Abstract
Mechanisms involving ammonium toxicity, excess light, and photosynthesis are scarcely known in plants. We tested the hypothesis that high NH4+ supply in presence of high light decreases photosynthetic efficiency of rice plants, an allegedly tolerant species. Mature rice plants were previously supplied with 10 mM NH4+ or 10 mM NO3− and subsequently exposed to 400 µmol m−2 s−1 (moderate light—ML) or 2000 µmol m−2 s−1 (high light—HL) for 8 h. HL greatly stimulated NH4+ accumulation in roots and in a minor extent in leaves. These plants displayed significant delay in D1 protein recovery in the dark, compared to nitrate-supplied plants. These responses were related to reduction of both PSII and PSI quantum efficiencies and induction of non-photochemical quenching. These changes were also associated with higher limitation in the donor side and lower restriction in the acceptor side of PSI. This later response was closely related to prominent decrease in stomatal conductance and net CO2 assimilation that could have strongly affected the energy balance in chloroplast, favoring ATP accumulation and NPQ induction. In parallel, NH4+ induced a strong increase in the electron flux to photorespiration and, inversely, it decreased the flux to Rubisco carboxylation. Overall, ammonium supply negatively interacts with excess light, possibly by enhancing ammonium transport towards leaves, causing negative effects on some photosynthetic steps. We propose that high ammonium supply to rice combined with excess light is capable to induce strong delay in D1 protein turnover and restriction in stomatal conductance, which might have contributed to generalized disturbances on photosynthetic efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ammonium (NH4+) is an important N-source particularly for plant species cultivated in environments such as flooded and paddy soils and/or after NH4+/urea fertilization (Ishiyama et al. 2004a; Miller and Cramer 2005). Although roots are able to uptake large amounts of this cation and of its deprotonated form (NH3), commonly they are toxic for the majority of the cultivated plants (Li et al. 2012; Britto and Kronzucker 2013), whereas toxicity and N-source physiological preference are species-dependent (Britto and Kronzucker 2005). In most aerated soils, NO3− is the prevailing N-source and plant species are capable to uptake and store this anion in high concentrations in their vacuoles (Guan et al. 2016). Nitrate and ammonium assimilation affects several biochemical and molecular mechanisms, altering various specific physiological processes throughout plant development (Liu and Von Wirén 2017). Photosynthesis is affected by ammonium toxicity but the underlying mechanisms are less understood, especially in tolerant plant species (Velthuys 1975; Sharma and Sirohi 1987, 1988; Bendixen et al. 2001; Silva et al. 2001; Lopes et al. 2004; Podgórska et al. 2013).
For more than 40 years, several studies have supported that ammonia in very high concentrations is able to bind to oxygen evolving complex (OEC), decreasing the PSII quantum efficiency. In a pioneer study, Velthuys (1975) have suggested that NH3 binds to OEC, probably competing with H2O in the S2 and S3 oxidation states. Later in the 1980s, evidence emerged that chloride and ammonia can compete for the same site and, consequently, ammonia could inhibit oxygen evolution by displacing chloride from an essential binding site in the OEC (Sandusky and Yocum 1983, 1984). Subsequently, Beck et al. (1986) demonstrated that, in fact, the coordination binding of ammonia to Mn site occurs suddenly after formation of the S2 state. More recently, these conclusions were corroborated by crystallography studies involving PSII of cyanobacteria, which revealed that a secondary binding also occurs in the outer shell of OEC amino acids, which might directly compete with chloride and decrease PSII activity (Askerka et al. 2015; Vinyard et al. 2016).
Despite several works have promoted the understanding of biochemical mechanisms of ammonia interaction with OEC, the physiological consequences of these processes are much less investigated to date. Indeed, NH3-OEC binding might induce a delay in the electron transference to reaction center, causing photodamage to PSII (Dai et al. 2014). Currently, some studies employing the cyanobacteria Arthrospira and the microalgae Chlorella have evidenced that high NH4+ levels induce reduction in the PSII and PSI activities and gradual inhibition in the O2-evolution complex (Markou et al. 2016). In addition, some results have suggested that Fv/Fm recovery kinetics display a clear trend to display a more intense delay in NH4+-treated thylakoids compared to nitrate, suggesting that D1 protein turnover could also be affected by excess ammonium in cyanobacteria (Crawford et al. 2016). However, these toxic effects at physiological concentrations (1–10 mM), on specific aspects of photosynthesis in higher plants, such as inhibition in D1 protein turnover, have been scarcely reported.
The majority of cultivated species are sensitive to excess ammonium because in high concentrations this molecule might trigger many metabolic disorders (Li et al. 2012; Britto and Kronzucker 2013). In general, plants exposed to excess NH4+ display reduced growth, increased oxidative stress and modifications in the mitochondrial and chloroplast metabolism (Ariz et al. 2010; Cruz et al. 2011; Bittsánszky et al. 2015; Yang et al. 2015). Plants that are highly NH4+ tolerant are able to activate efficient mechanisms to avoid toxicity in roots, especially excluding it from leaf tissues (Bittsánszky et al. 2015). Rice is considered an NH4+ very tolerant plant species (Wang et al. 1993). This main feature is largely attributed to leaf-NH4+ exclusion and triggering of an efficient GS/GOGAT cycle (glutamine synthetase/glutamate synthase) in roots, avoiding the toxic effects of this molecule/ion (Ishiyama et al. 2004b; Balkos et al. 2010). However, several others protective mechanisms to prevent NH4+ toxicity such as an efficient antioxidant system, have been amply reported (Szal and Podgórska 2012; Esteban et al. 2016).
Paradoxically, despite the great importance of ammonium nutrition and photosynthesis for plant growth, especially for rice grown in paddy soils, few studies have been devoted to these issues (Guo et al. 2007; Li et al. 2009; Ding et al. 2015). This problem deserves a special attention in the case of rice plants cultivated in tropical regions, where is very common the occurrence of high light intensities (Murchie et al. 2015) and the co-occurrence of excess ammonium due to anaerobic conditions of paddy soils (Britto and Kronzucker 2002). Indeed, under HL conditions, NH4+ flux from roots towards leaves could be intensified (von Wirén et al. 2000). Moreover, these environmental circumstances also should favor the photorespiratory cycle since excess light greatly stimulates photorespiration (Peterhansel and Maurino 2011) as well as high ammonia concentrations inside chloroplasts (Frantz et al. 1982). However, studies involving rice and ammonium toxicity in presence of excess photochemical energy have been neglected. Besides, the scarce published reports commonly have been conducted with moderate NH4+ concentrations, short-term exposure, and presence of mild light conditions. Overall, these studies are focused on comparing the effects of NO3− and NH4+ nutrition on photosynthetic performance in different species and not for evaluating toxicity (Guo et al. 2007; Li et al. 2009; Ding et al. 2015).
Despite the interaction between NH3 and OEC is biochemically well established, several gaps involving ammonium toxicity and photosynthesis in higher plants remain to be solved. This problem is especially meaningful in physiological terms, since important crops might intensely utilize that N-source in field conditions. In particular, the utilization of rice plants, as a model, is essential since it is an allegedly ammonium-tolerant species widely cultivated in soil conditions where NH4+ might be predominant. Particularly, one important question could be raised here. Is excess NH4+ capable to affect some specific photosynthetic mechanism such as D1 protein turnover in rice plants and could these constraints be aggravated by high light?
In this study we tested the hypothesis that high NH4+ supply in presence of excess light is able to induce disturbances on photosynthetic apparatus of rice plants, affecting D1 protein turnover and decreasing PSII and PSI activities. Our data provide evidence that in these conditions high ammonium supply in presence of excess light causes generalized disorders on the rice photosynthetic apparatus. These effects are non-specific and widespread, reaching several photosynthesis-related processes, including delay in D1 protein turnover and stomatal conductance, which reflected in increases of non-photochemical quenching (NPQ) and photorespiration. These finds are discussed in terms of physiological importance of the interaction between ammonium and high light and its consequences for the photosynthetic efficiency of rice, an NH4+-tolerant plant species.
