Abstract
Productivity of most macroalgae is not currently considered limited by dissolved inorganic carbon (DIC), as the majority of species have CO2-concentrating mechanisms (CCM) allowing the active uptake of DIC. The alternative, diffusive uptake of CO2 (non-CCM), is considered rare (0–9 % of all macroalgal cover in a given ecosystem), and identifying species without CCMs is important in understanding factors controlling inorganic carbon use by eukaryotic algae. CCM activity has higher energetic requirements than diffusive CO2 uptake, therefore when light is low, CCM activity is reduced in favour of diffusive CO2 uptake. We hypothesized that the proportional cover of macroalgae without CCMs (red and green macroalgae) would be low (<10 %) across four sites in Tasmania, southern Australia at two depths (4–5 and 12–14 m); the proportion of species lacking CCMs would increase with decreasing depth; the δ13C values of macroalgae with CCMs would be more depleted with depth. We found the proportion of non-CCM species ranged from 0 to 90 % and included species from all three macroalgal phyla: 81 % of red (59 species), 14 % of brown (three species) and 29 % of green macroalgae (two species). The proportion of non-CCM species increased with depth at three of four sites. 35 % of species tested had significantly depleted δ13C values at deeper depths. Non-CCM macroalgae are more abundant in some temperate reefs than previously thought. If ocean acidification benefits non-CCM species, the ramifications for subtidal macroalgal assemblages could be larger than previously considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
By the year 2100, mean aqueous CO2 concentrations in surface waters will increase by 225 % under current business as usual scenarios (RCP 8.5; Ciais et al. 2013). This is due to the absorption by seawater of anthropogenic CO2 (i.e. ocean acidification) (Raven et al. 2005b; Caldeira and Wickett 2003). Currently, less than 1 % of seawater-dissolved inorganic carbon (DIC) is comprised of CO2, 92 % is HCO3 −, and 7 % is CO3 2− at pH 8.05 and total alkalinity (AT) of 2200 µmol kg−1. Macroalgae provide food and habitat for an ecologically diverse array of species, supporting commercially important coastal fisheries (Steneck et al. 2002; Tegner and Dayton 2000). The effects of ocean acidification on macroalgal-based ecosystems could be large (Hall-Spencer et al. 2008; Hepburn et al. 2011). It has been proposed that many species of non-calcareous macroalgae may benefit from ocean acidification due to the increase in concentrations of CO2, to the detriment of calcareous species that may decline in abundance (e.g. Connell et al. 2013; Kroeker et al. 2013; Hepburn et al. 2011; Cornwall et al. 2012). This could either enhance or reduce the productivity of coastal ecosystems, depending on the ecological role of species that benefit or suffer adverse effects from ocean acidification.
Macroalgae utilize DIC via a variety of complex pathways during organic carbon fixation (see reviews by Raven 1997; Giordano et al. 2005; Raven et al. 2011). CO2 concentrating mechanisms (CCMs) elevate CO2 concentration relative to O2 concentration at the site of RuBisCO, and involve the active direct or indirect uptake of DIC (as HCO3 − or CO2) for photosynthesis during periods of relatively low concentrations of CO2 in the seawater in contact with the macroalgae. This reduces the photorespiration, i.e. the wasteful competing oxygenase activity of RuBisCO with carboxylase (Kübler et al. 1999). This can occur via several direct uptake mechanisms, involving symports, antiports, the active pumping of H+ or local acidic zones, where external HCO3 − usually is taken into the cell (Raven et al. 2005a, 2014; Giordano et al. 2005). Some species of macroalgae do not possess functional CCMs and must rely on the uptake of CO2 via diffusion over cellular membranes (Kübler et al. 1999; Giordano et al. 2005; Kevekordes et al. 2006; Raven et al. 2005a). These macroalgae most likely exclusively use CO2 in seawater, either as CO2 or via the conversion of HCO3 − by external carbonic anhydrase (exCA; which is also used by some CCM species to facilitate diffusion), and include members of the red and green macroalgae (phyla Rhodophyta and Chlorophyta). Although no brown macroalgae (phylum Ochrophyta) have been identified as exclusively relying on diffusive CO2, one species (Desmarestia anceps from Antarctica) has inconclusive δ13C values ranging from both above and below −30 ‰ (Fischer and Wiencke 1992), which is often regarded as the value separating the two mechanisms (Raven et al. 2005a).
