 4 °C for 5 min to remove insolubilized materials, and the resulting supernatants were used for the following pigment analysis. The pigments in the supernatant were separated by ultra-performance liquid chromatography (UPLC) using an H-class system (Waters Corp., Milford, MA, USA) equipped with an 2.1 × 150 mm ACQUITY UPLC HSS C18 column (Waters Corp.), as described previously by Tokutsu and Minagawa (2013).
Results
Figure 1 shows the separation of LHCII complexes from Chlamydomonas. The PSI-LHCII complex was separated from the PsaA-His mutant by nickel-affinity column chromatography, and the fraction that passed through the affinity column was subjected to SDG ultracentrifugation. A major, clear green band, corresponding to LHCIIs, was obtained (inset in Fig. 1a), and then fractionated by anion-exchange chromatography (Fig. 1a). Four distinct peaks, namely fractions 1–4, were separated, and each of the four fractions was positioned at the same layer in the SDG (inset in Fig. 1a), indicating that the four LHCII trimers have similar molecular weights but different surface charges properties. Each fraction was purified further by SDG ultracentrifugation (Fig. 1b). Similar molecular weights among the four LHCII trimer fractions were also observed by blue native PAGE (Fig. 1c). The main band in each of the fractions appeared slightly above the molecular weight marker of 146 kDa, which is the characteristic molecular weight for LHCII trimers (García-Cerdán et al. 2011). Two bands at a lower molecular weight in the LHCII trimer band were thought to be LHCII monomer and dimer, respectively (Janik et al. 2017). The compositions of the subunits of the four LHCII trimers were analyzed by SDS-PAGE (Fig. 1d). The LHCII trimers isolated in the first SDG ultracentrifugation (mixture of LHCIIs) exhibited three characteristic bands corresponding to subunit Types I, III, and IV (lane 1), whereas fractions 1–4 had different subunit compositions (lanes 2–5). Fraction 1 contained two subunits, Types I and III, at a ratio of 2:1 but did not contain Type IV. Fraction 2 contained subunit Types I, III, and IV with a similar stoichiometry. Fraction 3 contained subunit Types I and III at a ratio of 1:2, although faint bands corresponding to Type IV were detected. Fraction 4 contained subunit Types I, III, and IV at a ratio of 1:2:1. These results clearly indicate that Chlamydomonas accumulates at least four trimeric forms of LHCII with different combinations of Types I, III, and IV subunits. The subunit compositions of fractions 1–4 are summarized in Table 1.
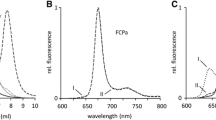
Figure 2 shows absorption spectra measured at room temperature for fractions 1–4 obtained by anion-exchange chromatography. The peaks around 672 and 650 nm represent the Qy bands of Chls a and b, respectively, and the peaks in the shorter wavelength region were assigned as the Soret bands of Chl a (435 nm) and of Chl b (470 nm) and carotenoids (450–500 nm) (panel A). The spectra were normalized to the Qy peak intensity of Chl a. In the Qy band of Chl a, the peak positions were very similar (panel B). The relative intensity of the Qy peak of Chl b increased in the order fraction of 3 > 2 = 1 > 4 (panel B). In the Soret region (panel A), the relative intensity among the trimers differed slightly, and the intensity tended to decrease, similar to the intensity of the Chl b-Qy band. Figure 3 and Table 2 show the pigment composition (based on the molarity of Chl a) of each LHCII trimer after UPLC. The contents of Chl b and various carotenoids (neoxanthin, violaxanthin, lutein, and β-carotene) among in the four fractions were very similar; however, there was a slight difference in the spectral intensity of the Qy peak of Chl b (Fig. 2). Although β-carotene was detected in fractions 1 and 2, it was considered a contaminant. Figure 4 shows the fluorescence spectra of fractions 1–4 at 77 K. The spectra exhibited a single peak at approximately 680 nm. No fluorescence peaks associated with photosystem reaction centers I and II were observed. The overall shapes of the spectral peaks of the four fractions were almost identical, and the spectral intensity of the longer wavelength region was very similar, ranging from 700 to 760 nm, which is regarded as indicative of Chl vibration bands (Lamb et al. 2018) (Fig. 4a). On the other hand, the fluorescence peaks were slightly shifted to a shorter wavelength in the order of fractions of 4 > 2 > 3 > 1 (Fig. 4b).
