Abstract
In this study, we have compared the photosynthetic characteristics of two contrasting species of Tradescantia plants, T. fluminensis (shade-tolerant species), and T. sillamontana (light-resistant species), grown under the low light (LL, 50–125 µmol photons m−2 s−1) or high light (HL, 875–1000 µmol photons m−2 s−1) conditions during their entire growth period. For monitoring the functional state of photosynthetic apparatus (PSA), we measured chlorophyll (Chl) a emission fluorescence spectra and kinetics of light-induced changes in the heights of fluorescence peaks at 685 and 740 nm (F 685 and F 740). We also compared the light-induced oxidation of P700 and assayed the composition of carotenoids in Tradescantia leaves grown under the LL and HL conditions. The analyses of slow induction of Chl a fluorescence (SIF) uncovered different traits in the LL- and HL-grown plants of ecologically contrasting Tradescantia species, which may have potential ecophysiological significance with respect to their tolerance to HL stress. The fluorometry and EPR studies of induction events in chloroplasts in situ demonstrated that acclimation of both Tradescantia species to HL conditions promoted faster responses of their PSA as compared to LL-grown plants. Acclimation of both species to HL also caused marked changes in the leaf anatomy and carotenoid composition (an increase in Violaxanthin + Antheraxantin + Zeaxanthin and Lutein pools), suggesting enhanced photoprotective capacity of the carotenoids in the plants grown in nature under high irradiance. Collectively, the results of the present work suggest that the mechanisms of long-term PSA photoprotection in Tradescantia are based predominantly on the light-induced remodeling of pigment-protein complexes in chloroplasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In photosynthetic systems of oxygenic type, two pigment-protein complexes, photosystem I (PSI) and photosystem II (PSII), provide electron transfer from the water molecule oxidized by PSII to the terminal electron acceptor of PSI, NADP+ (Barber 2008; Eberhard et al. 2008; Mamedov et al. 2015). PSI and PSII are interconnected via the cytochrome (Cyt) b 6 f complex and mobile electron carriers: plastoquinone (PQ) and plastocyanin (Pc). Electron transport through the intersystem electron transport chain (ETC) is accompanied by acidification of the thylakoid lumen and alkalization of stroma, thereby generating the trans-thylakoid difference in electrochemical potentials of protons \(\left( \Delta {{{\tilde{\mu }}}_{{{\text{H}}^{+}}}} \right)\), which serves as the driving force for ATP synthesis (Mitchell 1966). The products of the light-induced reactions of photosynthesis, ATP and NADPH, are used mainly in biosynthetic processes of the Calvin–Benson cycle (CBC) (Edwards and Walker 1983). Several mechanisms of electron transport control provide a well-balanced performance of photosynthetic apparatus (PSA) and ensure its protection against light stress (Eberhard et al. 2008; Horton 2012). Short-term response of chloroplasts to dark-to-light transients includes (a) the light-induced activation of the CBC (Woodrow and Berry 1988; Buchanan 1980, 1991; Michelet et al. 2013); (b) pH-dependent regulation of the intersystem electron transport (Kramer et al. 2003; Foyer et al. 2012; Järvi et al. 2013; Tikhonov 2013, 2015); (c) re-distribution of electron fluxes between alternative pathways of electron transport (Miyake 2010; Johnson 2011); (d) light energy partitioning between PSI and PSII, the so-called “state transitions” (Allen 2003; Lemeille and Rochaix 2010); and (e) the light-induced remodeling of PSA (Horton 2012; Kirchhoff 2013). Variations in the environment conditions may cause the long-term changes in photosynthetic apparatus, termed acclimation, which are associated with the adjustment of stoichiometry of light-harvesting and electron transport complexes by synthesis and/or degradation of chloroplast components (Chow et al. 1990, 1991; Adamson et al. 1991; Liu et al. 1993; Demmig-Adams 1998; Anderson et al. 2001; Lichtenthaler and Babani 2004; Lichtenthaler et al. 2007a, b). Acclimation-dependent changes in PSA may improve the quantum efficiency of photosynthesis, providing a high photosynthetic performance of chloroplasts and sustainability of plants under variable environmental conditions.
One of the key mechanisms of PSA protection against excessive light stress is based on enhanced thermal dissipation of excess light energy in the light-harvesting complex II (LHCII), commonly termed the non-photochemical quenching (NPQ). In general, however, the term NPQ comprises several events associated with a decrease in PSII activity: (1) ΔpH-induced quenching of excitation in the light-harvesting antenna of PSII (qE component of NPQ); (2) state transition caused by migration of mobile LHCII complexes from PSII to PSI (qT); and (3) partly reversible or irreversible photoinhibition of PSA (qI) (Ruban 2012). In plants, the key role in rapid generation of NPQ (qE component of NPQ) belongs to the PsbS subunit of PSII and the xanthophylls cycle (Li et al. 2000, 2002, 2004). The medium phase (~5–15 min) of the light-induced generation of NPQ, observed at sufficiently strong irradiance (the so-called qM-component of NPQ, sometimes denoted as qZ), is induced predominantly by the ΔpH-dependent de-epoxidation of V to Z (Jahns and Holzwarth 2012). The light-induced protonation of lumen facing acidic residues of the PsbS protein and violaxanthin de-epoxidase (VDE) induce remodeling of the PSII-LHCII supercomplex and structural re-arrangements in thylakoid membranes creating a channel for heat dissipation of excess light energy in LHCII and thereby protecting the photosynthetic antenna against harmful over-excitation (Li et al. 2009; Ruban 2012). According to Dall’Osto et al. (2014), in Arabidopsis, nearly 50% of qM is accounted for by the mechanism of chloroplast photorelocation within the plant cell (the avoidance effect). NPQ development is regulated by the needs of photosynthetic machinery (Tikkanen et al. 2012; Kono and Terashima 2014). At low intensities of actinic light (AL) which are insufficient to saturate photosynthesis, NPQ is usually insignificant. If the irradiance exceeds the capacity of the chloroplast ETC, NPQ increases.
The mechanisms of plant photoprotection under abrupt fluctuations in ambient irradiance have been in the focus of numerous studies for several decades (for review, see Allakhverdiev and Murata 2004; Horton 2012; Tikkanen et al. 2012; Anderson et al. 1988, 2001; Thayer and Björkman 1990; Demmig-Adams et al. 2012; Matsubara et al. 2012; Murata et al. 2012; Suorsa et al. 2012). Nevertheless, this problem still presents a challenge to biophysics and biochemistry of photosynthesis. The capacity of plants for acclimation to irradiance is specific for species and their habitat (Bjorkman and Demmig 1987; Johnson et al. 1993; Terashima et al. 2006; Matsubara et al. 2009, 2012). Therefore, investigations of species-specific difference between the plants of the same genus related to contrasting ecological groups (Samoilova et al. 2011; Ptushenko et al. 2013; Mishanin et al. 2016) are of particular interest for elucidation of acclimation mechanisms. The genus Tradescantia containing species with contrasting environmental preferences is a convenient model for comparative study of PSA plasticity and its response to environmental light. In our previous works (Ptushenko et al. 2013; Mishanin et al. 2016), we used two Tradescantia species of contrasting ecological groups, T. fluminensis (shade-tolerant) and T. sillamontana (light-resistant), as the convenient models for elucidation of species-specific peculiarities of photoprotection mechanisms in plants. In particular, we have found that acclimation to HL augments the level of PsbS (by a factor of ≈ 1.7–1.8) with respect to photoreaction centers P700. In the light-resistant species, T. sillamontana, the ratio PsbS/P700 is about two times higher than in shade-tolerant species T. fluminensis grown under the same conditions. This should enhance the capacity of their leaves for protection against the light stress.
Fluorescence of Chl a is a convenient reporter of the redox state of the chloroplast ETC and structural reorganization of the PSII-LHCII supercomplex (for review, see Lazar 1999; Strasser et al. 2004; Stirbet and Govindjee 2011, 2012, 2016; Ruban 2012; Kalaji et al. 2014; Schansker et al. 2014). In this work, in continuation of our previous study of the two contrasting Tradescantia species (Mishanin et al. 2016), we focused on the comparison of chlorophyll a (Chl a) fluorescence emission spectra and peculiarities of fluorescence induction simultaneously recorded at the two peaks of the emission spectrum, F 685 and F 740. We have demonstrated that in HL-acclimated plants of both species, the light-induced changes in fluorescence spectra occur faster than in the LL-grown plants. The HL plants also revealed marked changes in carotenoid content and composition, manifesting an increase in the relative content of violaxanthin cycle pigments involved into phoprotective responses. In the meantime, the steady-state ratio of electron fluxes from PSII to PSI and beyond PSI (as measured by the EPR method) was virtually independent of the growth conditions.