The response of macroalgae to ocean acidification will likely be dependent on the physiological mechanism employed to use DIC, hence understanding the composition of different DIC use strategies in any given macroalgal assemblage is important when predicting how those assemblages might respond to ocean acidification. Hepburn et al. (2011) stated there would be two broad responses of non-calcareous macroalgae with different DIC-utilization strategies to ocean acidification: (1) all macroalgae which express a CCM allowing active uptake of DIC (hereafter “CCM-macroalgae”) will likely display neutral or positive responses. This is because theoretically their photosynthetic rates are already saturated for DIC, as they utilize energized uptake of some form to increase the concentration of CO2 relative to O2 at the site of RuBisCO above that of the bulk medium (Raven 2010; Raven et al. 2011). However, CCM activity requires more energy than diffusive uptake, and so the reliance on the CCM declines in low light or under high CO2 concentrations, where a switch to greater diffusive uptake of CO2 can be observed for many autotrophs, including macroalgae (Hepburn et al. 2011; Raven and Beardall 2014). (2) All macroalgae which do not possess or use a CCM (those that rely exclusively on diffusive uptake of CO2 are hereafter “non-CCM macroalgae”) will likely respond positively, as their rates of photosynthesis could currently be limited by CO2 concentrations (Raven et al. 2005a; Kübler et al. 1999; Raven 1991). Most calcareous species have δ13C values indicating that they possess a CCM (Lee and Carpenter 2001; Hepburn et al. 2011), except for the lightly calcified green Udotea flabellum (Lepoint et al. 2000). However, because the functioning of their CCM is relatively unexplored, and that they take up DIC for calcification that could be used for photosynthesis, and because they will respond very differently to ocean acidification than non-calcareous CCM species, Hepburn et al. (2011) categorized calcareous species as a third DIC using group distinct from non-calcareous species.
Hepburn et al. (2011) is the only quantitative description of the abundance of species with an active CCM versus non-CCM macroalgae in any ecosystem. Non-CCM macroalgae have been described as relatively rare when compared to CCM macroalgae (Raven et al. 2002b, 2005a), making up around 50 % of all non-calcareous red species and comprising 0–8.9 % (at 2 and 10 m, respectively) of available substrate in a Macrocystis pyrifera forest on the east coast of the South Island, New Zealand (Hepburn et al. 2011). This suggests that the abundance of macroalgae without a CCM could increase as photosynthetically active radiation (PAR) declines, where the operation of a CCM has more energetic costs (Hepburn et al. 2011; Raven et al. 2014).
The degree to which CCM species will rely on diffusive uptake of CO2 over changing light can be measured using several techniques (Giordano et al. 2005). Macroalgal δ13C becomes more depleted with increasing depth and/or decreasing light for a variety of species (Wiencke and Fischer 1990; Hepburn et al. 2011; Fischer and Wiencke 1992). δ13C values from one species cannot be compared with another to determine the degree to which one species relies on diffusive CO2 over another (Raven et al. 2002b). However, shifts in δ13C values between individuals of the same species can be used to determine changes in the active uptake of HCO3 − via the CCM versus the uptake of CO2 via diffusion (Cornelisen et al. 2007; Raven and Beardall 2014; Korb et al. 1996). The role of singular environmental variables (such as water motion or light) on the general activity of the CCM can be suggested if one variable is altered, while other variables are kept constant or controlled for (Cornelisen et al. 2007; Hepburn et al. 2011; Raven and Beardall 2014). It has been hypothesized that macroalgae that are light limited in deeper habitats (i.e. those that reduce their reliance on energetically expensive CCMs, as shown by depleted δ13C values at deeper depths) could be as DIC limited as species that do not possess a functional CCM (Hepburn et al. 2011). pH-drift techniques, which estimate the ability of macroalgae to continue to photosynthesise above pH 9 when [CO2] is functionally absent (Maberly 1990), can be combined with macroalgal δ13C values to assess the difference between a species that does not have a functional CCM versus a species that possesses a CCM but has a lack of activity in situ. This can occur when a species δ13C value is more depleted than −30 ‰, but it possesses the physiological machinery to raise pH above 9 (Hepburn et al. 2011). The pH-drift technique works on the premise that the concentration of CO2 is effectively 0 at pHNBS 9, therefore photosynthetic activity that increases pH above 9 should be a result of the uptake of HCO3 −. This final pH value that an individual macroalga can increase seawater to in known as the “pH compensation point”.