Fluorescence spectra at 77 K of the four fractions of LHCII excited at 440 nm. Fractions 1–4 are depicted as black, blue, red, and green, respectively. The spectra were normalized to the maximum fluorescence intensity. Panel A, whole region; Panel B, expanded view of the fluorescence maximum around 680 nm
Discussion
In this study, we isolated four distinct trimeric forms of LHCII from Chlamydomonas. A detailed biochemical characterization (Fig. 1) showed that the four LHCII trimers are composed of different combinations of Type I, III, and IV subunits, indicating that the four LHCIIs form different trimers. Fractions 1 and 2 contained heterotrimers consisting of subunit Types I and III at a ratio of 2:1 and subunit Types I, IV, and III at a ratio of 1:1:1, respectively, whereas fraction 3 contained subunit Types I and III at stoichiometry of 1:2. Fraction 4 contained an LHCII trimer composed mainly of the Type III subunit and a small amount of Type I and IV subunits, which were considered to be contaminants (Table 1). The separation pattern in Fig. 1a suggests that Type I and IV subunits in fraction 4 may have come from fraction 3 (Fig. 1d), indicating that fraction 4 is an LHCII trimer consisting of subunit Types III, III, and IV or only Type III. The plant-type LHCII trimers, S-trimer and M-trimer, were identified as a homotrimer and heterotrimer, respectively (Jackowski et al. 2001; Caffarri et al. 2004; Su et al. 2017). Thus, the present findings provide solid evidence that the subunit compositions and organizations of LHCII trimers differ in green lineage oxygenic photosynthetic organisms.
Several studies have examined the function of LHCII subunits. Type I (LhcbM9) acts as a quencher under conditions of sulfur deficiency (Nguyen et al. 2008; Grewe et al. 2014). We clearly showed that Type I subunit was present in all four fractions (Fig. 1), suggesting that the four LHCII trimers are involved in the quenching of excitation energy, but in different ways. Type IV (LhcbM1) plays a role in thermal dissipation, but not state transition (Elrad et al. 2002; Natali et al. 2015). Type IV subunit was contained in fraction 2 (Fig. 1d), but it was considered a contaminant in fractions 1, 3, and 4. This suggests that the Type IV subunit contained in fraction 2 is involved in thermal dissipation. Type III subunit (LhcbM2 and M7) was suggested to be involved in state transition (Ferrante et al. 2012; Takahashi et al. 2014; Natali et al. 2015). In our preparation, Type III subunit was present in all LHCII trimers to different extents (Fig. 1d), indicating that Type III is the ubiquitous subunit of LHCII trimer in Chlamydomonas. In a previous study, Type II subunit was not observed within the PSII-LHCII supercomplex but rather constituted an “extra” LHCII population (Drop et al. 2014). This feature may explain why we could not identify the Type II subunit in the four LHCII trimers obtained from the PSII-LHCII supercomplex.
There were minimal differences in the pigment composition of each LHCII trimer (Fig. 3, Table 2). Fractions 1 and 2 contained β-carotene, which may have been present due to contamination of the reaction center and/or LHCII complexes, as β-carotene is usually not a component of LHCII trimers in land plants. The absorption and fluorescence spectra of the four LHCII trimers were similar, except for very minor differences in peak positions and intensities (Figs. 2 and 4). Further investigations of the excitation energy dynamics in the LHCII preparations are required to elucidate the function of the four LHCIIs in Chlamydomonas.
One of the major questions remaining to be addressed concerns the localization of the four LHCII trimers in thylakoids. It has been reported that Chlamydomonas PSII-LHCII complexes are C2S2M2L2-type supercomplexes and a certain amount of Type I, III, and IV LHCII is involved in the C2S2M2L2-type supercomplex (Tokutsu et al. 2012). To clarify whether those four LHCII trimers (fractions 1–4) are practically associated with PSII, it is necessary to try the purification of LHCII trimers from the C2S2M2L2-type supercomplex by a careful and reproducible method.