Materials and methods
Plant material and growth conditions
Plants of two Tradescantia species (T. fluminensis and T. sillamontana) were cultivated in soil and grown under the same experimental conditions at 24–26 °C and 40–50% humidity, with a photoperiod close to natural (≈16-h light in the midday and ≈ 8-h dark) as described in Mishanin et al. (2016). Plants were grown either at a low light (LL, 50−125 µmol photons m−2 s−1) or at a high light (HL, 875−1000 µmol photons m−2 s−1) irradiation. All measurements were taken using fully expanded mature leaves of the same age and developmental stage from the plants grown for 2–3 month. The shade-tolerant species T. fluminensis Vell (often regarded as a synonym of T. albiflora Kunth) is a habitant of tropical rainforests and other humid and shaded areas in south-eastern Brazil, Argentina, and Uruguay, which is tolerant of heavy shade (Randall 2012). T. sillamontana, a habitant of semi-deserts, is endemic to arid areas of the State of Nuevo León in Mexico. This species is almost succulent and nearly xerophytic; its fleshy leaves are covered with dense semi-transparent grayish-white spider web-like hairs (trichomes). Figure 1 presents photos of leaves of the plants used in our work. Note that young leaves of HL-grown plants of both species are green in color and may acquire red coloration with aging. It is likely that the leaf reddening reflects one of the protective mechanisms against light stress associated with accumulation of photoprotective pigments (Solovchenko 2010). In order to minimize scattering of data, we always used green leaves of the same age.
Fluorescence measurements
Chl fluorescence spectra were taken with a spectrofluorometer CM2203 (Solar, Belarus) capable of simultaneous recordings of slow induction of fluorescence (SIF) at two wavelengths. Chl a fluorescence was excited by the actinic light (AL) produced by a light-emitting diode LXHL-LB3C (Phillips Lumileds, USA; λmax = 475 nm, Δλ1/2 = 20 nm). Intensity of AL on the sample surface could be varied in the interval from 50 to 500 µmol photons m−2 s−1 (13−130 W m−2).
EPR measurements
The relative content of PSI centers in leaves and kinetics of \(\text{P}_{700}^{{}}\) redox transients were measured by the EPR method as described earlier (Trubitsin et al. 2015). Freshly cut segments of leaves (5 × 15 mm) were placed in a transparent quartz holder positioned at the center of the rectangular TM110 resonator of a Varian (USA) E-4 X-band spectrometer. EPR signals from oxidized centers \(\text{P}_{700}^{+}\) were registered at room temperature (22–24 °C), microwave power of 10 mW, and magnetic field modulation amplitude H m = 0.4 mT.
Cuttings from different leaves may have different thickness and water content, which could influence the quality factor of the EPR spectrometer cavity. Therefore, for accurate quantification of the EPR signals of \(\text{P}_{700}^{+}\), we normalized them to the reference EPR signal given by Mn2+ ions embedded into the MgO lattice as it was described earlier (Mishanin et al. 2016). DCMU was administered into the leaf by means of vacuum infiltration of the water–ethanol solution of DCMU (250 µM), which was enough to inhibit PSII completely (Trubitsin et al. 2015). The final concentration of ethanol in the solution was ≤1%.
Samples were illuminated by white light (WL) from an incandescent tungsten lamp as described earlier (Kuvykin et al. 2011; Trubitsin et al. 2015). Infrared radiation was cut off with a 5-cm-thick water filter. The intensity of WL focused on the specimen surface was about 300 W m−2, which corresponds to ~800–1000 µmol photons m−2 s−1.
HPLC analysis of carotenoids
A spot (6 mm diameter) on a dark-adapted half of the leaf (see above) was irradiated by AL (λ max = 475 nm, 800 µmol photons m−2 s−1) for 20 min. The other half of the leaf blade remained in darkness. After the irradiation of the sample, acetone extracts were prepared from both the irradiated part and the darkened part of the leaf (Solovchenko et al. 2001). The extracts were then injected into a Waters Alliance 2695 chromatograph equipped with a Waters Sunfire RP C18 column (150 × 4.6 mm, 3.5 μm) and Waters 2995 diode-array detector (Waters, Milford, USA) according to the protocol reported in Merzlyak et al. (2005). Xanthophyll de-epoxidation index was calculated as DE = (Z + 0.5 A)/(Z + A + V), where Z, A, and V are zeaxanthin, antheraxanthin, and violaxanthin content, respectively.
Results
Fluorescence spectra
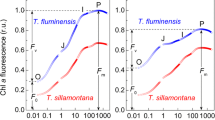
Chl a fluorescence spectrum is a “fingerprint” of structural and functional peculiarities of PSA (for review, see Adams and Demmig-Adams 2004; Baker and Oxborough 2004; Govindjee 2004; Lichtenthaler and Babani 2004; Strasser et al. 2004; Baker 2008; Stirbet and Govindjee 2011, 2012, 2016; Allorent et al. 2013; Wientjes et al. 2013; Kalaji et al. 2014). Figure 2 shows the normalized emission spectra of Chl a fluorescence in dark-adapted (15-min) Tradescantia leaves (designated as “Dark”) recorded before continuous AL irradiation and immediately after 6-min illumination with strong AL (400 µmol quanta m−2 s−1, termed “Light”). The fluorescence emission spectra of the leaves of both Tradescantia species have two bands, with their maxima at 685 and at 740 nm. The fluorescence spectra of dark-adapted samples (“Dark”) were normalized to the height of the “red” band, F 685 (see Fig. 2a for definition). The fluorescence spectra of illuminated samples (“Light”) were normalized by the same way as the corresponding spectra of dark-adapted samples.
Fluorescence emission spectra of T. fluminensis (a, c) and T. sillamontana (b, d) leaves of plants grown under the HL (a, b) or the LL (c, d) irradiation. Spectra of the dark-adapted (15 min) leaves (closed symbols) are normalized at the height of the “red” peak F 685. Open symbols show the corresponding spectra recorded immediately after 6-min illumination (400 µmol photons m−2 s−1). The fluorescence spectra of dark-adapted samples (denoted as “dark”) were normalized to the height of the “red” band, F 685 (for definition of F 685, a). The fluorescence spectra of illuminated samples (denoted as “light”) were normalized using the same coefficients as for the corresponding spectra of dark-adapted samples
The “red” band F 685 originates in the light-harvesting antenna of PSII (for review, see Govindjee 2004). Most of F 685 belongs to Chl a in core PSII complexes. The “far-red” band (F 740) is usually related to fluorescence emitted from PSI with a certain contribution of PSII, \({{F}_{740}}=F_{\text{740}}^{\text{PSI}}+F_{\text{740}}^{\text{PSII}}\). The ratio between the amplitudes of the fluorescence emission band at 685 nm and at 740 nm, F 685/F 740, depends on plant growth conditions (for review, see Lichtenthaller and Babani 2004). The LL-acclimated plants of both species reveal higher amplitudes of the far-red peak F 740 (with respect to normalized peak F 685) as compared to HL-grown plants (Fig. 2).
The shape of the Chl fluorescence emission spectra depends on a number of factors, i.e., the Chl content of the leaves and the re-absorption effect (for review, see Lichtenthaler and Babani 2004). The red fluorescence emission band F 685 overlaps with the absorption bands of the in vivo forms of Chl a, which would cause a preferential re-absorption of the light emitted in the F 685 band, whereas the far-red band F 740 is little affected. Thus, the fluorescence ratio F 685/F 740 can serve as indicator of the Chl content in leaves. Variation of the F 685/F 740 ratio may reflect relative changes in the contents of PSII and PSI complexes. A relative increase in the far-red peak in LL-acclimated plants correlates with a significant increase in the content of Chl (per unit leaf area) in both Tradescantia species (Mishanin et al. 2016). With increasing Chl content of Tradescantia leaves upon acclimation of plants to LL irradiation, the relative intensity of the far-red fluorescence band F 740 markedly increases (Fig. 2), which may be caused, at least partly, by the light re-absorption effect: the light quanta emitted by PSII are absorbed by chloroplasts and then re-emitted as the far-red quanta of Chl a fluorescence.