The identification of “rare” CO2-only species has been identified as one of the 4 most important questions in inorganic carbon use by eukaryotic algae (Raven 2010), and determining which species rely exclusively on diffusive CO2 could aid current predictions of how macroalgal assemblages will respond to ocean acidification. Our aim was to determine the extent to which diffusive CO2 is the mode of DIC acquisition in macroalgal communities that inhabit a variety of subtidal temperate reef habitats in Tasmania, southern Australia. We hypothesized that (1) the abundance of species relying solely on diffusive uptake of CO2 will be low (<10 % in all locations) and non-CCM species will be comprised solely of Rhodophytes and Caulerpa spp. (Chlorophyta) based on prior knowledge (Hepburn et al. 2011; Kevekordes et al. 2006; Murru and Sandgren 2004); (2) the abundance of species relying solely on diffusive uptake of CO2 will increase at depth; and (3) macroalgae which possess a CCM will decrease its activity at depth, which will be facultative (Hepburn et al. 2011), whereby their δ13C values will become more depleted, but their capacity to raise pH will remain equal. We tested these hypotheses for macroalgal assemblages across four different sites, one characteristic of a sea urchin barrens dominated east coast site (Saint Helens Island), an Ecklonia radiata forest on the east coast (Fortescue Bay), a site heavily influenced by tannin-rich surface water near the Huon River estuary with reduced wavelengths below 500 and above 600 nm (Ninepin point; see Clementson et al. 2004), and one wave-sheltered location further from the Huon River (Tinderbox; see Fig. 1a for site locations). Current and prior measurements of irradiance at three of the four sites (Fortescue Bay, Saint Helens and Tinderbox) indicated that irradiance was significantly reduced between 4–5 and 12–15 m (see ESM 1). Hence, the sites represent two sheltered low-light habitats with large inputs of terrestrial influences that could be expected to favour non-CCM species (Tinderbox and Ninepin point), and two exposed sites that could be expected to favour non-CCM species (Fortescue Bay and Saint Helens Islands). Sites were not chosen to test differences between sites, rather to see how general patterns in abundance for the three physiological groups would be at these sites.
Methods
Characterisation of sites
Surveys and collections for macroalgae were conducted between November 2013 and March 2014 at four sites. Collections of macroalgae and surveys took place at Saint Helens Island (S41°20′43.364E148°20′14.809) 1st November 2013, Fortescue Bay (S43°7′22.945E147°58′30.748) 28th November 2013, Tinderbox (S43°3′15.522E147°20′28.199) 10th March 2014, Ninepin point (S43°16′38.222E147°11′16.86) 10th March 2014. Surveys were conducted using SCUBA along 2 × 15 m transects at each site and depth combination. Estimates of canopy coverage were conducted in situ for 3, 5 × 1 m sections on each transect. Understorey cover and species abundance was assessed using 10, 50 × 50 cm photoquadrats that were taken on each transect. Visibility limited species identification for larger photoquadrats in some instances (mostly at Ninepins point and Tinderbox at 12–14 m). Therefore, 10, 10 × 10 cm photoquadrats were also taken on each transect to identify the species present in all transects at all sites. Ideally, biomass estimates of each species from each site and depth would have been conducted along the transects, as the CCM species are most likely larger than species lacking a CCM. However, legislation at the study sites restricts the removal of any given species of macroalgae to 200 g on any given day, meaning that the area surveyed would be particularly small. We therefore considered this the most appropriate approach.