Abbreviations
- CBB:
-
Coomassie brilliant blue
- Chl:
-
Chlorophyll
- CV:
-
Column volume
- α-DDM:
-
Dodecyl-α-D-maltoside
- β-DDM:
-
Dodecyl-β-D-maltoside
- LHCII:
-
Light-harvesting complex II
- MES:
-
2-(N-morpholino) ethanesulfonic acid
- PSII:
-
Photosystem II
- PSI:
-
Photosystem I
- SDG:
-
Sucrose density gradient
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- UPLC:
-
Ultra-performance liquid chromatography
References
Caffarri S, Croce R, Cattivelli L, Bassi R (2004) A look within LHCII: differential analysis of the Lhcb1-3 complexes building the major trimeric antenna complex of higher-plant photosynthesis. Biochemistry 43:9467–9476
Drop B, Webber-Birungi M, Yadav SKN, Filipowicz-Szymanska A, Fusetti F, Boekema EJ, Croce R (2014) Light-harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii. Biochim Biophys Acta 1837:63–72
Elrad D, Niyogi KK, Grossman AR (2002) A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14:1801–1816
Ferrante P, Ballottari M, Bonente G, Giuliano G, Bassi R (2012) LHCBM1 and LHCBM2/7 polypeptides, components of major LHCII complex, have distinct functional roles in photosynthetic antenna system of Chlamydomonas reinhardtii. J Biol Chem 287:16276–16288
García-Cerdán JG, Kovács L, Tõth T et al (2011) The PsbW protein stabilizes the supramolecular organization of photosystem II in higher plants. Plant J 65:368–381
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA 54:1665–1669
Grewe S, Ballottari M, Alcocer M et al (2014) Light-harvesting complex protein LHCBM9 is critical for photosystem II activity and hydrogen production in Chlamydomonas reinhardtii. Plant Cell 26:1598–1611
Gulis G, Narasimhulu KV, Redding KE (2008) Purification of His(6)-tagged photosystem I from Chlamydomonas reinhardtii. Photosynth Res 96:51–60
Ikeuchi M, Inoue Y (1988) A new 4.8-kDa polypeptide intrinsic to the PS II reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular-weight proteins. Plant Cell Physiol 29:1233–1239
Jackowski G, Kacprzak K, Jansson S (2001) Identification of Lhcb1/Lhcb2/Lhcb3 heterotrimers of the main light-harvesting chlorophyll a/bprotein complex II of Photosystem II (LHCII). Biochim Biophys Acta 1504:340–345
Janik E, Bednarska J, Sowinski K, Luchowski R, Zubik M, Grudzinski W, Gruszecki WI (2017) Light-induced formation of dimeric LHCII. Photosynth Res 132:265–276
Jansson S (1999) A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci 4:236–240
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lamb JJ, Røkke G, Hohmann-Marriott MF (2018) Chlorophyll fluorescence emission spectroscopy of oxygenic organisms at 77 K. Photosynthetica 56:105–124
Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
Minagawa J, Takahashi Y (2004) Structure, function and assembly of Photosystem II and its light-harvesting proteins. Photosynth Res 82:241–263
Natali A, Croce R (2015) Characterization of the major light-harvesting complexes (LHCBM) of the green alga Chlamydomonas reinhardtii. PLoS ONE 10:1–18
Nguyen AV, Thomas-Hall SR, Malnoë A et al (2008) Transcriptome for photobiological hydrogen production induced by sulfur deprivation in the green alga Chlamydomonas reinhardtii. Eukaryot Cell 7:1965–1979
Standfuss J, Van Scheltinga ACT, Lamborghini M, Kühlbrandt W (2005) Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J 24:919–928
Su X, Ma J, Wei X, Cao P, Zhu D, Chang W, Liu Z, Zhang X, Mei L (2017) Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science 357:815–820
Takahashi H, Iwai M, Takahashi Y, Minagawa J (2006) Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103:477–482
Takahashi H, Okamuro A, Minagawa J, Takahashi Y (2014) Biochemical characterization of photosystem I-associated light-harvesting complexes I and II isolated from state 2 cells of Chlamydomonas reinhardtii. Plant Cell Physiol 55:1437–1449
Tokutsu R, Minagawa J (2013) Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 110:10016–10021
Tokutsu R, Kato N, Bui KH, Ishikawa T, Minagawa J (2012) Revisiting the supramolecular organization of photosystem II in Chlamydomonas reinhardtii. J Biol Chem 287:31574–31581
Wei X, Su X, Cao P, Liu X, Chang W, Li M, Zhang X, Liu Z (2016) Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 534:69–74
Wittig I, Braun HP, Schägger H (2006) Blue native PAGE. Nat Protoc 1:418–428
Acknowledgements
We thank Ms. Rie Uno (Osaka City University), Mr. Daisuke Namba (Osaka City University), and Ms. Chiyo Noda (National Institute for Basic Biology) for their assistance with purification and analysis. We are also grateful to Prof. Nobuo Kamiya (Osaka City University) and Associate Prof. Ikuko Miyahara (Osaka City University) for providing laboratory access. This work was funded by the Joint Usage/Research Program of the Artificial Photosynthesis, Osaka City University. K.K. and J.M. are grateful for the continued support of JST CREST, Japan (Grant No. JPMJCR13M4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawakami, K., Tokutsu, R., Kim, E. et al. Four distinct trimeric forms of light-harvesting complex II isolated from the green alga Chlamydomonas reinhardtii. Photosynth Res 142, 195–201 (2019). https://doi.org/10.1007/s11120-019-00669-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00669-y