Illumination of dark-adapted leaves induces reversible changes in their fluorescence spectra (Fig. 2). Along with the light-induced decrease in the intensity of fluorescence, there are marked changes in the line-shape of the spectrum: the “red” peak F 685 decreases more significantly than the “far-red” peak F 740. The alterations of the fluorescence spectra reflect functional and structural changes in PSA. In particular, they may be associated with the light-induced remodeling of thylakoid membranes and PSII-LHCII complex induced by the light-induced acidification of the thylakoid lumen (for review, see Ruban 2012; Kaiser et al. 2015; Albanese et al. 2016). The effect of the trans-thylakoid pH difference (ΔpH) on the light-induced changes in the shape of Chl a emission spectra was confirmed by our observation that the injection into Tradescantia leaves of uncouplers (100 µM monensin or nigericin), which destroyed ΔpH, prohibited the light-induced changes in the shape of Chl a fluorescence spectra, F 685/F 740 ≈ const (Fig. 3). The light-induced decrease in the ratio between the fluorescence emission bands at 685 nm (PSII) and 740 (PSI) may also be indicative of state transitions, in particular, the state 1 → state 2 transition due to migration of phosphorylated LHCII complexes from PSII to PSI (for review, see Allen 1992, 2003; Lemeille and Rochaix 2010; Rochaix 2014).
Normalized fluorescence emission spectra of the leaves of T. fluminensis and T. sillamontana grown under the LL irradiation. Cuttings of the leaves were treated with 100 µM monensin (the uncoupler which destroys ΔpH). Closed symbols show the fluorescence spectra of dark-adapted samples, and open symbols demonstrate spectra recorded immediately after 6-min illumination (400 µmol photons m−2 s−1). We normalized “dark” spectra to the height of peak F 685; the fluorescence spectra of illuminated samples (“light”) were normalized using the same coefficients as for the corresponding “dark” spectra
Kinetics of slow induction of Chl a fluorescence
Illumination of dark-adapted leaves induces reversible multiphase changes in the yield of Chl a fluorescence, the so-called OJIPSMT curve (for references, see Govindjee 1995; Lazar 1999; Strasser et al. 2004; Papageorgiou et al. 2007; Stirbet and Govindjee 2011, 2012, 2016; Kalaji et al. 2014). In response to a sufficiently strong AL, the fluorescence intensity rapidly (within ~1–2 s) rises to the extreme level P (this fast phase of Chl a fluorescence induction is usually termed as the OJIP transition). The patterns of the OJIP kinetics, and their differences in T. fluminensis and T. sillamontana, have been described in our recent work (Mishanin et al. 2016). After reaching the maximal level F P (Fig. 4), the intensity of Chl a fluorescence gradually decays to the steady-state level T. The slow phase of Chl fluorescence induction (PSMT) is usually termed as the slow induction of fluorescence (SIF) (for the nomenclature of the OJIPS(M)T transient, see Govindjee 1995). Note that the temporary rise of fluorescence intensity on the phase SMT is not always observed (for references, see Stirbet and Govindjee 2016).
Fluorescence induction curves for dark-adapted (15 min) leaves of LL-acclimated and HL-acclimated T. fluminensis. Traces of the light-induced changes in the amplitudes of peaks F 685 and F 740 were recorded in response to strong AL (500 µmol photons m−2 s−1, a, b) or weak AL (50 µmol photons m−2 s−1, c, d)
Three basic factors determine SIF: (a) the light-induced activation of the CBC enzymes, (b) acidification of the thylakoid lumen, and (c) state transitions associated with the re-distribution of absorbed light energy between PSII and PSI. Acceleration of electron drain from PSI to the activated CBC will stimulate the intersystem electron flow, thereby enhancing photochemical quenching of Chl a fluorescence due to re-oxidation of electron carriers on the acceptor side of PSII (QA and QB). The lumen acidification (pHin ↓) induces quenching of Chl a fluorescence due to the enhancement of NPQ associated with remodeling of PSII-LHCII supercomplexes (Jahns and Holzwarth 2012; Ruban 2012). The rate of fluorescence decay characterizes the PSA capacity for rapid (short-term) response to variations of light conditions (for the sake of brevity, we will term this property of PSA as its “reactivity”). A difference in the kinetics of SIF in contrasting Tradescantia species may reflect their ecophysiological peculiarities (Ptushenko et al. 2013). Below we compare the peculiarities of SIF in Tradescantia leaves recorded for two peaks (685 and 740 nm) of the Chl a emission spectra. This allowed us to judge about dynamics of light-induced changes in the shape of fluorescence spectra.
Figure 4 presents the typical patterns of SIF in dark-adapted (15-min) T. fluminensis leaves as measured simultaneously at 685 and 740 nm during illumination with the strong AL (panels a, b) or weak AL (panels c, d). In response to AL, the intensities of both peaks rapidly increase to their maximal values (F P) and then gradually decline toward steady-state levels (F T). The rates of SIF depend on the plant growth conditions and AL intensity. During the action of the strong AL (500 µmol photons m−2 s−1, Fig. 4a, b), the HL-acclimated plants show more rapid quenching of Chl a fluorescence (t HL ~0.5 min for F 685) than the LL-grown plants (t LL ~0.9 min for F 685). The rate of SIF decay decreases with attenuation of the AL intensity (50 µmol photons m−2 s−1, Fig. 4c, d). In the latter case, both the HL- and LL-grown plants show somewhat slower rates of SIF decay (t HL ~0.8 min and t LL ~1.3 min, respectively). Thus, T. fluminensis acclimated to high growth irradiance reveal more rapid kinetics of SIF decay, both upon the action of strong or low AL.
Figure 5 shows the representative time courses of SIF in T. sillamontana leaves measured simultaneously at 685 nm and at 740 nm during illumination with the strong (panels a, b) or weak AL (panels c, d). Kinetics of SIF in T. sillamontana depends on the plant growth conditions, but less significantly than in T. fluminensis. Upon the action of strong AL, the HL-acclimated plants reveal somewhat more rapid quenching of Chl a fluorescence than the LL-grown plants (t HL ~0.5 min and t LL ~0.6 min, respectively, for F 685). During the action of the weak AL, the fluorescence decay in the LL-grown T. sillamontana is markedly slower than in the HL-acclimated plants (t HL ~0.5 min and t LL ~1.2 min, respectively, for F 685). Thus, in T. sillamontana, we observed the same trend as in T. fluminensis, the HL-acclimated plants revealed somewhat more rapid response (the light-induced decay of Chl a fluorescence) as compared to the LL-grown plants.
Fluorescence induction curves for dark-adapted (15 min) leaves of LL-acclimated and HL-acclimated T. sillamontana. Traces of the light-induced changes in the amplitudes of peaks F 685 and F 740 were recorded in response to strong AL (500 µmol photons m−2 s−1, a, b) or weak AL (50 µmol photons m−2 s−1, c, d)
The difference between the LL- and HL-grown plants was more distinct upon comparison of the kinetics of the light-induced decrease in the F 685/F 740 ratio characterizing the shape of Chl a fluorescence spectrum. The light-induced changes in the F 685/F 740 ratio during the induction phase can be caused by structural reorganization of PSII-LHCII complexes in the thylakoid membrane. For instance, it has been demonstrated that formation of the photoprotective state is accompanied by a structural reorganization of the photosynthetic membrane involving dissociation of LHCII from PSII and its aggregation (Johnson et al. 2011; Jahns and Holzwarth 2012; Ruban 2012). Figure 6 shows the representative kinetics of F 685/F 740 decay during the action of the strong AL. As one can see, the rapid phase of F 685/F 740 decay is peculiar to HL-grown plants (see also Table 1). In LL-grown T. fluminensis (Fig. 6a), we usually observed the relatively slow decay of F 685/F 740 with the characteristic time t ≈ 3.5 ± 0.24 min. Note that the spectral parameter F 685/F 740 decays more slowly than the amplitudes of peaks F 685 and F 740. In HL-grown T. fluminensis (Fig. 6a), we observed the two-exponential decay of F 685/F 740 with the dominant rapid component (t 1 ≈ 0.5 ± 0.05 min) and the minor slow component (t 2 ≈ 3.5 ± 1.22 min). The light-induced decrease in the F 685/F 740 ratio may reflect several events: (a) the light-induced remodeling of the PSII-LHCII supercomplex associated with the NPQ generation (Johnson et al. 2011; Jahns and Holzwarth 2012; Ruban et al. 2012), and/or (b) a state 1 → state 2 transition (Derks et al. 2015). Thus, we may conclude that acclimation of T. fluminensis to HL-growth conditions provides the noticeable acceleration of the PSA response to illumination. Similar trend of accelerating F 685/F 740 decay (although less noticeable) was observed in the light-resistant species T. sillamontana, which is the inhabitant of semi-deserts (Fig. 6b). Both LL-grown and HL-grown plants demonstrate the mono-exponential decay of F 685/F 740 with somewhat different times, t LL ≈ 1.3 ± 0.04 min (LL) and t HL ≈ 1.00 ± 0.03 min (HL). Bearing in mind that a decrease in the F 685/F 740 ratio reflects the light-induced structural changes in PSA, we can suggest that the long-term acclimation of plants to HL facilitates accelerated remodeling of the thylakoid membrane in response to AL. Accelerated response of HL-grown plants to AL is likely to reflect their elevated resistance to hazardous rapid fluctuations of solar irradiation.