Materials and methods
Plant material and growth conditions
Rice (Oryza sativa L.) seedlings of the Nipponbare cultivar, which is adapted to lowland soils (Parent et al. 2010), with 10 days after germination, were transplanted to 3 L plastic pots filled with 1/4 strength Hoagland-Arnon’s nutrient solution (Hoagland and Arnon 1950), containing initially 2.5 mM NO3− and 0.5 mM NH4+ as N-sources. Previous studies carried out in our laboratory have demonstrated that the Nipponbare is a facultative cultivar able to complete its life cycle under exclusive nutrition of NO3− or NH4+ as a sole N-source. When these ions are supplied separately at 10 mM concentration, rice plants display similar growth under hydroponics and greenhouse conditions. The pH of the nutrient solution was adjusted every 2 days to 6.0 ± 0.5, with 1 M KOH or 1 M HCl, and it was completely changed weekly. After three weeks, plants were transferred to nutrient solution with full-strength (10 mM NO3− and 2 mM NH4+) for another 2 weeks (until 35-day-old) inside a greenhouse under the following conditions: day/night mean temperature of 30/25 °C, mean relative humidity of 65%, maximum photosynthetic photon flux density (PPFD) of 600 µmol m−2 s−1 at noon, and a photoperiod of 12 h.
Experiments
Initially, an experiment (Experiment I) was performed with 35-day-old intact plants previously grown in a complete nutrient solution, as described above, and further transferred to an N-free solution for 72 h in order to induce a transient N-deprivation to trigger the expression of nitrate and ammonium transporters. Afterwards, plants were transferred to a controlled growth chamber (28/24 °C day/night temperature, 60% relative humidity, 400 µmol m−2 s−1 PPFD, and 12 h photoperiod) and exposed to a complete nutritive solution containing 10 mM NO3− or 10 mM NH4+, as a sole N-source, for 7 days. After this acclimation period, in the last day the plants were exposed to moderate light—ML (400 µmol m−2 s−1) or high light—HL (2000 µmol m−2 s−1) for 8 h. Subsequently, specific in vivo assays of PSII and PSI kinetics and gas exchanges analyses were performed in presence of different light intensities.
A second experiment (Experiment II) was performed in order to analyze the PSII quantum efficiency and D1 protein dark-recovery kinetics employing a single detached mature leaf containing its respective sheath. These individual leaves were obtained from plants previously grown in 10 mM NO3− or 10 mM NH4+ in a growth chamber as described above. These leaves were incubated in tubes containing water (control) or 2 mM lincomycin in the dark during 24 h. Afterwards, the maximum quantum efficiency of PSII (Fv/Fm) was measured and then the detached leaves were exposed to high light (2000 µmol m−2 s−1) throughout 60 min. Subsequently, the effective quantum efficiency was measured immediately after the illumination phase and periodically (over 80 min) during the dark-recovery. Detached leaves (without sheath) were sampled for the D1 protein immunodetection in the following conditions: (1) before the light exposure (dark), (2) after 1 h of high light exposure, and (3) after 30 min of dark-recovery.
Finally, a third experiment (Experiment III) was performed in rice leaf segments in order to evaluate the more direct effects of NO3− and NH4+ on the activity of glutamine synthetase isoforms (GS1 and GS2). Three cm length segments from fully expanded leaves were incubated in a solution containing 10 mM NO3− or 10 mM NH4+ dissolved in 10 mM Hepes buffer (pH 6.0) and 0.01% (v/v) Triton X-100 and kept under PPFD of 400 µmol m−2 s−1 or 2000 µmol m−2 s−1 for 8 h. A previous vacuum-infiltration for 5 min was applied to allow a more effective absorption into the leaf tissues. After light treatments, the activities of GS1 and GS2 were assessed.
Measurements of NO3 − and NH4 + contents in root and leaf tissues
To quantify nitrate and ammonium contents in plant tissues, lyophilized samples were incubated with distilled water at 90 °C for 1 h and filtered to obtain the crude extract. Subsequently, the nitrate concentration was measured by the salicylic acid method of Cataldo et al. (1975) and ammonium concentration was determined by the phenol-hypochlorite method (Felker 1977).
Gas exchange and photochemical measurements
The gas exchange parameters were measured by using a portable infrared gas analyzer system (LI-6400XT, LI-COR, Lincoln, NE, USA), equipped with a leaf chamber fluorometer (LI-6400-40, LI-COR, Lincoln, NE, USA), in mature leaves of plants previously acclimated to growth light conditions. For A–Ci and gS–Ci curves, PPFD and temperature inside the measurement chamber were kept at 1500 µmol m−2 s−1 and 28 °C, respectively, and the CO2 partial pressure was changed from 5 to 120 Pa involving the following 11 steps: 40, 30, 20, 10, 5, 40, 50, 70, 100, 120, and 40 Pa. The curve fitting was performed according to Ethier and Livingston (2004) and the Vcmax [maximum carboxylation rate of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco)], and Jmax (maximum electron transport rate) were estimated. For A-PPFD, Ci-PPFD and gS-PPFD curves, CO2 partial pressure and temperature inside leaf chamber were maintained at 40 Pa and 28 °C, respectively, and PPFD was changed from 2000 to 0 µmol m−2 s−1 according to Huang et al. (2016). The A-PPFD fitting curve was performed using a non-rectangular hyperbola, employing the platform available in: http://www.landflux.org/Tools.php (Marshall and Biscoe 1980; Thornley and Johnson 1990). In all measurements, the amount of blue light was set up to 10% of the PPFD to maximize stomatal aperture (Flexas et al. 2008) and the leaf-to-air vapor pressure difference was 1.85 ± 0.14 kPa. Measurements were recorded when the total coefficient of variation was lower than 5% and temporal stability was achieved (on average, 3 min after the beginning of each step).
After the light curve measurements, photosynthetic electron flow parameters were estimated according to the Farquhar et al. (1980) models. The electron transport rate at PSII (ETRII) was calculated as: ETRII = ΦPSII × PPFD × 0.5 × 0.84, where ΦPSII represents the effective quantum yield of PSII, 0.5 represents the distribution ratio of light absorbed by chloroplast to PSII and 0.84 represents the ratio of light absorbed by chloroplasts. The electron flow devoted to RuBP oxygenation (Jo) or RuBP carboxylation (Jc) and the photorespiration rate (Pr) were determined according to Valentini et al. (1995): Jo = 2/3 × [ETRII − 4 × (A + Rd)]; Jc = 1/3 × [ETRII + 8 × (A + Rd)], Pr = 1/12 × [ETRII − 4 × (A + Rd)], where A represents the net CO2 assimilation and Rd represents the rate of mitochondrial respiration in the dark.
The following in vivo chlorophyll a fluorescence and the P700+ absorbance were measured using a Dual-PAM 100 (Walz, Germany). The photochemical light curves were performed employing increasing light intensities from 0 to 2000 µmol m−2 s−1 and the leaves remained by 5 min under each light intensity. For induction/recovery kinetics, 2000 µmol m−2 s−1 of PPFD was employed for 40 min (induction), followed by 40 min dark (recovery) and subsequently 40 min at 2000 µmol m−2 s−1 of PPFD (re-induction). The fluorescence parameters were measured using the saturation pulse method (Schreiber 2004) and leaves were previously acclimated to dark for 30 min. The intensity and duration of the saturation pulse were 8000 µmol m−2 s−1 and 0.6 s, respectively. The following parameters were assessed: maximum quantum yield of PSII [Fv/Fm = (Fm − Fo)/Fm] and effective quantum yield of PSII [ΦPSII = (Fm − Fs)/Fm′]. Photochemical and non-photochemical quenching coefficient were calculated as qP = (Fm′ − Fs)/(Fm′ − Fo′) and NPQ = (Fm/Fm′) − 1, respectively, whereas Fm was determined at the onset of light induction kinetics. Fm and Fo are the maximum and minimum fluorescence of dark-adapted leaves, respectively; Fm′ and Fs are the maximum and steady-state fluorescence in the light-adapted leaves, respectively; Fo′ is the minimum fluorescence after the far-red illumination of the previously light-exposed leaves (Schreiber 2004). The quinone pool redox state was estimated as 1-qP and ETRII were calculated as reported before.