δ13C and % C analysis
Three replicates of all available species of macroalgae were collected at the four sites and two depths on the dates mentioned in the previous section during 30 min searches by one diver on SCUBA for δ13C analysis. This equalled 464 individual samples and a total of 106 species. Upon collection, macroalgae were placed in a zip-lock bag in situ with seawater, and then these bags were placed within a black non-transparent bag. Care was taken to remove specimens by the holdfast to reduce wounding effects. At the surface, the black bags were placed into a cooler container to reduce stress to the macroalgae. Samples were then taken to the laboratory within 4 h of collection, except for Saint Helens Island samples, which were frozen at −10 °C due to the distance between the site and the laboratory. Prior trials indicate that freezing macroalgae for δ13C analysis did not influence the data (Hurd and Cornwall, unpublished data). Samples were cleaned of epiphytes and identified to species level when possible before destructive sampling. Calcareous samples were placed in 10 % HCl until calcareous tissue was removed. All samples were then dried at 60 °C for 24 h before been ground into a fine powder using a porcelain mortar and pestle and weighed into pressed tin capsules (0.2 mg; Sercon, UK). Isotope values were determined using a Fisons NA1500 elemental analyser coupled to a Thermo Scientific Delta V Plus via a Conflo IV. Combustion and reduction were achieved at 1020 and 650 °C, respectively. Percent composition was calculated by comparison of mass spectrometer peak areas to those of standards with known concentrations. Isotopic values for carbon are reported as δ-values (‰) relative to VPDB and were corrected via a 4-point calibration using certified standards. Reproducibility was also monitored by the use of long-term internal reference materials. Both precision and accuracy were ±0.1 ‰ (1 SD).
pH drift
Collection of macroalgae for pH-drift analysis was identical to that mentioned in the δ13C analysis section, except that pH-drift assays were not conducted for the Saint Helens Island site, due to its greater distance from the laboratory and due to the small number of species in the deep habitat. Also, whole specimens that weighed 2 g of wet weight (±0.1 g SE) were selectively collected, because cutting some species of macroalgae (especially E. radiata) tended to cause them to leak polysaccharides and other substances that could affect the final pH value (Cornwall and Hurd, unpublished data; Hepburn et al. 2011). Specimens were placed into a large aquarium of seawater for 24 h at 18 µmol photons m−2 s−1 and 14 °C with constant bubbling from four air stones before pH-drift assays begun. Where possible, three replicates of each species from each site and depth combination were placed into a 100-ml sealed, transparent plastic containers with seawater of mean pH 8.05, an oxygen concentration of 253 µmol kg−1, total AT 2210 µmol kg−1, calculated DIC of 2000 µmol kg−1 and salinity 35 S A. This gave a total of 242 individuals. Seawater pH was measured using metre (Orion Star A216 pH/RDO/DO) and pH electrode (Orion 8107BNUMD—Ross Ultra pH/AIC triode), calibrated with pH 7.0 and 9.0 NBS buffers, cross referenced with Tris and Amp seawater buffers to convert to the total scale (Dickson et al. 2007). All mention of pH throughout the manuscript is on the total scale unless otherwise noted. Oxygen was measured using an Orion RDO probe (ORI087100MDW) attached to the same metre and was calibrated using 0 and 100 % air saturation standards made by bubbling seawater with N2 or air, respectively, for 10 min. AT was measured using an open-cell titration using a Metrohm 809 Titrando and Metrohm 800 Dosino. AT measurements were made at 25 °C using a Circu temperature bath, and were measured against a CRM supplied by Andrew Dickson (see Dickson et al. 2007 for more details).
Containers were placed under 250 µmol photons m−2 s−1 at 14 °C on a bench, randomly ordered so that replicates of species and depths (and sites in the case of Tinderbox and Ninepins point assays) were mixed to avoid artefacts due to segregation. Three control containers without seaweed were also used to control for changes in seawater pH not due to the macroalgae. After 24 h, the containers were removed from the bench and final pH and oxygen concentration was recorded. Macroalgae were also removed and weighted to the nearest 0.01 g. Samples were poisoned with HgCl2 and kept in a dark container without light until later AT measurements, which were done to determine the extent of changes in seawater AT occurred independent of DIC uptake (Axelsson 1988). 24 h were used because previous experiments had indicated that this was the optimal time for measuring maximal pH change for these and similar species (Cornwall and Hurd, unpublished data; Hepburn et al. 2011). Containers used in the assays are left open to the atmosphere for 24 h after the experiment has concluded, and individual replicates where pH does not return to ambient (~8) are excluded from the analysis, as they indicate the likely leaching of substances that alter pH.
Note that previous pH-drift assays have usually reported data on the NBS scale (Murru and Sandgren 2004; Hepburn et al. 2011; Maberly 1990), while here we report on the more accurate total scale (pHT), which is 0.12 units lower than pHNBS 9.0 at AT 2210 µmol kg−1. We also accounted for measured reductions in AT of between 180 and 400 µmol kg−1 when the CO2 compensation point was calculated (i.e. the point at which [CO2] < 1 µmol kg−1), which meant the pH compensation point was between pH 8.83 for most species, but as low as 8.76 for calcifying species that decreased AT during calcification. Specimens that visibly bleached or leached compounds were excluded from analysis.