Time courses of the light-induced changes in the peak height ratio F 685/F 740 in dark-adapted (15 min) leaves of HL- and LL-acclimated T. fluminensis (a) and T. sillamontana (b). The AL intensity was 500 µmol photons m−2 s−1. Characteristic times of the mono- or bi-exponential decay fits are indicated near the corresponding curves
Photo-oxidation of P700
Figure 7 depicts the time course of the light-induced oxidation of P700 in dark-adapted (t ad = 10 min) leaves of T. fluminensis grown at HL conditions. Kinetics of P700 photo-oxidation reveals three distinct phases (A–C) peculiar to intact chloroplasts in plants (Tikhonov 2015; Trubitsin et al. 2015). After the relatively small initial jump (phase A), we observed more significant rise of the EPR signal from \(\text{P}_{700}^{+}\) (phase B) followed by relatively slow rise of \(\text{P}_{700}^{+}\) to the steady-state level C. After ceasing the illumination, \(\text{P}_{700}^{+}\) reduced due to electrons donated by the intersystem ETC. The multiphase kinetics of \(\text{P}_{700}^{+}\) induction in control samples can be explained by the interplay of several feedbacks that regulate the rates of electron flow in the intersystem ETC between PSII and PSI, on the one hand, and on the acceptor of PSI, on the other hand (for review, see Tikhonov 2015). Regulation of electron transport in the ETC beyond the PSI complex is associated with the light-induced activation of the CBC reactions, which accelerates the efflux of electrons from PSI, thereby promoting oxidation of P700. The light-induced re-distribution of light energy in favor of PSI (state 1 → state 2 transition, Lemeille and Rochaix 2010; Allorent et al. 2013) should also facilitate photo-oxidation of P700. On the other hand, the light-induced acidification of the thylakoid lumen (pHin ↓) would lead to a decrease in the capacity of PSII to donate electrons into the intersystem ETC. This will occur due to the enhancement of thermal energy dissipation in the light-harvesting antenna of PSII (generation of qE). Besides, the lumen acidification would slow down oxidation of PQH2 by the Cyt b 6 f complex (for review, see Tikhonov 2013, 2014), thereby hindering the electron flow to PSI and stimulating the rise of the EPR signal from \(\text{P}_{700}^{+}\). In DCMU-treated samples with inhibited PSII, we observed the rapid monotonous rise of \(\text{P}_{700}^{+}\) to the level P 0, which was markedly higher than the steady-state level P in control samples. Along with a marked increase in the level of \(\text{P}_{700}^{+}\), DCMU-treatment caused a decrease in the rate of the post-illumination reduction of \(\text{P}_{700}^{+}\) centers. By analogy with the leaves of other plants (Trubitsin et al. 2015), we may assume that the contribution of cyclic electron flow around PSI was comparatively small in the presence of DCMU. Since parameter P 0 characterizes the total content of P700 in the specimen, the ratio P/P 0 is indicative of PSII activity in chloroplasts in situ.
In Fig. 8, we compare the P/P 0 ratio in the two Tradescantia species grown under the LL and HL conditions. In all cases, we found close values P/P 0 ≈ 0.65. Despite a tendency for a small decrease in the P/P 0 ratio in the HL plants, we did not observe statistically significant distinctions between the HL and LL leaves of both species. The steady-state level of oxidized centers \(\text{P}_{700}^{+}\) should be determined by efficient rate constants of the outflow of electrons from P700 (k 1) and the electron inflow to \(\text{P}_{700}^{+}\)(k 2). A simple kinetic equation \(\text{d}\left[ P \right]/\text{d}t={{k}_{1}}\left( {{\left[ P \right]}_{0}}-\left[ P \right] \right)-{{k}_{2}}\left[ P \right]\), where \({{\left[ P \right]}_{0}}\) and \(\left[ P \right]\) stand for the total and current concentrations of \(\text{P}_{700}^{+}\), respectively, yields the steady-state ratio P/P 0 = k 1/(k 1 + k 2). Taking into account the experimental value P/P 0 ≈ 0.65 (Fig. 8), we obtain that under the steady-state conditions the ratio of the apparent rate constants equals to k 1/k 2 ≈ 1.9.
Effects of the growth irradiance (LL or HL) on the ratio P/P 0 measured by the EPR method (for definition, see Fig. 7) in the leaves of T. fluminensis and T. sillamontana, as indicated (n = 8–12, mean values ± SE)
Composition of carotenoids in shade and sun leaves
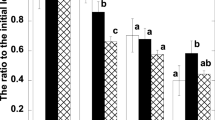
Figure 9 shows the carotenoid composition, including the proportions of β-carotene (β-Car), lutein (Lut), the xanthophyll cycle pigments (V, A, Z), and neoxanthin (Neo), in the plants acclimated either to the LL or to the HL conditions. In the HL-grown plants of both species, the relative amount of β-Car is lower than in the LL-grown plants (Fig. 9a). A decrease in the percentage of β-Car molecules occurs in favor of V + A + Z (Fig. 9b) and lutein (Fig. 9c). Changes in the proportion of neoxanthin are insignificant (Fig. 9d). Statistically valid rise in the proportion of the total amount of xanthophylls (V + A + Z and Lut) in HL plants should be relevant to the mechanism of plant protection against light stress associated with the xanthophylls cycle reactions (Demmig-Adams 1998; Jahns et al. 2009; Matsubara et al. 2009; Demmig-Adams et al. 2012; Jahns and Holzwarth 2012). It is interesting to note that HL-grown T. sillamontana (sun species) is characterized by more significant increase in the relative level of xanthophylls (V + A + Z + Lut) than that in shade-tolerant species T. fluminensis.
The percentage of major carotenoids in the dark-adapted (D) and AL-illuminated (AL) leaves of HL-acclimated and LL-acclimated T. sillamontana and T. fluminensis. V violaxanthin, A antheraxanthin, Z zeaxanthin, βCar β-carotene, Lut lutein, and Neo neoxanthin (n = 3; mean values ± SE). The brackets connect the bars representing significantly different values. The asterisks denote P value according to Student’s t test (*P < 0.10; **P < 0.05; ***P < 0.01)
Figure 10 shows how the short-term illumination of T. fluminensis and T. sillamontana leaves (20 min, 800 µmol quanta m−2 s−1) induces the conversion of violaxanthin (V) to zeaxanthin (Z). The light-induced decrease in the relative level of V (Fig. 10a) is accompanied by the concomitant enhancement of the percentage of Z molecules (Fig. 10b). Relative amounts of antheraxanthin (A), the xanthophyll intermediate of the violaxanthin cycle (V → A → Z), are negligible (Fig. 10c). These data allowed us to derive the de-epoxidation index, DE = (Z + 0.5 A)/(V + A + Z) (Fig. 10d). In dark-adapted leaves of both species, the de-epoxidation indexes are close, DE dark ≈ 25–32%. Illumination induces a marked rise of violaxanthin de-epoxidation, DE light ≈ 51–61%. Although the HL-grown plants revealed a certain trend in the rise of DE light, this effect was not statistically significant. In the meantime, as we reported earlier (Mishanin et al. 2016), generation of NPQ in HL-grown plants was usually higher than in LL-grown plants. We also demonstrated that the relative levels of the regulatory protein PsbS, calculated as the amount of PsbS per PSI complexes, increased with acclimation of T. fluminensis and T. sillamontana to HL conditions (Mishanin et al. 2016). This suggests that there may be the synergetic effect of the PsbS and violaxanthin de-epoxidase (VDE) proteins, which enforces the protection of photosynthetic apparatus against excessive light.