The redox state of the PSI primary donor (P700) was measured and the following parameters were assessed: (1) photochemical quantum yield of PSI by [ΦPSI = (Pm′ − P)/Pm] and (2) estimated electron transport rate of PSI as [ETRI = ΦPSI × PPFD × 0.5 × 0.84]. The donor side limitation of PSI was calculated by [Φ(ND) = (P − Po)/Pm] and the acceptor side of PSI limitation as [Φ(NA) = (Pm − Pm′)/Pm] (Klughammer and Schreiber 2008). In the second experiment, for determination of induction/recovery kinetics a PPFD of 2000 µmol m−2 s−1 was employed for 1 h (induction phase) followed by 80 min of dark-recovery.
Leaf membrane damage and lipid peroxidation
Leaf membrane damage (MD) was measured as described previously by Lima Neto et al. (2014). Leaf segments (5 cm length) were placed in tubes containing 10 mL of deionized water and incubated in a shaking water bath at 25 °C for 24 h. After, the electric conductive in medium (L1) was measured. Next, the segments were boiled at 95 °C for 1 h, cooled to 25 °C, and the electric conductivity (L2) was measured and the MD was calculated using the following equation: MD = (L1/L2) × 100. The lipid peroxidation was measured based on the formation of thiobarbituric acid-reactive substances (TBARS) in accordance with Cakmak and Horst (1991). TBARS concentrations were calculated using its absorption coefficient (155 mM−1 cm−1) and the results were expressed as ηmol MDA g−1 FM.
Activities of glutamine synthetase GS1 and GS2 isoforms
Fresh leaves were ground until obtaining a fine powder in presence of liquid N2, 200 mM Tris–HCl buffer (pH 7.5) containing 1 mM EDTA and 1 mM MgCl2. All extraction stages were carried out at 4 °C. The activities of total GS (EC 6.3.1.2) and GS1 were determined by the hydroxamate biosynthetic method as described by Hirel and Gadal (1980). For the GS total activity, the assay buffer consisted of 50 mM Tris–HCl buffer, pH 7.8 containing 5 mM ATP, 12.5 mM MgSO4, and 25 mM Na-glutamate. For GS1 activity, 5 mM glucosamine 6-phosphate was added in the assay buffer to inhibit the GS2 activity. The concentration of the brown complex was determined by measuring the absorbance at 540 ηm. The blank consisted of the reaction mixture in the absence of enzymatic extract. A control was performed from omitting of hydroxylamine from the reaction mixture. A standard curve was made with γ-glutamyl hydroxamate and the GS activity was expressed as µmol γ-glutamyl hydroxamate (GGH) g−1 FW h−1. The GS2 activity was calculated as follow: GS2 = GS Total − GS1.
D1 protein immunoblotting
Fresh leaves samples were ground until obtaining a fine powder in presence of liquid N2, ice-cold 100 mM K-phosphate buffer (pH 7.0) containing 1 mM EDTA and 2 mM ascorbic acid. After centrifugation at 14,000×g for 30 min, the supernatant was collected and used as protein extract. The total soluble protein was measured according to the Bradford’s method. Leaf protein extracts were first separated by SDS-PAGE (Laemmli 1970). Equal amounts of protein (10 µg) were electrophoretically transferred to a nitrocellulose membrane (Towbin et al. 1979). Polypeptide detection was performed using specific polyclonal antibodies against PsbA (AS05084-Agrisera©, Sweden). Membranes were blocked for 3 h with 5% non-fat milk in saline Tris–HCl buffer (100 mM Tris–HCl, pH 7.6, 150 mM NaCl), incubated with PsbA antibody overnight and afterwards with alkaline phosphatase-conjugated secondary antibody (A3812-Sigma-Aldrich©, USA) by 6 h. The protein detection was performed using NBT/BCIP (Sigma-Aldrich©, USA) by adding one tablet to 10 mL of deionized water until bands were visualized. The bands abundance was calculated using the SmartView Pro 1200 Imager System Version 1.0.0.3 (Major Science).
Statistical analysis and experimental design
The experiments were arranged in a completely randomized design, with four replicates per treatment. In the leaf segment experiment, one replicate was represented by one Petri dish containing 40 leaf segments. In intact plants experiments, a pot containing two plants represented one replicate. For the detached leaves experiment a single detached leaf represented one replicate. The data were subjected to analysis of variance by ANOVA and averages were compared by Tukey’s test or t-test at 5% of probability (p ≤ 0.05), as mentioned in figure captions. All statistical analyses were conducted using SigmaPlot 12.0 (Systat Software, San Jose, USA).
Results
Rice plants exposed to high ammonium supply under moderate light displayed an effective NH4 + exclusion mechanism from leaves but not when they were exposed to high light
In order to evaluate NH4+ accumulation in roots and leaves of rice plants exposed to high exogenous ammonium supply for a long-term exposure (7 days), was performed in presence of moderate or high light. When ammonium content was measured in NH4+-supplied plants and subsequently exposed to HL, the results show increases in ammonium accumulation of 32% and 40% in roots and leaves, respectively, compared to ML, reaching contents of approximately 180 µmol g−1 DW and 52 µmol g−1 DW (Fig. S1). Therefore, the interaction of HL with high NH4+ supply was capable to significantly enhance ammonium accumulation in both roots and leaves of rice plants (Fig. S1). Leaf NH4+ accumulation in nitrate-supplied plants was much lower compared to NH4+-supplied plants, reaching values threefold lower and ammonia content was not enhanced by HL in this combination (Fig. S1). In addition, nitrate content in leaves was similar in all N-treatments and it was slightly decreased under high light conditions, when compared to ML (Fig. S2).
Interestingly, after 7 days of exposure to high ammonium concentrations, rice plants exhibited some leaf senescence symptoms, especially in the older leaves, which were not detected in nitrate-supplied plants (Fig. S3). This result is interesting because most of the reports related to rice and ammonium nutrition consider this species as an ammonium specialist and this senescence phenomenon has not been reported yet. Since changes in nutritive solution pH are constantly referred as important side effects caused by ammonium uptake, we performed a rigorous procedure of periodically to change the nutritive solution and to correct the pH at every 2 days to adequate levels (Fig. S4). Therefore, the leaf senescence presented by rice plants in response to ammonium was not related to pH side effects in nutrient solution.
High NH4 + supply generated a delay in PSII dark-recovery and impairment in PSII and PSI quantum efficiencies
Since rice plants exhibited a light-dependent ammonium accumulation in leaves, it was investigated if ammonium toxicity could have induced imbalances in PSII and PSI activities. An experiment using 35-day-old rice plants previously grown for 7 days under 10 mM NH4+ or 10 mM NO3− was performed. Initially, a long-term kinetics revealed that ammonium supplying induced an intense decrease in effective quantum yield efficiencies of both PSII and PSI (ΦPSII and ΦPSI) at the steady-state level of the illumination phase. During this stage ΦPSII and ΦPSI values of ammonium-treated plants corresponded to 65% of plants grown under nitrate supply (Fig. 1).