Data analysis
We tested for differences in the abundance of the three physiological functional groups (CCM, non-CCM and calcareous species) between the depths, controlling for site as a random factor using a crossed permutational multivariate analysis of variance (PERMANOVA) and an analysis of similarity (ANOSIM). Both were performed using PERMANOVA+ for PRIMER, PRIMER-E Ltd, Plymouth, UK. PERMANOVA was performed on square-root transformed data using Bray–Curtis similarity measures using 9999 permutations. Physiological groups were determined by the δ13C value of species’ tissue, and when all samples of a particular species had δ13C values more enriched than −29 ‰ we took that as indicating the presence of a CCM. One-way ANOVAs were used to detect differences among δ13C values, final pH and O2 compensation points for each species for instances where 3 replicates could be collected at both depths at a specific site. Linear models were used to determine correlations and R 2 coefficients between final pH and O2, and δ13C values in R 3.0.2 (R Core Development Team 2008).
Results
Characterization of sites
Saint Helens 5 m, Fortescue Bay 5 and 15 m, and Tinderbox 5 m all were characterized by high (96–100 %) cover of the canopy-forming brown macroalga E. radiata (and Phyllospora comosa at Fortescue Bay). Canopies of the green macroalga Caulerpa trifaria replaced E. radiata as the dominant species at 15 m at Tinderbox and 5 m at Ninepin point, but their cover was sparser than E. radiata (17–53 %). Crustose coralline algae covered most of the benthos at 15 m at Saint Helens (97–99 %). Understorey assemblages were dominated by crustose coralline algae at 5 m at Saint Helens (82–90 %) and Fortescue Bay (40–50 %). At 15 m at Fortescue Bay, the understorey was mix of crustose corallines, C. flexilis, the brown alga Halopteris paniculata and non-coralline red macroalgae (predominately Lenormandia marginata, Plocamium spp., Rhodymenia spp., and Sonderopelta coriacea). At both depths at Tinderbox, juvenile E. radiata dominated understorey assemblages during our surveys (22–66 %), while C. trifaria was also abundant at 15 m (40–42.5 %), and was replaced by a mix of crustose coralline algae and non-calcareous red macroalgal species at 5 m (predominately Callophyllis spp., Plocamium spp. and Thamnoclonium dichotomum). At both depths at Ninepin point, mixed communities of red macroalgae, the green Caulerpa spp. and filamentous brown macroalgae comprised the understorey; these assemblages were comprised predominately of C. trifaria, the reds (Callophyllis spp., Plocamium spp., species from the family Peyssonneliaceae) and the brown macroalga Sporochnus comosus.
δ13C and % C analysis
A total of 106 species were collected across all sites for analysis. Table S2 in ESM 2 contains the mean and SEs of species’ δ13C values from all sites. Fifty-nine of the 78 red macroalgae collected had δ13C values equal or more depleted than −30 ‰. Five of the remaining 19 red macroalgae with values more enriched than −30 ‰ were calcifying, while 14 were non-calcifying. This means 80.8 % of non-calcareous reds were non-CCM species. Three of the 18 brown and 2 of the 5 green macroalgae collected also had δ13C values equal to, or more depleted than, −30 ‰. 60.3 % of all collected species had δ13C values indicative of lacking a CCM.
There was a trend for δ13C values within species to be depleted with depth when individual species were collected from both depths at any given site. Seven out of 12 red (58.3 %), nine out of 12 brown (75 %) and four out of four green species examined across depths had more depleted δ13C values at 15 m. However, for only a smaller subset of species were there three replicates at each depth for any given site, due to the rarity of some species, and due to the restricted distribution of some species across depths and sites. A comparison of the δ13C values of 20 species could be made across both depths at all sites combined. This depletion was statistically significant (p < 0.05) only for seven out of 20 species examined (see Table S2, ESM 3). These species included the reds Curdiea angustata (Ninepin Point), Plocamium angustum (Fortescue Bay), the browns Dictyopteris muelleri (Tinderbox) and E. radiata (Fortescue Bay), and the Chlorophytes Caulerpa geminata (Tinderbox) and C. trifaria (both Tinderbox and at Ninepin point). It must be noted that Cu. angustata, P. angustum and C. trifaria all had δ13C values more depleted than −30 ‰ at both the 5- and 15-m depths at all examined sites.