Discussion
In this work, we have compared emission spectra of Chl a fluorescence and their changes during illumination in Tradescantia species of two contrasting ecological groups. Induction events in green leaves, monitored by the fluorescence method, are determined by a number of regulatory feedbacks (for recent review, see Tikhonov 2015). A slow decay of Chl a fluorescence may be caused by the following events taking place within the chloroplast: (a) photochemical quenching, (b) non-photochemical quenching, (c) re-distribution of light energy between PSII and PSI, and (d) reversible/irreversible inactivation of PSII. The enhancement of photochemical quenching occurs due to the light-induced acceleration of electron outflow from PSI to activated CBC (Buchanan 1980, 1991; Michelet et al. 2013), which would cause the re-oxidation of PSII electron acceptors (QA and QB) via the intersystem ETC, thereby stimulating photochemical quenching of Chl a fluorescence. Generation of NPQ and relocation of chloroplasts within the plant cell (the avoidance phenomenon, Zurzycki 1955; Park et al. 1996; Kasahara et al. 2002; Davis et al. 2011; Königer and Bollinger 2012) may be the other reasons for fluorescence decay, which provide protection of PSA against light stress. Below, we briefly consider ecophysiological aspects of induction events in Tradescantia leaves, starting with the consideration of Chl a fluorescence spectra.
In green leaves, Chl a fluorescence has heterogeneous origin, demonstrating two distinct peaks in the emission spectrum (in Tradescantia leaves, at 685 and 740 nm, Fig. 2). According to Govindjee (2004), at room temperature, the “red” fluorescence band F 685 and its vibrational satellite at 720–735 nm originate mostly in the PSII antenna complexes, the “far-red” band (F 740) comes from both PSI and PSII complexes. The ratio between peaks F 685 and F 740 depends on the growth conditions and may reflect relative changes in the contents of PSII and PSI complexes. With increasing Chl content of Tradescantia leaves upon acclimation of plants to LL irradiation (Mishanin et al. 2016), the relative contribution of the far-red fluorescence band F 740 markedly increases in both species (Fig. 2). The enhancement of peak F 740 may be explained by changes in relative sizes of the light-harvesting antennas of PSI and PSII (Mishanin et al. 2016; Ptushenko et al. 2017). The enhancement of peak F 740 in LL plants may also be explained, at least partly, by the re-absorption effect: the light quanta emitted by PSII will be absorbed and then re-emitted as the far-red quanta of Chl a fluorescence. This effect should increase at higher contents of Chls (for review, see Lichtenthaler and Babani 2004). In favor of the re-absorption mechanism may be considered well-known fact that the far-red band increases with the rise of Chl content in photosynthetic and model systems. Our data are consistent with this point of view: an increase in F 740 correlates with the rise of the Chl content (per leaf area) in LL-acclimated Tradescantia plants (Mishanin et al. 2016).
Illumination of dark-adapted leaves with a sufficiently intensive AL induces quenching of Chl a fluorescence, collectively referred to as the Kautsky effect (Govindjee 1995; Lazar 1999; Stirbet and Govindjee 2011). Along with a gradual decrease in the amplitudes of the red (F 685) and far-red (F 740) peaks (Figs. 4, 5), their ratio F 685/F 740 also decreases (Figs. 2, 6). It is noteworthy that peak \({{F}_{685}}\) originates almost entirely in PSII and variable fluorescence of Chl a is attributed predominantly to PSII, whereas the contribution of PSI to variable fluorescence is negligible (Govindjee 2004). Therefore, the “far-red” peak \({{F}_{740}}=F_{\text{740}}^{\text{PSI}}+F_{\text{740}}^{\text{PSII}}\) will show less significant decay than F 685. Since PSII is the sole source of light emission related to the F 685 band, the decrease in F 685 during the induction phase should be more indicative to generation of NPQ than F 740. For this reason, the NPQ-dependent quenching of Chl a fluorescence manifests itself more significantly in the “red” band F 685 than in the “far-red” band F 740 (Fig. 2).
We have found that in HL-grown plants of both species, the light-induced decrease in F 685/F 740 occurs faster than in corresponding LL-grown plants (Fig. 6). Thus, we can conclude that acclimation of plants to HL promotes more rapid generation of NPQ, increasing their capacity for survival upon rapid fluctuations of solar light. Along with the NPQ-dependent response of PSA, a decrease in F 685/F 740 might also reflect the “state 1 → state 2” transition associated with the re-distribution of absorbed light quanta between PSII and PSI (the qT component of NPQ, Horton et al. 1996). The light-induced decrease in sizes of PSII antenna in favor of PSI may lead to additional reduction of the F 685 band. The “state 1 → state 2” transition is a prominent phenomenon in C. reinhardtii cells, where significant amount of the light-harvesting complexes (up to 80%) can migrate from PSII to PSI (Delosme et al. 1996; Dall’Osto et al. 2014), providing a marked rise of the “far-red” band of Chl a fluorescence upon illumination (Allorent et al. 2013). It should be noted that in higher plants, the ΔpH-dependent component of NPQ (qE) is by far the most prominent (Horton et al. 1996; Horton 2012). Otherwise, quenching of Chl a fluorescence due to state transitions (qT) is usually insignificant (Eberhard et al. 2008). This is likely because state transitions are of limited amplitude in higher plants. According to Vener (2007), in higher plants, only ~25% of LHCII is phosphorylated in state 2. He also concluded that the dramatic remodeling of the antenna system observed during laboratory-induced state transitions (e.g., using far-red illumination) may not accurately represent the true nature of LHCII distribution under natural conditions.
The light-induced decay of F 685/F 740 might also be affected (at least partly) by the chloroplast avoidance phenomenon. Relocation of chloroplasts positioned along periclinal (top and/or bottom) to anticlinal cell walls should cause the self-shading of chloroplasts, thereby protecting the PSA against excess light (Augustynowicz and Gabrys 1999; Kasahara et al. 2002; Wada et al. 2003). In the Arabidopsis npq4 mutant, this effect may contribute up to ~30–40% of the overall value of NPQ (Dall’Osto et al. 2014). Inhibition of chloroplast movement has a drastic effect on stress tolerance to HL irradiation (Kasahara et al. 2002). However, the literature data concerning the avoidance response of different plants vary greatly among species (Königer and Bollinger 2011, 2012). Davis et al. (2011), who examined how leaf anatomy influenced chloroplast movements in 24 plant species, reported that chloroplast movement-dependent changes in leaf absorptance were greatest in shade species, in which absorptance changes of >10% were observed between HL and LL treatment. They concluded that the interplay between leaf anatomy, chloroplast position, and acclimation of photosynthetic apparatus will help maximize photosynthetic efficiency at light conditions change. Königer and Bollinger (2012) noted that sun plants showed a larger degree of accumulation response and faster changes in transmission than shade plants. However, they did not find correlation between the rate or the degree of leaf transparency changes under HL and stress tolerance. This suggests that plants can compensate for slow and limited changes of light trapping within the leaf using other photoprotective mechanisms.