Photochemical kinetics measured in leaves from intact rice plants supplied with 10 mM NO3− or 10 mM NH4+ in a controlled growth chamber for 7 days. During these experiments plants were kept under a 12 h photoperiod with 400 µmol m−2 s−1 of continuous light regime (Experiment I). a Effective quantum efficiency of PSII (ΦPSII) and b effective quantum efficiency of PSI (ΦPSI). The actinic light employed for induction kinetics was 2000 µmol m−2 s−1. Photochemical parameters were noted in response to time (120 min), with 40 min of light induction (0–40 min), 40 min of dark relaxation (40–80 min) and 40 min of light re-induction (80–120 min). Represented values indicate the average of four independent replicates (± SE)
During the dark-recovery phase, ammonium-treated plants exhibited a severe delay in the ΦPSII relaxing kinetics (Fig. 1a). On the other hand, the photosystem I relaxation kinetics in ammonium-supplied plants was much faster than that of nitrate supplying, reaching the maximum relaxation in the first minutes of dark exposure, whereas nitrate-treated plants exhibited this maximum only after 15 min (Fig. 1b). Remarkably, the re-induction kinetics revealed a greater decrease in ΦPSII and ΦPSI steady-state values of plants treated with ammonium compared to nitrate-supplied plants (Fig. 1).
The 1-qP parameter has been widely employed to estimate the pool of PSII acceptor redox state. Ammonium-treated plants exhibited a slight increase in the reduced state of quinones, after 40 min of HL exposure, in comparison to nitrate-treated leaves (Fig. 2a). During the relaxation phase, ammonium-supplied rice exhibited a severe delay in the oxidation dynamics of the quinone pool. Nevertheless, during the re-induction phase the ammonium-supplied rice displayed a more intense difference of quinone pool reduced state in comparison to induction phase, reaching 86% against 73% exhibited by nitrate references (Fig. 2a).
Photochemical kinetics measured in leaves from intact rice plants supplied with 10 mM NO3− or 10 mM NH4+ in a controlled growth chamber for 7 days. During these experiments plants were kept under a 12 h photoperiod with 400 µmol m−2 s−1 of continuous light regime (Experiment I). a quinone pool redox state (1-qP) and b non-photochemical quenching (NPQ). The actinic light employed for induction kinetics was 2000 µmol m−2 s−1. Photochemical parameters were noted in response to time (120 min), with 40 min of light induction (0–40 min), 40 min of dark relaxation (40–80 min) and 40 min of light re-induction (80–120 min). Represented values indicate the average of four independent replicates (± SE)
The non-photochemical quenching (NPQ) was greatly stimulated in rice after ammonium exposure. Indeed, during the first cycle of illumination, ammonium-treated plants exhibited prominent induction of NPQ in comparison to nitrate supplying (Fig. 2b). Moreover, during the relaxation phase the induced state of NPQ persisted for a longer time in high ammonium plants compared to nitrate-treated rice and during the re-induction phase ammonium-treated leaves also displayed higher NPQ values in comparison to nitrate-supplied plants (Fig. 2b).
In order to understand the dynamics observed in both PSII and PSI, two important parameters related to the extent of P700 oxidation state were assessed: the limitation of acceptor side of PSI – Φ(ND) and PSI acceptor side limitation – Φ(NA). PSI donor side limitation in plants supplied with ammonium reached higher values (circa 45%) in both induction phases compared to nitrate references, but during the relaxation stage Φ(ND) values of both treatments were similar (Fig. 3a). In parallel, Φ(NA) was higher in nitrate reference than in ammonium-supplied plants and this difference also occurred during the first 5 min of dark relaxation kinetics, but no significant differences were observed after 40 min of dark (Fig. 3b).
Photochemical kinetics measured in leaves from intact rice plants supplied with 10 mM NO3− or 10 mM NH4+ in a controlled growth chamber for 7 days. During these experiments, plants were kept under a 12 h photoperiod with 400 µmol m−2 s−1 of continuous light regime (Experiment I). a PSI donor side limitation (ΦND) and b PSI acceptor side limitation (ΦNA). The actinic light employed for induction kinetics was 2000 µmol m−2 s−1. Photochemical parameters were noted in response to time (120 min), with 40 min of light induction (0–40 min), 40 min of dark relaxation (40–80 min) and 40 min of light re-induction (80–120 min). Represented values indicate the average of four independent replicates (± SE)
To investigate the mechanisms underlying the delay of PSII recovery and role displayed by D1 protein in NH4+-treated plants, an experiment using detached leaves was performed. These leaves were subjected to a long-term PSII induction/recovery kinetics, in presence of lincomycin, a chloroplast protein synthesis inhibitor. Dark acclimated leaves of both NO3− and NH4+ treatments exhibited a similar content of D1 protein under reference condition (without lincomycin) but in the presence of this inhibitor the abundance of this protein was similarly decreased in both N-treatments (Fig. 4a).
Changes in a D1 protein abundance and b photochemical kinetics in detached rice leaves of plants supplied with 10 mM NO3− or 10 mM NH4+ in a growth chamber. After 7 days, detached leaves were exposed to lincomycin solution (L; 2 mM) or water (control; C) by 24 h under dark (Experiment II). To D1 immunodetection, samples from detached leaves were collected previous light exposure (dark), after 1 h of high light condition (HL; 2000 µmol m−2 s−1) and after 30 min of dark-recovery (30′; REC). The western blot image represents the most representative of three repetitions. Bars represent the average of four independent replicates (n = 4) ± SE. Different letters represent significant differences at 5% level according to Tukey’s test (p ≤ 0.05). For photochemical kinetics, Fv/Fm was previously measured in dark-adapted leaves. Subsequently, leaves were exposed to HL (2000 µmol m−2 s−1) and PSII quantum efficiency was determined immediately after illumination (60′) and during dark-recovery (dark for 80 min). Values represented indicate the average of three independent replicates (± SE). N nitrate, a ammonium, N + L nitrate + lincomycin, A + L ammonium + lincomycin
After 1 h of HL exposure, the D1 protein amount strongly decreased (by 51%) in ammonium + lincomycin compared to nitrate-supplied plants (Fig. 4a). After dark-recovery, the amount of D1 protein in ammonium-supplied leaves did change and it was lower than nitrate treatment, indicating that both single NH4+ and ammonium + lincomycin treatments did not display recovery in D1 synthesis (Fig. 4a). In parallel to those changes observed in D1 protein abundance, chlorophyll a fluorescence dynamics were also studied in these conditions. The results obtained from this experiment corroborated the previous data attained from short-time kinetics and D1 protein abundance. Fv/Fm values in both treatments were very similar, but PSII quantum efficiency and relaxation responses were decreased in ammonium-treated plants after 1 h of HL exposure and during dark-recovery, as compared to nitrate ones (Fig. 4b). In presence of lincomycin, these parameters (Fv/Fm and ΦPSII) decreased similarly in both N-sources (Fig. 4b).
High ammonium supply in presence of high light decreased CO2 assimilation and stimulated photorespiration and cytosolic GS1 activity, but did not affect plastidial GS2 activity
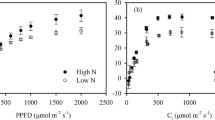
Considering that ammonium supply in presence of high light exhibited a prominent decrease in photochemical activities of PSII and PSI in parallel to decrease in the PSI acceptor side limitation, it was performed A-PPFD curves to calculate parameters related to CO2 assimilation and photorespiration. Under HL the ammonium-supplied plants displayed a decrease of 23% in net CO2 assimilation (A) and 15% in Amax, compared to nitrate-treated plants (Fig. 5a). The gS-PPFD and Ci-PPFD curves also revealed a great increase in the stomatal restriction and a slight decrease of Ci in ammonium-supplied plants, as compared to nitrate references, especially at high light intensities (Figs. 5b, S5). The electron flux for Rubisco carboxylation (Jc) in ammonium-treated plants was decreased by 16% whereas in opposition the electron flux for Rubisco oxygenation (Jo) was intensely increased by 104% (Fig. 5c). Jo/Jc ratios and Pr raised by 131% and 96%, respectively in ammonium-supplied rice compared to nitrate treatment (Fig. 5d). Thus, ammonium supply in presence of HL induced decreases in the PSII and PSI activities and affected the electron flux towards CO2 assimilation. Inversely, electron flux directed to photorespiration was significantly stimulated, probably contributing to decrease the PSI acceptor side limitation, previously noticed here.