Seven out of 20 species had statistically significant declines in % C between 5 and 15 m depth (see Table S2, ESM 3). These included the reds Gracilaria sp. and L. marginata (both species at Tinderbox) and Cu. angustata (Ninepin Point); the brown D. muelleri (Tinderbox) and E. radiata (Fortescue Bay) and the green macroalgae C. geminata (Tinderbox) and C. trifaria (Ninepins Point).
pH drift
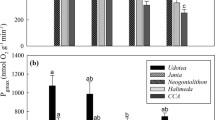
A total of 47 species of the 106 that were collected yielded reliable pH compensation points. The smaller subset here than in the δ13C analysis was due to the larger requirements for tissue for some species, and due to the fact that many species leached compounds during the experiment (e.g. some E. radiata and many Caulerpa spp. replicates). Five of the 28 species of red, 14 of the 15 brown and three of the four green macroalgae examined could raise pH past the compensation point (Fig. S1 in ESM 4). All species that could not raise pH beyond this point had δ13C values equal to or more depleted than −27 ‰, while all species that could raise pH beyond the compensation point had δ13C values more enriched than −27 ‰ (Fig. 2a). There was a weak correlation between final pH and δ13C values (R 2 = 0.37, p < 0.01).
There was a weak positive correlation between the final oxygen concentration and the pH compensation point (Fig. 3; R 2 = 0.54, p < 0.01), with species having a higher pH compensation point also raising oxygen concentrations more. There was an even weaker correlation between the final oxygen concentration and the δ13C value of species (Fig. 2b; R 2 = 0.26, p < 0.01), though all species which could increase oxygen concentrations over 500 µmol kg−1 also had δ13C values equal to or more enriched than −25 ‰.
Fourteen species were examined for differences in their final pH compensation points and final [O2] between the two depths at a particular site. Zonaria angustata was the only species whose final pH compensation point was statistically lower, decreasing from 8.93 to 8.70 at 5–15 m (F 1,4 = 10.7, p = 0.03). P. comosa and Jania roseum were the only two species whose final [O2] were statistically different falling from 217 to 182 and 219 to 177 µmol kg−1, respectively, between 5 and 15 m (F 1,4 = 18.8, p = 0.01 for P. comosa and F 1,4 = 201.6, p < 0.01 for J. roseum). See Table S3, ESM 3 for more details.
Proportion cover of carbon-uptake physiological groups
There was a large difference between the proportional abundance of the three different physiological groups across different depths (Fig. 3; ANOSIM Global R = 1.0, p = 0.01). At all sites, CCM macroalgal cover decreased with depth, and at all sites other than Saint Helens Island calcareous macroalgae decreased with depth and non-CCM macroalgal proportional abundance increased with depth (Fig. 4a; Table 1). Absolute cover of non-CCM macroalgae did not increase with depth at Ninepin Point, due to a decline of reef substrate at 12 m, leading to a significant interaction between depth and site for the cover of physiological groups (Table 1). The highest absolute abundance of non-CCM macroalgae was at Tinderbox 14 m, where they made up 117 ± 8 % cover (mean ± SE). The absolute abundance of non-CCM macroalgae was also high at 5 and 14 m at Ninepin Point (Fig. 3a; 75 ± 16 and 43 ± 6 %, respectively; mean ± SE), though its proportional abundance was higher at 14 m due to the lower percent cover of macroalgae at 14 m (Fig. 3b). CCM macroalgae had over 100 % cover at Saint Helens 5 m, Fortescue Bay 5 and 14 m, and Tinderbox 5 m, and they covered the majority of space in all sites and depths except 14 m Saint Helens Island (where calcareous macroalgae covered a larger percentage of area), and 14 m Tinderbox, 5 and 14 m Ninepin Point (where non-CCM macroalgae covered a larger percentage of area).