Recently, Ptushenko et al. (2016) observed exceptionally high enhancement of the leaf transmittance in T. fluminensis and T. sillamontana, which they attributed to chloroplast avoidance movements. The maximal effect (T light/T dark ≈ 4) was observed in LL-grown T. fluminensis, in other specimens they found T light/T dark ~2. Surprisingly, the enhancement of leaf transmittance in LL-acclimated T. fluminensis was almost two times stronger than in HL-grown leaves. In the meantime, the mesophyll cell widths were definitely smaller, and chloroplasts were somewhat larger in LL-grown than in HL-grown plants. Besides, T. fluminensis mesophyll cells had relatively small size and large chloroplasts compared to other species, but unexpectedly showed substantially more significant avoidance. In this work, we have found that LL-acclimated T. fluminensis showed relatively slow decay of F 685/F 740. In the meantime, this process was markedly faster (t ≈ 3.5 min) than the avoidance effect (t ~10 min) reported by Ptushenko et al. (2017) for LL- and HL-grown T. fluminensis and T. sillamontana. Of course, we cannot exclude that the mechanism to chloroplast photorelocation might affect the light-induced decay of F 685/F 740. However, the comparison of kinetic data suggests that it is the NPQ generation within the chloroplast PSA that determines the light-induced decay of F 685/F 740, while the relative contribution of the avoidance effect should be insignificant.
In our previous work (Mishanin et al. 2016), using the EPR method for monitoring the redox state of P700, we demonstrated the accelerated response of HL-grown Tradescantia to the dark–light–dark transitions. The dark-adapted leaves of HL-acclimated plants showed more rapid photo-oxidation of P700, as well as faster relaxation of their PSA in the dark, as compared to the LL-acclimated plants. These results correlate with the fluorescence data. In dark-adapted Tradescantia leaves, characteristic times of SIF and photo-oxidation of P700 are close (compare Figs. 4, 7). As noted above, the multiphase kinetics of \(\text{P}_{700}^{+}\) induction in dark-adapted leaves may be determined by two basic mechanisms of the feedback regulation of electron transport in chloroplasts: (a) the light-induced activation of the CBC and (b) generation of ΔpH (for review, see Tikhonov 2015). As a matter of fact, these mechanisms are virtually of the same nature as those determining the kinetics of photochemical and non-photochemical quenching of Chl a fluorescence. Therefore, it is not surprising that both the EPR and fluorescence methods of monitoring photosynthetic processes in leaves lead to the same results, demonstrating that the long-term acclimation of Tradescantia plants to HL conditions augments the PSA “reactivity,” which manifests itself upon the dark–light–dark transitions. This effect might stem, at least in part, by increased expression of the regulatory protein PsbS in HL-acclimated plants of both Tradescantia species (Mishanin et al. 2016). Protonation of PsbS caused by the lumen acidification is known to be responsible for rapid energy-dependent phase of NPQ generation (qE) (Li et al. 2002, 2004, 2009; Ruban 2012; Correa-Galvis et al. 2016). Results of our previous study (Mishanin et al. 2016), which showed that PsbS is essential for the activation of NPQ in Tradescantia, are in close agreement with the literature data on enhanced expression of the regulatory protein PsbS in HL-acclimated plants of other species (Ballottari et al. 2007; Zia et al. 2011). These data suggest that PsbS is essential for the activation of NPQ, promoting conformational changes in PSA, which provide for thermal quenching of excess energy in the antenna of PSII. Enhanced expression of PsbS would enlarge the capacity of HL-acclimated plants for their protection against light stress.
In chloroplasts, efficient photoprotection of PSA is provided by synergetic action of two basic factors of NPQ induction, the light-induced protonation of the regulatory protein PsbS and conversion of V to Z (Rees et al. 1989; Zia et al. 2011; Jahns and Holzwarth 2012; Ruban et al. 2012). Formation of Z is known to promote the generation of NPQ; lutein is also involved in photoprotection of plants (for references, see Adams and Demmig-Adams 2004; Jahns and Holzwarth 2012). The content of xanthophylls in photosynthetic systems increases, as a rule, under the light stress conditions. In this context, it was interesting to compare the induction events in Tradescantia with alterations of the content of their xanthophylls upon acclimation to different growth light conditions (Fig. 9). Similarly to other photosynthetic organisms (Adams and Demmig-Adams 2004; Matsubara et al. 2009, 2012), in Tradescantia grown under LL and HL conditions, the relative contents of different carotenoid species change with the intensity of light to which plants were exposed during their growth period (Adamson et al. 1991). The HPLC analysis of carotenoids revealed a distinct rise in the proportion of xanthophylls (V + A + Z and Lut) in HL-acclimated plants of both species (Fig. 9). Enhancement of the xanthophyll content augments photoprotective capability of carotenoids in the plants grown under the solar stress conditions. Illumination of dark-adapted leaves for a rather long time (~20 min) induced the conversion of V to Z (Fig. 10), which is accompanied by a further rise of NPQ (the qM phase of NPQ). In HL-acclimated plants, the qM-component of NPQ induced by rather strong AL is usually higher than in LL-acclimated plants (for more details, see Ptushenko et al. 2013; Mishanin et al. 2016). The light-induced conversion of V to Z and binding of Z to the PsbS protein is known to enhance the sensitivity of PsbS to the lumen acidification (for review, see Jahns and Holzwarth 2012; Ruban et al. 2012). The synergetic action of PsbS-dependent mechanism of NPQ generation, sensitized by Z, should enhance the capacity of HL-grown plants for protection against the light stress. Along with the reactions of the xanthophyll cycle, slow components of NPQ development in the light, as well as its relaxation in the dark, may reflect other events in chloroplasts: re-distribution of light quanta between PSI and PSII (“state transitions”, Allen 1992; Lemeille and Rochaix 2010; Tikkanen and Aro 2012, 2014; Kim et al. 2015) and photoinhibition (qI-component of NPQ, Öquist et al. 1992; Baker 2008). These factors, however, provide less significant contribution to NPQ generation in PSA of higher plants (Lemeille and Rochaix 2010).
Noteworthy that the steady-state levels of \(\text{P}_{700}^{+}\) induced by a strong white light were close in the LL- and HL-grown plants (Fig. 8). This fact means that the ratio between the apparent rate constants of the electron outflow from P700 (k 1) and the electron inflow to \(\text{P}_{700}^{+}\) (k 2) remained almost the same (k 1/k 2 ≈ const). This implies that there may be synergetic effects of multifarious factors maintaining optimal balance between photosynthetic activities of PSI and PSII upon variations of the growth conditions. Bearing in mind more significant decrease in the photosynthetic performance of PSII in HL-grown Tradescantia plants caused by NPQ generation (Mishanin et al. 2016), we may suggest that the overall electron flow through PSI will be balanced, maintaining the ratio k 1/k 2 ≈ const at various growth conditions. One of the mechanisms, which compensate the NPQ-dependent decrease in the overall performance of PSII at high actinic light, may be associated with the fact that the rate-limiting step in the chain of electron transfer from PSII to PSI lies beyond the PSII. It is well-known fact that the inhibition of a significant portion of PSII complexes (inhibition up to ≈ 70−80% of PSII units) by DCMU suppresses but only insignificantly the electron flux from PSII to PSI through the common pools of PQ and Pc molecules (Siggel et al. 1972; Tikhonov and Ruuge 1979; Tikhonov and Vershubskii 2017). Efficient interaction between the intersystem ETCs can be explained not only by “electronic” interactions but also due to “excitonic” connectivity between different PSII units (Antal et al. 2013; Stirbet 2013). Thus, the NPQ-dependent attenuation of PSII capability to donate electrons into the intersystem ETC should not lead to dramatic reduction of electron flux between PSII and PSI, provided the rate-limiting step of electron transfer is beyond the stage of PQH2 formation in PSII.
Another mechanism supporting the optimal balance between the operation of PSII and PSI may be associated with variations of the relative contents of PSI and PSII (Murchie and Horton 1998; Schöttler and Tόth 2014). In particular, Ballottari et al. (2007) reported about contrasting behavior of PSI and PSII during acclimation of Arabidopsis to different light. They observed, in particular, that the PSI/PSII ratio increased in LL conditions and shifted to lower values in HL conditions. In this case, NPQ-dependent modulation of PSII activity will be compensated by variations of the PSI/PSII ratio.
Concluding remarks
Comparative study of ecologically contrasting Tradescantia species (T. fluminensis, shade-tolerant, and T. sillamontana, light-resistant) revealed distinct traits of their PSA, which have potential ecophysiological significance with respect to their tolerance to high light stress. Acclimation of plants to different growth irradiances involved changes in their leaf anatomy and carotenoid composition (increase in V + A + Z and Lut pools) in HL-acclimated plants of both species, suggesting enhanced photoprotective capacity of the carotenoids in the plants grown in nature under high irradiance. The fluorometry and EPR studies of the induction events in Tradescantia chloroplasts in situ demonstrated that irradiance acclimation of the plants promoted a faster “reactivity” of PSA in HL-acclimated plants as compared to LL-acclimated plants. Collectively, the results of the present work suggest that the mechanisms of long-term PSA photoprotection in Tradescantia are based predominantly on the light-induced remodeling of pigment-protein complexes in chloroplasts.