Light-response curves of net CO2 assimilation (A-PPFD), stomatal conductance (gS-PPFD) and energy fluxes (Jc- and Jo-PPFD) measured in leaves from intact rice plants previously grown with 10 mM NO3− or 10 mM NH4+ in a controlled growth chamber for 7 days. During these experiments, plants were kept under a 12 h photoperiod with 400 µmol m−2 s−1 of continuous light regime (Experiment I). a Net CO2 assimilation, b stomatal conductance, c electron flux directed to Rubisco carboxylation activity (JC) and d electron flux directed to Rubisco oxygenation activity (Jo). In graph A is highlighted maximum net CO2 assimilation rate (Amax; µmol m−2 s−1) for nitrate-N and ammonium-A supplied plants. In d is showed (Jo/Jc) ratios and the photorespiration rate parameter (Pr). Each measurement represents the average of four replicates (± SE). Different letters represent significant differences at 5% level according to t-test (p ≤ 0.05)
Changes in A and gs in response to different intercellular CO2 partial pressure concentrations (A–Ci and gS–Ci curves) were also evaluated. Net CO2 assimilation was lower in NH4+-treated plants, especially under high Ci concentrations, which was supported by reductions in Jmax (by 34%), whereas Vcmax decreased by 25% (Fig. 6a, b). A–Ci curves revealed that stomatal conductance was much lower in ammonium-supplied plants mainly in presence of high Ci concentrations, as compared to NO3− plants (Fig. 6c).
Intercellular CO2 partial pressure-response curves of net CO2 assimilation (A–Ci) and stomatal conductance (gS–Ci) and parameters estimated from A–Ci curves (Vcmax and Jmax) measured in leaves from intact rice plants supplied with 10 mM NO3− or 10 mM NH4+ in a controlled growth chamber for 7 days. During these experiments, plants were kept under a 12 h photoperiod with 400 µmol m−2 s−1 of continuous light regime (Experiment I). a Net CO2 assimilation, b maximum Rubisco carboxylation rate, maximum electron transport rate and c stomatal conductance. Each measurement represents the average of four replicates (± SE)
In order to evaluate if the induction of photorespiration by high ammonium supply in presence of HL was related to ammonium accumulation and induction of GS activity, an experiment was performed employing leaf segments directly in contact with NH4+ and NO3−. The activities of both GS isoforms were not altered by N-sources under ML but in the presence of HL, the GS1 isoform activity was stimulated in ammonium-supplied leaf segments and, inversely, it was inhibited in nitrate treatment (Fig. S6). Differently, GS2 activity did not change by the effect of light regimes and N-sources despite it had presented higher values in all treatments compared to GS1 activities (Fig. S6).
Photosynthetic disturbances in ammonium-treated rice plants were not related to increase in oxidative stress indicators in leaves
Considering that in this current study rice plants decreased photosynthesis and increased photorespiration, we investigated if ammonium supply could have induced accumulation of reactive oxygen species (ROS) in comparison to nitrate reference plants. Two stress indicators related to ROS accumulation (oxidative stress) and cellular integrity were evaluated in leaves: thiobarbituric acid-reactive species content—TBARS (lipid peroxidation indicator) and membrane damage index (indicated by electrolyte leakage), respectively. None of these stress markers pointed for differences between leaves of nitrate- and ammonium-supplied plants under ML conditions (Fig. 7). In HL conditions, membrane damage increased in NH4+-treated plants as compared to NO3− plants in the same light regime (Fig. 7a). This stress indicator was not affected by the light regimes within of each N-source (Fig. 7a). The concentration of TBARS increased in both N-treatments under HL conditions when compared to ML but it was not affected by N-sources regardless of light regimes (Fig. 7b).
Changes in a membrane damage index (electrolyte leakage) and b thiobarbituric acid-reactive species (TBARS) content measured in leaves from intact rice plants previously supplied with 10 mM NO3− or 10 mM NH4+ in a controlled growth chamber for 7 days. In the last day, plants were exposed to 8 h of moderate light (ML—400 µmol m−2 s−1) or high light (HL—2000 µmol m−2 s−1) (Experiment I). Each bar represents the average of four replicates (± SE) and different capital and lowercase letters represent significant differences between light and N-treatments, respectively, according to Tukey’s test (p ≤ 0.05)
Discussion
In this study rice plants exposed to high ammonium supply were able to accumulate high NH4+ levels in roots and, in a minor extent in leaves and this response was strongly stimulated by excess light. In parallel, generalized disturbances in photosynthesis were evidenced in some processes such as PSII quantum yield, D1 protein turnover, quinone redox state, and PSI quantum yield. These responses are unexpected since rice is an allegedly NH4+-tolerant species and additionally no report has been published reporting similar results. This fact could have occurred simply because most of the works involving rice and photosynthesis have employed low ammonium concentrations in root medium, combined with moderate light regimes (Guo et al. 2007; Li et al. 2009; Gao et al. 2010; Ding et al. 2015). Thus, our previously raised hypothesis that high light is able to aggravate toxic effects induced by ammonium on the photosynthetic apparatus should be accepted and PSII is an important target for ammonium toxicity.
Balance encompassing D1 protein degradation and de novo synthesis are the main mechanisms responsible by changes in PSII quantum yield during illumination (Tikkanen and Aro 2012). Accordingly, we hypothesized that D1 turnover could be a potential target for ammonium toxicity in rice plants. Indeed, evidence assembled here involving photochemical induction/recovery in presence of lincomycin suggests that high ammonium supply induces a delay in D1 dark-recovery. Investigating ammonia toxicity in the cyanobacteria Synechocystis, Drath et al. (2008) have reported that high ammonia concentrations are able to trigger a rapid photodamage on PSII and that the FtsH2-deficient mutant is more sensitive to NH4+ and these responses are related to a prominent decrease in PSII activity. Moreover, the increased sensitivity to ammonia exhibited by that mutant suggests an important role for the D1 repair mechanism to avoid ammonium-induced photodamage. These responses are in accordance with our obtained data, suggesting that a similar negative effect related to D1 repair could have also occurred in rice plants.
A more recent work with isolated thylakoids of the same cyanobacteria stripe has evidenced that ammonium directly accelerates photodamage of PSII and also affects the repair of photodamaged PSII, but in a minor extent (Dai et al. 2014). In that study, the psbA expression was essential for ammonium tolerance and PSII repair, evidencing that D1 protein is important for photosynthetic protection against NH4+ toxicity. These results are in agreement with our current study since during the illumination phase the PSII activity is drastically decreased by ammonium. Moreover, during dark-recovery, ammonium-treated plants exhibit a significant delay in PSII relaxation. In addition, in presence of lincomycin these plants display a similar performance compared to nitrate, evidencing that PSII repair involving D1 synthesis represents a crucial target for ammonium toxicity. In other plant species, in vivo photochemical studies in response to NH4+ supply are relatively superficial and controversial (Zhu et al. 2000; Bendixen et al. 2001; Podgórska et al. 2013) and they do not allow establishing a confident assumption on the importance of D1 protein.
Since 1970s several studies have reported that ammonia at very high concentrations can bind OEC and consequently generating impairment in PSII efficiency (Velthuys 1975; Delrieu 1976; Sandusky and Yocum 1984; Beck et al. 1986; MacLachlan et al. 1994; Askerka et al. 2015). In fact, these important studies provided several insights concerning a direct molecular mechanism of ammonia toxicity in the PSII core. However, exhaustive studies addressing an integrative view of photosynthesis during a condition of ammonium supplying to higher plants directly in their root medium are still lacking, especially in combination with an excess light environment. Indeed, this current study provides evidence for a toxicity mechanism involving high ammonium, excess light, and D1 protein repair. The link between occurrence of NH3 coordinate binding to OEC and PSII dark-recovery, however, is still a remaining open question and deserves further investigation, especially in higher plants exposed to high but physiological ammonium concentrations.