Cover of macroalgae with CCMs, without CCMs, and calcareous macroalgae at two depths at four sites. a Proportion of macroalgal cover comprised of the three inorganic carbon-use groups. b Absolute percentage cover of macroalgae grouped by their use of inorganic carbon. Note that cover equates to the percentage of understorey plus canopy macroalgae, which can be greater than 100 % on some occasions
Even while using 10 cm2 photoquadrats, some macroalgae could not be identified visually to species level. This is because of low visibility or the fact that several species are morphologically similar (e.g. Stenogramme interrupta/Rhodymenia spp. and Kallymenia/Halymenia spp.). These data were removed from the analysis. The cover of understorey macroalgae that fell into this category was relatively low (0 % at Saint Helens, 3 % at Fortescue Bay and 1 % at Tinderbox) for all sites other than Ninepin point (13 % cover).
Discussion
This is only the second study to quantify the carbon-use strategies of seaweed assemblages, and our results are strikingly different from those of Hepburn et al. (2011) who studied a temperate reef at similar latitude in New Zealand: the percentage cover of non-CCM macroalgae at all depths at Fortescue Bay, Tinderbox and Ninepin point is larger (20–110 %). This was surprising, because non-CCM macroalgae have traditionally been thought of as being rare (Raven et al. 2002a, b). Red macroalgae have the highest proportion of species that do not possess a CCM amongst the three macroalgal phyla (Raven et al. 2005a; Raven 2010). The largest proportion of red macroalgae lacking a CCM observed in any one given area is 60 % of the species examined in Antarctica (Dunton 2001; Raven et al. 2002a); this is much lower than the 95 % of non-calcareous red macroalgae examined at Ninepin Point, 83 % at Tinderbox and 84 % examined at Fortescue Bay. The study of non-CCM species is of particular importance in understanding inorganic carbon acquisition by eukaryotic algae (Raven 2010); the lack of other quantitative studies and the identification of 64 non-CCM species in south and east Tasmania begs the question, how prevalent are ecosystems with such high proportions of non-CCM macroalgae?
Low-light environments favour the use of CO2 via diffusion over direct uptake of DIC via the CCM due to the higher energetic requirements of operating a functional CCM (Hepburn et al. 2011; Wiencke and Fischer 1990; Raven and Beardall 2014; Kübler and Raven 1994). Subtidal reefs in Tasmania (this study), southern New Zealand (Hepburn et al. 2011), and Sicily (Marconi et al. 2011) and the intertidal in Washington (Murru and Sandgren 2004) all show that the proportion of species expressing a CCM decreases with depth (i.e. with decreasing PAR; Lüning and Dring 1979). Also, within some species, individuals at lower depths rely more on diffusive CO2 than individuals of the same species growing above them, or have lower capacities to increase pH during pH-drift assays. This has previously been found with species that possess a CCM: the green macroalga Cladophora sp., the red macroalgae Mastocarpus papillatus and the brown macroalgae Padina pavonica, Desmerestia ligulata, M. pyrifera, Landsburgia quercifolia, Marginariella boryana and Colpomenia sinuosa and the non-CM red Anotrichium crinitum (Marconi et al. 2011; Hepburn et al. 2011; Murru and Sandgren 2004). Here, this was also found for species both with and without CCMs: the green macroalgae C. geminata (CCM) and C. trifaria (non-CCM), the red macroalgae Cu. angustata and P. angustum (both non-CCM), and the brown macroalgae D. muelleri and E. radiata (both CCM). The fact that non-CCM species also showed significantly depleted δ13C signatures could indicate an increased reliance on the recycling of respiratory CO2 at greater depths; however, this requires further investigation as factors such as decreased storage of carbohydrates, increased storage of lipids or increased selectivity for 12C over 13C by RuBisCO at lower irradiance/decreased growth could all cause depleted tissue δ13C values (Raven et al. 2005a, 2002b).