Abbreviations
- AL:
-
Actinic light
- A:
-
Antheraxanthin
- Car:
-
Carotenoid(s)
- CBC:
-
Calvin–Benson cycle
- Chl:
-
Chlorophyll(s)
- Cyt:
-
Cytochrome
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,10-dimethyl urea
- DE :
-
Xanthophyll de-epoxidation index
- EPR:
-
Electron paramagnetic resonance
- ETC:
-
Electron transport chain
- F 685 :
-
Intensity of chlorophyll fluorescence at 685 nm
- F 740 :
-
Intensity of chlorophyll fluorescence at 740 nm
- HL:
-
High light
- LHCII:
-
Light-harvesting complex II
- LL:
-
Low light
- Lut:
-
Lutein
- NPQ:
-
Non-photochemical quenching
- OJIP:
-
Polyphasic fast chlorophyll fluorescence induction
- PAM:
-
Pulse amplitude modulation
- Pc:
-
Plastocyanin
- PSA:
-
Photosynthetic apparatus
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- PQ:
-
Plastoquinone
- P700 :
-
Primary electron donor in photosystem I
- QA, QB :
-
Electron acceptors of PSII; primary and secondary plastoquinones
- qE:
-
Rapid (energy-dependent) component of NPQ
- qI:
-
NPQ component related to inactivation of PSII
- qM:
-
Medium component of NPQ
- qT:
-
NPQ component related to state transitions
- SIF:
-
Slow induction of fluorescence
- VDE:
-
Violaxanthin de-epoxidase
- V:
-
Violaxanthin
- WL:
-
White light
- Z:
-
Zeaxanthin
References
Adams WW III, Demmig-Adams B (2004) Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. A signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 583–604
Adamson HY, Chow WS, Anderson JM, Vesk M, Sutherland MW (1991) Photosynthetic acclimation of Tradescantia albiflora to growth irradiance: morphological, ultrastructural and growth responses. Physiol Plant 82:353–359
Albanese P, Manfredi M, Meneghesso A, Marengo E, Saracco G, Barber J, Morosinotto T, Pagliano C (2016) Dynamic reorganization of photosystem II supercomplexes in response to variations in light intensities. Biochim Biophys Acta 1857:1651–1660
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Allen JF (2003) State transitions: a question of balance. Science 299:1530–1532
Allorent G, Tokutsu R, Roach T, Peers G, Cardol P, Girard-Bascou J, Seigneurin-Berny D, Petroutsos D, Kuntz M, Breyton C, Franck F, Wollman FA, Niyogi KK, Krieger-Liszkay A, Minagawa J, Finazzi G (2013) A dual strategy to cope with high light in Chlamidomonas reinhardtii. Plant Cell 25:545–557
Anderson J, Chow WS, Goodchild DJ (1988) Thylakoid membrane organisation in sun/shade acclimation. Funct Plant Biol 15:11–26
Anderson JM, Chow WS, Park Y-I, Franklin LA, Robinson SP-A, van Hasselt PR (2001) Response of Tradescantia albiflora to growth irradiance: change versus changeability. Photosynth Res 67:103–112
Antal TK, Kolacheva A, Maslakov A, Riznichenko GYu, Krendeleva TE, Rubin AB (2013) Study of the effect of reducing conditions on the initial chlorophyll fluorescence rise in the green microalgae Chlamydomonas reinhardtii. Photosynth Res 114:143–154
Augustynowicz J, Gabrys H (1999) Chloroplast movement in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant Cell Environ 22:1239–1248
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baker NR, Oxborough K (2004) Chlorophyll fluorescence as a probe of photosynthetic productivity. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. A signature of photosynthesis. Advances in Photosynthesis and Respiration, vol 19. Springer, Dordrecht, pp 65–82
Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282:8947–8958
Barber J (2008) Crystal structure of the oxygen-evolving complex of photosystem II. Inorg Chem 47:1700–1710
Bjorkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170:489–504
Buchanan BB (1980) Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31:341–374
Buchanan BB (1991) Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys 288:1–9
Chow WS, Melis A, Anderson JM (1990) Adjustment of photosystems stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87:7502–7505
Chow WS, Adamson HY, Anderson JM (1991) Photosynthetic acclimation of Tradescantia albiflora to growth irradiance: lack of adjustment of light-harvesting components and its consequences. Physiol Plant 81:175–182
Correa-Galvis V, Redekop P, Guan K, Griess A, Truong TB, Wakao S, Niyogi KK, Jahns P (2016) Photosystem II subunit PsbS is involved in the induction of LHCSR protein-dependent energy dissipation in Chlamydomonas reinhardtii. J Biol Chem 291:17478–17487
Dall’Osto L, Cazzaniga S, Wada M, Bassi R (2014) On the origin of a slow reversible fluorescence decay component in the Arabidopsis npq4 mutant. Phyl Trans R Soc B 369:20130221
Davis PA, Caylor S, Whippo CW, Hangarter RP (2011) Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environment 34:2047–2059
Delosme R, Olive J, Wollman F-A (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta 1273:150–158
Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39:474–482
Demmig-Adams B, Cohu CM, Muller O, Adams WW (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Derks A, Schaven K, Bruce D (2015) Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation to rapid environmental change. Biochim Biophys Acta 1847:468–485
Eberhard S, Finazzi G, Wollman FA (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515
Edwards GE, Walker DA (1983) C3, C4: mechanisms, and cellular and environmental regulation of photosynthesis. Blackwell, Oxford
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:1637–1661
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence. A signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 1–42
Horton P (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Phil Trans R Soc B 367:3455–3465
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Jahns P, Holzwarth AR (2012) The role of the xanthophylls cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Jahns P., Latowski D., Strzalka K. (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Järvi S, Gollan PJ, Aro E-M (2013) Understanding the roles of the thylakoid lumen in photosynthetic regulation. Front Plant Sci. doi:10.3389/fpls.2013.00434
Johnson GN (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807:906–911
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679
Johnson MP, Goral TK, Duffy CDP, Bran APR, Mullineaux CW, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complex in the grana membranes of spinach chloroplasts. Plant Cell 23:1468–1479
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM (2015) Dynamic photosynthesis in different environmental conditions. J Exp Botany 66:2415–2426
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI et al (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kim E, Ahn TK, Kumazaki S (2015) Changes in antenna sizes of photosystems during state transitions in granal and stroma-exposed thylakoid membrane in intact chloroplasts in Arabidopsis mesophyll protoplasts. Plant Cell Physiol 56:759–768
Kirchhoff H (2013) Architectural switches in plant thylakoid membranes. Photosynth Res 116:481–487
Königer M, Bollinger N (2011) Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environ 34:2047–2059
Königer M, Bollinger N (2012) Chloroplast movement behavior varies widely among species and does not correlate with high light stress tolerance. Planta 236:411–426
Kono M, Terashima I (2014) Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J Photochem Photobiol B 137:89–99
Kramer DM, Sacksteder CA, Cruz JA (2003) Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 8:27–32
Kuvykin IV, Ptushenko VV, Vershubskii AV, Tikhonov AN (2011) Regulation of electron transport in C3 plant chloroplasts in situ and in silico: short-term effects of atmospheric CO2 and O2. Biochim Biophys Acta 1807:336–347
Lazar D (1999) Chlorophyll a fluorescence induction. Biochim Biophys Acta 1412:1–28
Lemeille S, Rochaix J-D (2010) State transitions at the crossroad of thylakoid signaling pathways. Photosynth Res 106:33–46
Li X-P, Bjorkman O, Shih C, Grossmann AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Li X-P, Muller-Moule P, Gilmore AM, Niyogi KK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99:15222–15227
Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Lichtenthaler HK, Babani F (2004) Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 713–736
Lichtenthaler HK, Ac A, Marek MV, Kalina J, Urban O (2007a) Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol Biochem 45:577–588
Lichtenthaler HK, Babani F, Langsdorf G (2007b) Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth Res 93:235–241