In this study, a decrease in the quantum yield of PSI occurred in parallel to decreased PSII activity of ammonium-supplied plants. This response can be related to two distinct restriction mechanisms: the donor side limitation—Φ(ND) and the acceptor side limitation—Φ(NA) of the PSI (Klughammer and Schreiber 2008). The data obtained here reveal that changes in Φ(ND) are much larger than that displayed by complementary Φ(NA), evidencing that modifications in this late PSI limitation could have been simply caused by the much higher Φ(ND). In addition, we have postulated that at least in part these alterations induced by ammonium and HL on PSI activity are related to reduction in PSII activity. In fact, the responses displayed in Φ(ND) are consistent with previous observation that PSII reaction center activity is affected by restriction in the D1 protein synthesis, restringing the electron flux to PSI.
The obtained data concerning the quinone pool redox state (1 − qP) reinforce that, during HL illumination, the ammonium supply induces a highly reduced state of the PSII reaction centers. Two processes could have strongly aggravated this condition: stomatal restriction and impairment in CO2 assimilation induced by NH4+, which might have contributed to decrease the electron flow in thylakoids, increasing the lifetime of P700+, and consequently rendering higher Φ(ND) (Bukhov et al. 2002). In parallel, a decrease in electron sink for CO2 assimilation could have induced ATP accumulation associated with an energy unbalance, lowering the rate of ATP synthesis due to a decrease in ADP concentration and thus, leading to enlargement in the proton gradient that could favor NPQ formation (Ruban 2018). Moreover, since ATP synthase activity is reversible, excess ATP may become hydrolyzed during the return to darkness, maintaining a high proton gradient enough to sustain increased NPQ over a longer period of time in ammonium-supplied plants at relaxation phase (Gilmore and Yamamoto 1992). Thus, besides the delay in D1 turnover, other processes could have contributed to alter PSII activity in NH4+-treated plants.
Intriguingly, high ammonium-supplied plants showed lower stomatal conductance (gS) under different light intensities and CO2 concentrations (Figs. 5, 6). Nitrate is a well-known counter ion of K+ during stomatal opening mechanism (Guo et al. 2003). This could suggest that NO3−-enriched plants have higher gS compared to NH4+-supplied plants given their high amount of NO3− in leaves. However, no difference in nitrate content between NO3−- and NH4+-supplied plants was observed in both light regimes (Fig. S2), evidencing that gS is not strictly dependent on nitrate or ammonium supply during 7 days. These results suggest that the effects induced by high ammonium supply on the stomatal closure in rice plants are probably indirect. Indeed, high NH4+ levels in the root medium might reduce K+ uptake and alter the ionic balance in leaves (Voigt et al. 2009; Coskun et al. 2017) changing the regulation of stomatal movement. As expected, the reduction in gs greatly affected CO2 assimilation but the noted reduction in Vcmax suggests that biochemical restrictions displayed by Rubisco activity also should have occurred. Further experiments are needed to better understand the role of NH4+ supply in stomatal movement regulation.
Other important aspect highlighted in this current study lies in the fact that ammonium-treated plants display higher photorespiration (Hall et al. 1984). Certainly, HL and ammonia are two environmental factors that individually are known to stimulate this process (Zhu et al. 2000; Busch et al. 2018). Electron flux towards photorespiration (Jo) was strongly enhanced in ammonium-supplied inversely to the electron transport rates to Rubisco carboxylation as revealed by Jc and Jo/Jc ratios. It is interesting to highlight that supplying and recycling of ammonia and amino acids in chloroplast during photorespiration is an important mechanism to sink excess energy from PETC, as this pathway is responsible for the consumption of electrons and ATP (Busch et al. 2018). Thus, this mechanism could mitigate, at least in part, the stressful effects caused by high ammonium levels on photosynthesis of rice plants (Heber et al. 1996). Interestingly, high ammonium supply in presence of excess light strongly stimulated GS1 activity, but did not change GS2, highlighting the importance of the cytosolic isoform in ammonium detoxification in leaves, as previously reported for Arabidopsis and tobacco plants (Oliveira et al. 2002; Guan et al. 2016).
Previous studies have reported that NH4+ toxicity in plants is related to an over-accumulation of reactive oxygen species (ROS) and oxidative stress (Podgórska et al. 2013). Indeed, ROS are frequently reported as important chemical species involved with photoinhibition of PSII, especially due to generating protein carbonylation at the reaction centers (Kale et al. 2017) and inhibition of the activity of the elongation factor Tu, delaying the synthesis of chloroplastic proteins (Jimbo et al. 2018). In this vein, we analyzed whether ammonium supply was able to induce ROS over-accumulation in leaves, to establish if these substances could have contributed to delayed D1 turnover. Based on the absence of any significant signals of oxidative stress in leaves of NH4+-treated plants, either in moderate- or in high light conditions, this likelihood is currently rejected. However, further accurate studies employing additional and more sensitive tools are needed to definitely rule out the possibility of an NH4+-induced ROS accumulation in the PSII reaction center.
In conclusion, our results evidence that high ammonium supply in presence of high light causes disturbances in some important photosynthetic processes in rice plants, an allegedly tolerant species. We are proposing some of the possible mechanisms underlying these responses (Fig. 8). These stressful effects could be explained mainly by two non-excluding mechanisms: (1) an NH4+-induced delay in the D1 protein recovery which could have affected the PSII integrity, limiting electron transport to PSI and contributing to increasing donor side limitation of PSI; and (2) a decrease in Calvin-Benson cycle functioning as a consequence of restrictions in stomatal conductance and Rubisco activity, which can have provoked an energy unbalance between light capture and utilization by CO2 assimilation, inducing ATP accumulation and subsequently contributing to increase NPQ. In parallel, ammonium also stimulated electron transport to photorespiration in detriment of Rubisco carboxylation. Further studies are needed to explain completely how high ammonium concentrations but, at physiological levels, can directly affect specific photosynthetic targets in higher plants exposed to high light environments.