Liu L-X, Chow WS, Anderson JM (1993) Light quality during growth of Tradescantia albiflora regulates photosystem stoichiometry, photosynthetic function and susceptibility to photoinhibition. Physiol Plant 89:854–860
Mamedov M, Govindjee, Nadtochenko V, Semenov A (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth Res 125:51–63
Matsubara S, Krause GH, Aranda J, Virgo A, Beisel K, Jahns P, Winter K (2009) Sun–shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol 36:20–36
Matsubara S, Förster B, Waterman M, Robinson SA, Pogson BJ, Gunning B, Osmond B (2012) From ecophysiology to phenomics: some implications of photoprotection and shade–sun acclimation in situ for dynamics of thylakoids in vitro. Phil Trans R Soc B 367:3503–3514
Merzlyak M, Solovchenko A, Pogosyan S (2005) Optical properties of rhodoxanthin accumulated in Aloe arborescens Mill. leaves under high-light stress with special reference to its photoprotective function. Photochem Photobiol Sci 4:333–340
Michelet L, Zaffagnini M, Morisse S, Sparla F, Perez-Perez ME, Francia F, Danon A, Marchand CH, Fermani S, Trost P, Lemaire SD (2013) Redox regulation of the Calvin–Benson cycle: something old, something new. Front Plant Sci 4:470. doi:10.3389/fpls.2013.00470
Mishanin VI, Trubitsin BV, Benkov MA, Minin AA, Tikhonov AN (2016) Light acclimation of shade-tolerant and light-resistant Tradescantia species: induction of chlorophyll a fluorescence and P700 photooxidation, expression of PsbS and Lhcb1 proteins. Photosynth Res 130:275–291
Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev 41:445–502
Miyake C (2010) Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Murata N, Allakhverdiev SI, Nishiyama Y (2012) The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, α-tocopherol, nonphotochemical quenching, and electron transport. Biochim Biophys Acta 1817:1127–1133
Murchie EH, Horton P (1998) Contrasting patterns of photosynthetic acclimation to the light environment are dependent on the differential expression of the responses to altered irradiance and spectral quality. Plant Cell Environ 21:139–148
Őquist G, Chow WS, Anderson JM (1992) Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186:450–460
Papageorgiou GC, Tsimilli-Michael M, Stamatakis K (2007) The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynth Res 94:275–290
Park Y-I, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111:867–875
Ptushenko VV, Ptushenko EA, Samoilova OP, Tikhonov AN (2013) Chlorophyll fluorescence in the leaves of Tradescantia species of different ecological groups: induction events at different intensities of actinic light. BioSystems 114:85–97
Ptushenko VV, Ptushenko OS, Samoilova OP, Solovchenko AE (2016) An exceptional irradiance-induced decrease of light trapping in two Tradescantia species: an unexpected relationship with the leaf architecture and zeaxanthin-mediated photoprotection. Biol Plant 60:385–393
Ptushenko VV, Ptushenko OS, Samoilova OP, Solovchenko AE (2017) Analysis of photoprotection and apparent nonphotochemical quenching of chlorophyll fluorescence in Tradescantia leaves based on the rate of irradiance induced changes in optical transparence. Biochemistry (Moscow) 82:67–74
Randall RP (2012) A global compendium of weeds, 2nd edn. Department of Agriculture and Food, Western Australia, pp 1125
Rees D, Young A, Noctor G, Britton G, Horton P (1989) Enhancement of the pH-dependent dissipation of excitation energy in spinach chloroplasts by light activation: correlation with the synthesis of zeaxanthin. FEBS Lett 256:85–90
Rochaix J-D (2014) Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 65:287–309
Ruban A (2012) The photosynthetic membrane: molecular mechanisms and biophysics of light harvesting. Wiley-Blackwell, Oxford
Ruban AV, Johnson MP, Dufy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Samoilova OP, Ptushenko VV, Kuvykin IV, Kiselev SA, Ptushenko OS, Tikhonov AN (2011) Effects of light environment on the induction of chlorophyll fluorescence in leaves: a comparative study of Tradescantia species of different ecotypes. BioSystems 105:41–48
Schansker G, Tόth SZ, Holzwarth AR, Garab G (2014) Chlorophyll a fluorescence: beyond the limits of the QA model. Photosynth Res 120:43–58
Schöttler MA, Tόth SZ (2014) Photosynthetic complex stoichiometry dynamics in higher plants: environmental acclimation and photosynthetic flux control. Front Plant Sci 5(188):1–15
Siggel U, Renger G, Stiehl HH, Rumberg B (1972) Evidence for electronic and ionic interaction between electron transport chains in chloroplasts. Biochim Biophys Acta 256:328–335
Solovchenko A (2010) Photoprotection in plants, Springer series in biophysics, vol 14. Springer, Berlin
Solovchenko AE, Chivkunova OB, Merzlyak MN, Reshetnikova IV (2001) A spectrophotometric analysis of pigments in apples. Russ J Plant Physiol 48:693–700
Stirbet A (2013) Excitonic connectivity between photosystem II units: what is it, and how to measure it? Photosynth Res 116:189–214
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Stirbet A, Govindjee (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I-P rise. Photosynth Res 113:15–61
Stirbet A, Govindjee (2016) The slow phase of chlorophyll a fluorescence induction in silico: origin of the S–M fluorescence rise. Photosynth Res 1303:193–213
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transients. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 321–362
Suorsa M, Jarvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjarvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro E-M (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24:2934–2948
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to CO2 diffusion. J Exp Bot 57:343–354
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Tikhonov AN (2013) pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynth Res 116:511–534
Tikhonov AN (2014) The cytochrome b 6 f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol Biochem 81:163–183
Tikhonov AN (2015) Induction events and short-term regulation of electron transport in chloroplasts: an overview. Photosynth Res 125:65–94
Tikhonov AN, Ruuge EK (1979) Electron paramagnetic resonance study of electron transport in photosynthetic systems. VIII. The interplay between two photosystems and kinetics of P700 redox transients under various conditions of flash excitation. Molec Biol (Moscow) 13:1085–1097
Tikhonov AN, Vershubskii AV (2017) Connectivity between electron transport complexes and modulation of photosystem II activity in chloroplasts. Photosynth Res. doi:10.1007/s11120-017-0349-z
Tikkanen M, Aro E-M (2012) Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim Biophys Acta 1817:232–238
Tikkanen M, Aro E-M (2014) Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci 19:10–17
Tikkanen M, Grieco M, Nurmi M, Rantala M, Suorsa M, Aro E-M (2012) Regulation of the photosynthetic apparatus under fluctuating growth light. Philos Trans R Soc Lond B 367:3486–3493
Trubitsin BV, Vershubskii AV, Priklonskii VI, Tikhonov AN (2015) Short-term regulation and alternative pathways of photosynthetic electron transport in Hibiscus rosa-sinensis leaves. J Photochem Photobiol B 152:400–415
Vener AV (2007) Environmentally modulated phosphorylation and dynamics of proteins in photosynthetic membranes. Biochim Biophys Acta 1767:449–457
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Wientjes E, van Amerongen H, Croce R (2013) LHCII is an antenna of both photosystems after long-term acclimation. Biochim Biophys Acta 1827:420–426
Woodrow IE, Berry JA (1988) Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annu Rev Plant Physiol Plant Mol Biol 39:533–594
Zia A, Johnson MP, Ruban AV (2011) Acclimation- and mutation-induced enhancement of PsbS levels affects the kinetics of non-photochemical quenching in Arabidopsis thaliana. Planta 233:1253–1264
Zurzycki J (1955) Chloroplast arrangement as a factor in photosynthesis. Acta Soc Bot Pol 24:27–63
Acknowledgements
This work was supported in part by the Russian Foundation for Basic Research (RFBR Project 15-04-03790a). Analysis of carotenoid composition was funded by Russian Science Foundation (RSCF Project 14-50-00029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishanin, V.I., Trubitsin, B.V., Patsaeva, S.V. et al. Acclimation of shade-tolerant and light-resistant Tradescantia species to growth light: chlorophyll a fluorescence, electron transport, and xanthophyll content. Photosynth Res 133, 87–102 (2017). https://doi.org/10.1007/s11120-017-0339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0339-1