Hypothetical model highlighting the main effects induced by high ammonium supply and excess light on photosynthesis of rice plants. Initially, high light stimulates the accumulation of NH4+ in rice leaves. Subsequently, two important and non-excluding mechanisms are involved in ammonium toxic effects on photosynthesis: (1) NH4+ accumulation induces delay in D1 dark-recovery, which could affect PSII activity, limiting electron transport to PSI and contributing to increasing donor side limitation of PSI; (2) NH4+ stimulates decrease in stomatal conductance and subsequently reduction in Ci and Vcmax, generating an energetic unbalance between light capture and utilization in Calvin-Benson cycle, leading to ATP accumulation and subsequently contributing to increase NPQ and donor side limitation of PSI. In parallel, ammonium accumulation also stimulated greatly electron transport to photorespiration in detriment of Rubisco carboxylation
Abbreviations
- Amax :
-
Maximum net CO2 assimilation rate
- Ci:
-
Intercellular CO2 partial concentration
- ETRI:
-
Electron transport rate at PSI
- ETRII:
-
Electron transport rate at PSII
- Fm:
-
Dark maximum fluorescence
- Fm′:
-
Light maximum fluorescence
- Fo:
-
Dark minimum fluorescence
- Fo′:
-
Light minimum fluorescence after the far-red illumination
- Fs:
-
Light steady-state fluorescence
- Fv/Fm:
-
Maximum quantum efficiency of PSII
- Jc:
-
Electron flux to Rubisco carboxylation
- Jmax:
-
Maximum electron transport rate
- Jo:
-
Electron flux to Rubisco oxygenation
- NPQ:
-
Non-photochemical quenching
- OEC:
-
Oxygen evolving complex
- PPFD:
-
Photosynthetic photon flux density
- Vcmax:
-
Maximum Rubisco carboxylation rate
- Φ(NA):
-
Acceptor side limitation of PSI
- Φ(ND):
-
Donor side limitation of PSI
- PETC:
-
Photosynthetic electron transport chain
References
Ariz I, Esteban R, García-Plazaola JI et al (2010) High irradiance induces photoprotective mechanisms and a positive effect on NH4 + stress in Pisum sativum L. J Plant Physiol 167:1038–1045. https://doi.org/10.1016/j.jplph.2010.02.014
Askerka M, Vinyard DJ, Brudvig GW, Batista VS (2015) NH3 binding to the S2 state of the O2-evolving complex of photosystem II: analogue to H2O binding during the S2S3 transition. Biochemistry 54:5783–5786. https://doi.org/10.1021/acs.biochem.5b00974
Balkos KD, Britto DT, Kronzucker HJ (2010) Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ 33:23–34. https://doi.org/10.1111/j.1365-3040.2009.02046.x
Beck WF, De Paula JC, Brudvig GW (1986) Ammonia binds to the manganese site of the oxygen-evolving complex of photosystem II in the S2 state. J Am Chem Soc 108:4018–4022. https://doi.org/10.1021/ja00274a027
Bendixen R, Gerendás J, Schinner K et al (2001) Difference in zeaxanthin formation in nitrate- and ammonium-grown Phaseolus vulgaris. Physiol Plant 111:255–261. https://doi.org/10.1034/j.1399-3054.2001.1110218.x
Bittsánszky A, Pilinszky K, Gyulai G, Komives T (2015) Overcoming ammonium toxicity. Plant Sci 231:184–190. https://doi.org/10.1016/j.plantsci.2014.12.005
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584. https://doi.org/10.1078/0176-1617-0774
Britto DT, Kronzucker HJ (2005) Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new views on old paradigms. Plant Cell Environ 28:1396–1409. https://doi.org/10.1111/j.1365-3040.2005.01372.x
Britto DT, Kronzucker HJ (2013) Ecological significance and complexity of N-source preference in plants. Ann Bot 112:957–963. https://doi.org/10.1093/aob/mct157
Bukhov N, Egorova E, Carpentier R (2002) Electron flow to photosystem I from stromal reductants in vivo: The size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 215:812–820. https://doi.org/10.1007/s00425-002-0808-3
Busch FA, Sage RF, Farquhar GD (2018) Plants increase CO2 uptake by assimilating nitrogen via the photorespiratory pathway. Nat Plants 4:46–54. https://doi.org/10.1038/s41477-017-0065-x
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83(3):463–468
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Coskun D, Britto DT, Kronzucker HJ (2017) The nitrogen–potassium intersection: membranes, metabolism, and mechanism. Plant Cell Environ 40:2029–2041. https://doi.org/10.1111/pce.12671
Crawford TS, Hanning KR, Chua JPS et al (2016) Comparison of D1´- and D1-containing PS II reaction centre complexes under different environmental conditions in Synechocystis sp. PCC 6803. Plant Cell Environ 39:1715–1726. https://doi.org/10.1111/pce.12738
Cruz C, Domínguez-Valdivia MD, Aparicio-Tejo PM et al (2011) Intra-specific variation in pea responses to ammonium nutrition leads to different degrees of tolerance. Environ Exp Bot 70:233–243. https://doi.org/10.1016/j.envexpbot.2010.09.014
Dai G-Z, Qiu B-S, Forchhammer K (2014) Ammonium tolerance in the cyanobacterium Synechocystis sp. strain PCC 6803 and the role of the psbA multigene family. Plant Cell Environ 37:840–851. https://doi.org/10.1111/pce.12202
Delrieu MJ (1976) Inhibition by ammonium chloride of the oxygen yield of photosynthesis. Biochim Biophys Acta 440:176–188. https://doi.org/10.1016/0005-2728(76)90122-5
Ding L, Gao C, Li Y et al (2015) The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP). Plant Sci 234:14–21. https://doi.org/10.1016/j.plantsci.2015.01.016
Drath M, Kloft N, Batschauer A et al (2008) Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol 147:206–215. https://doi.org/10.1104/pp.108.117218
Esteban R, Ariz I, Cruz C, Moran JF (2016) Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci 248:92–101. https://doi.org/10.1016/j.plantsci.2016.04.008
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant Cell Environ 27:137–153. https://doi.org/10.1111/j.1365-3040.2004.01140.x
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. https://doi.org/10.1007/BF00386231
Felker P (1977) Microdetermination of nitrogen in seed protein extracts with the salicylate-dichloroisocyanurate color reaction. Anal Chem 49:1080–1080. https://doi.org/10.1021/ac50015a053
Flexas J, Ribas-Carbó M, Diaz-Espejo A et al (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621. https://doi.org/10.1111/j.1365-3040.2007.01757.x
Frantz TA, Peterson DM, Durbin RD (1982) Sources of ammonium in oat leaves treated with tabtoxin or methionine sulfoximine. Plant Physiol 69:345–348. https://doi.org/10.1104/pp.69.2.345
Gao Y, Li Y, Yang X et al (2010) Ammonium nutrition increases water absorption in rice seedlings (Oryza sativa L.) under water stress. Plant Soil 331:193–201. https://doi.org/10.1007/s11104-009-0245-1
Gilmore AM, Yamamoto HY (1992) Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA 89:1899–1903. https://doi.org/10.1073/pnas.89.5.1899
Guan M, de Bang TC, Pedersen C, Schjoerring JK (2016) Cytosolic glutamine synthetase Gln1.2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity. Plant Physiol 171:1921–1933. https://doi.org/10.1104/pp.16.01195
Guo F-Q, Young J, Crawford NM (2003) The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plat Cell 15:107–117. https://doi.org/10.1105/tpc.006312
Guo S, Chen G, Zhou Y, Shen Q (2007) Ammonium nutrition increases photosynthesis rate under water stress at early development stage of rice (Oryza sativa L.). Plant Soil 296:115–124. https://doi.org/10.1007/s11104-007-9302-9
Hall NP, Reggiani R, Franklin J et al (1984) An investigation into the interaction between nitrogen nutrition, photosynthesis and photorespiration. Photosynth Res 5:361–369. https://doi.org/10.1007/BF00034980
Heber U, Bligny R, Streb P, Douce R (1996) Photorespiration is essential for the protection of the photosynthetic apparatus of C3 plants against photoinactivation under sunlight. Bot Acta 109:307–315. https://doi.org/10.1111/j.1438-8677.1996.tb00578.x
Hirel B, Gadal P (1980) Glutamine synthetase in rice. Plant Physiol 66:619–623
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32. citeulike-article-id:9455435
Huang W, Yang YJ, Hu H, Zhang SB (2016) Response of the water-water cycle to the change in photorespiration in tobacco. J Photochem Photobiol B 157:97–104. https://doi.org/10.1016/j.jphotobiol.2016.02.006
Ishiyama K, Inoue E, Tabuchi M et al (2004a) Biochemical background and compartmentalized functions of cytosolic glutamine synthetase for active ammonium assimilation in rice roots. Plant Cell Physiol 45:1640–1647. https://doi.org/10.1093/pcp/pch190
Ishiyama K, Inoue E, Watanabe-Takahashi A et al (2004b) Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J Biol Chem 279:16598–16605. https://doi.org/10.1074/jbc.M313710200
Jimbo H, Yutthanasirikul R, Nagano T et al (2018) Oxidation of translation factor EF-Tu inhibits the repair of photosystem II. Plant Physiol 176:2691–2699. https://doi.org/10.1104/pp.18.00037
Kale R, Hebert AE, Frankel LK et al (2017) Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc Natl Acad Sci USA 114:2988–2993. https://doi.org/10.1073/pnas.1618922114
Klughammer C, Schreiber U (2008) Saturation pulse method for assessment of energy conversion in PS I. PAM Appl Notes 1:11–14
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Li Y, Gao Y, Ding L et al (2009) Ammonium enhances the tolerance of rice seedlings (Oryza sativa L.) to drought condition. Agric Water Manag 96:1746–1750. https://doi.org/10.1016/j.agwat.2009.07.008
Li G, Dong G, Li B et al (2012) Isolation and characterization of a novel ammonium overly sensitive mutant, amos2 in Arabidopsis thaliana. Planta 235:239–252. https://doi.org/10.1007/s00425-011-1504-y
Lima Neto MC, Lobo AKM, Martins MO et al (2014) Dissipation of excess photosynthetic energy contributes to salinity tolerance: A comparative study of salt-tolerant Ricinus communis and salt-sensitive Jatropha curcas. J Plant Physiol 171:23–30. https://doi.org/10.1016/j.jplph.2013.09.002
Liu Y, Von Wirén N (2017) Ammonium as a signal for physiological and morphological responses in plants. J Exp Bot 68:2581–2592. https://doi.org/10.1093/jxb/erx086
Lopes MS, Nogués S, Araus JL (2004) Nitrogen source and water regime effects on barley photosynthesis and isotope signature. Funct Plant Biol 31:995–1003. https://doi.org/10.1071/FP04031
MacLachlan DJ, Nugent JHA, Warden JT, Evans MCW (1994) Investigation of the ammonium chloride and ammonium acetate inhibition of oxygen evolution by Photosystem II. Biochim Biophys Acta 1188:325–334. https://doi.org/10.1016/0005-2728(94)90052-3
Markou G, Depraetere O, Muylaert K (2016) Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: A study on chlorophyll fluorescence and electron transport. Algal Res 16:449–457. https://doi.org/10.1016/j.algal.2016.03.039
Marshall B, Biscoe PV (1980) A model for C3 leaves describing the dependence of net photosynthesis on irradiance. J Exp Bot 31:29–39. https://doi.org/10.1093/jxb/31.1.29
Miller AJ, Cramer MD (2005) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36. https://doi.org/10.1007/s11104-004-0965-1
Murchie EH, Ali A, Herman T (2015) Photoprotection as a trait for rice yield improvement: status and prospects. Rice 8:31. https://doi.org/10.1186/s12284-015-0065-2
Oliveira IC, Brears T, Knight TJ et al (2002) Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiol 129:1170–1180. https://doi.org/10.1104/pp.020013
Parent B, Suard B, Serraj R, Tardieu F (2010) Rice leaf growth and water potential are resilient to evaporative demand and soil water deficit once the effects of root system are neutralized. Plant Cell Environ 33:1256–1267. https://doi.org/10.1111/j.1365-3040.2010.02145.x
Peterhansel C, Maurino VG (2011) Photorespiration redesigned. Plant Physiol 155:49–55. https://doi.org/10.1104/pp.110.165019
Podgórska A, Gieczewska K, Lukawska-Kuźma K et al (2013) Long-term ammonium nutrition of Arabidopsis increases the extrachloroplastic NAD(P)H/NAD(P)+ ratio and mitochondrial reactive oxygen species level in leaves but does not impair photosynthetic capacity. Plant Cell Environ 36:2034–2045. https://doi.org/10.1111/pce.12113
Ruban AV (2018) Light harvesting control in plants. FEBS Lett 1–10. https://doi.org/10.1002/1873-3468.13111
Sandusky PO, Yocum CF (1983) The mechanism of amine inhibition of the photosynthetic oxygen evolving complex. Amines displace functional chloride from a ligand site on manganese. FEBS Lett 162:339–343. https://doi.org/10.1016/0014-5793(83)80784-4
Sandusky PO, Yocum CF (1984) The chloride requirement for photosynthetic oxygen evolution. Analysis of the effects of chloride and other anions on amine inhibition of the oxygen-evolving complex. Biochim Biophys Acta 766:603–611. https://doi.org/10.1016/0005-2728(84)90121-X
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 279–319
Sharma SN, Sirohi GS (1987) The effect of ammonium and nitrate on CO2 assimilation, RuBP and PEP carboxylase activity and dry matter production in wheat. Photosynth Res 12:265–272. https://doi.org/10.1007/BF00055126
Sharma SN, Sirohi GS (1988) The effect of ammonium and nitrate on carbondioxide compensation point and enzymes associated with carbondioxide exchange in wheat. Photosynth Res 17:267–275. https://doi.org/10.1007/BF00035453
Silva LM, Dos Santos CP, Chaloub RM (2001) Effect of the respiratory activity on photoinhibition of the cyanobacterium Synechocystis sp. Photosynth Res 68:61–69. https://doi.org/10.1023/A:1011890200229
Szal B, Podgórska A (2012) The role of mitochondria in leaf nitrogen metabolism. Plant Cell Environ 35:1756–1768. https://doi.org/10.1111/j.1365-3040.2012.02559.x
Takagi D, Hashiguchi M, Sejima T et al (2016) Photorespiration provides the chance of cyclic electron flow to operate for the redox-regulation of P700 in photosynthetic electron transport system of sunflower leaves. Photosynth Res 129:279–290. https://doi.org/10.1007/s11120-016-0267-5
Thornley JHM, Johnson RL (1990) Plant and crop modeling. A mathematical approach to plant and crop physiology. Oxford Science Publications, Oxford
Tikkanen M, Aro EM (2012) Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim Biophys Acta 1817:232–238. https://doi.org/10.1016/j.bbabio.2011.05.005
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Valentini R, Epron D, Deangelis P et al (1995) In-Situ estimation of Net CO2 assimilation, photosynthetic electron flow and photorespiration in turkey oak (Q. cerris L.) leaves—diurnal cycles under different levels of water-supply. Plant Cell Environ 18:631–640. https://doi.org/10.1111/j.1365-3040.1995.tb00564.x doi
Velthuys BR (1975) Binding of the inhibitor NH3 to the oxygen-evolving apparatus of spinach chloroplasts. Biochim Biophys Acta 396:392–401. https://doi.org/10.1016/0005-2728(75)90145-0
Vinyard DJ, Askerka M, Debus RJ et al (2016) Ammonia binding in the second coordination sphere of the oxygen-evolving complex of photosystem II. Biochemistry 55:4432–4436. https://doi.org/10.1021/acs.biochem.6b00543
Voigt EL, Caitano RF, Maia JM et al (2009) Involvement of cation channels and NH4 +-sensitive K+ transporters in Na+ uptake by cowpea roots under salinity. Biol Plant 53:764–768. https://doi.org/10.1007/s10535-009-0140-x
von Wirén N, Lauter F, Ninnemann O et al (2000) Differential regulation of three functional transporter genes by nitrogen in root hairs and by light in tomato. Plant J 21:167–175. https://doi.org/10.1046/j.1365-313x.2000.00665.x
Wang MY, Siddiqi MY, Ruth TJ, Glass ADM (1993) Ammonium uptake by rice roots (I. Fluxes and subcellular distribution of 13NH4 +). Plant Physiol 103:1249–1258. https://doi.org/10.1104/pp.103.4.1249
Yang H, Von D Fecht-Bartenbach, Friml J J, et al (2015) Auxin-modulated root growth inhibition in Arabidopsis thaliana seedlings with ammonium as the sole nitrogen source. Funct Plant Biol 42:239–251. https://doi.org/10.1071/FP14171
Zhu Z, Gerendas J, Bendixen R et al (2000) Different tolerance to light stress in NO3 –- and NH4 +-grown Phaseolus vulgaris L. Plant Biol 2:558–570. https://doi.org/10.1055/s-2000-7498
Acknowledgements
The authors are grateful to Prof. Danilo M. Daloso for the manuscript revision and important suggestions. Authors also acknowledge to Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES), National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq), INCT Plant Stress Biotech (Conselho de Desenvolvimento Científico e Tecnológico) Proc. 465480/2014-4 and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) for funding. FELC is supported by FUNCAP/CAPES (Bolsista CAPES/BRASIL—Proc. 88887.162856/2018-00). AKML is supported by CNPq (Proc. 154471/2018-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alencar, V.T.C.B., Lobo, A.K.M., Carvalho, F.E.L. et al. High ammonium supply impairs photosynthetic efficiency in rice exposed to excess light. Photosynth Res 140, 321–335 (2019). https://doi.org/10.1007/s11120-019-00614-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00614-z