Three species in the brown macroalgal family Sporochnales (Perithalia caudata, S. comosus and Bellotia eriphorum) had δ13C values that indicated they did not possess a CCM. pH-drift data for S. comosus confirmed that this was correct. These are the only brown macroalgae found whose δ13C values were more depleted than −30 ‰ on all occasions across different light gradients (with P. caudata found in both 4 and 14 m at Fortescue Bay). D. anceps from Antarctica is the only other brown species that has been examined with a δ13C values more depleted than −30 ‰ from the field, and its δ13C values are inconclusive, ranging between −25.3 and −30.7 ‰ (Dunton 2001; Fischer and Wiencke 1992). Raven and colleagues (Marconi et al. 2011; Raven et al. 2005a) discuss grouping species that have an inefficient or less active CCM as having values more depleted than −25 ‰. Using −25 ‰ to indicate the absence of the CCM (or lack of activity) would be sensible for D. anceps, as well as the Antarctic brown macroalga Cystsphacea jacquinotii (−27.9 to −28.9 ‰) (Wiencke and Fischer 1990). While evidence suggests the three Sporochnales species do not have a CCM, one member of this family did (Carpomitra costata). More long-term work is needed to determine whether other specimens of the three non-CCM brown macroalgae also show evidence of a lack of a CCM in different geographic localities, or whether growing these species under higher irradiances enriches their δ13C values. This would further elucidate if these species are completely down-regulating the use of their CCM, or if they do not possess a CCM. If they do not possess a CCM, it would indicate the likely loss of the CCM in an ancestral member of this family, most likely before the split between Carpomitra and the genera of the other family members examined here (Sporochnus, Perithalia and Bellotia) estimated at ~38 million years ago (Silberfeld et al. 2010). If these species of macroalgae from the Sporochnales can indeed persist without a CCM, it indicates that the presence of a CCM is not required in low-light habitats for any type of macroalgae, regardless of their evolutionary origin. It is also possible that other unrelated brown macroalgae whose CCM status has not been examined lack CCMs, though this requires further investigation.
pH-drift assays used here were more difficult to interpret than the δ13C values, with some species having pH compensation values less than the traditional pHNBS 9, but with δ13C values more enriched than −25 ‰. One of these species (J. roseum) significantly altered AT to a point where CO2 concentrations would be equivalent to that measured at pHNBS 9 with no change in AT. We also noted that some species bleached during the assays, while others exuded substances that reduced pH and data for those species had to be excluded. In addition, it is not known how our standardized pre-treatment of all individuals at low light influenced their final compensation values, nor is it known what time-frame the up-regulation of the CCM requires to accurately measure its capacity to elevate pH. In addition, super-saturation of seawater oxygen concentrations could influence photosynthesis in these assays. While we do not caution against using pH-drift assays, these are factors for future research to consider.
It is interesting that such a large proportion of macroalgae surveyed here lacked a CCM. Low-light habitats, with large inputs of terrestrial influences, such as the Ninepin Point and Tinderbox sites here, would be expected to favour the persistence of non-CCM species. Indeed, the diversity of red macroalgae in temperate Australia, and especially of rare red species, is highest in regions near large river inputs of freshwaters (Scott 2012), likely indicating favourable conditions for non-CCM species. The fact that the E. radiata forest site (Fortescue Bay) also contained large numbers and proportions of non-CCM species was contrary to our initial expectations. This could indicate that other southern Australian kelp forest ecosystems also possess large proportions of non-CCM species. However, it must be noted that here and in Hepburn et al. (2011) that abundance rather than biomass was measured; the proportional biomass of non-CCM species will be lower than their proportional cover, due to their small size relative to some larger CCM species. To date, most research on inorganic carbon uptake has focused on easily accessible shallow systems (Raven et al. 2002b), though some exceptions exist (e.g. Runcie et al. 2008; Hepburn et al. 2011; Fischer and Wiencke 1992). Higher priority should be given to examining trends in deeper reefs that may make up the majority of kelp forest ecosystems (Nelson et al. 2014).
The abundance of non-CCM macroalgae in temperate macroalgal communities could be higher than previously thought, which has important implications for predicting the effects of ocean acidification. It has been hypothesized that non-CCM macroalgae will benefit from ocean acidification due to release from DIC limitation (Hepburn et al. 2011; Raven and Beardall 2014). If this is correct, then the proportional effects in some ecosystems, and the numbers of species affected, could be larger than previously considered. However, this hypothesis needs more empirical testing for marine macroalgae. The growth rates of the non-CCM red macroalga Lomentaria articulata increased with elevated CO2, although results were variable (Kübler et al. 1999), and at saturating irradiance the photosynthetic rates of two non-CCM species (Rhodophyllis gunnii and Schizoseris sp.) did not increase with elevated CO2 over very short time scales (Cornwall et al. 2012). The possession of strategies other than the active HCO3 − uptake (i.e. different numbers of aquaporins or high exCA activity to enable enhanced diffusion of CO2 over the plasmalemma) could decrease the magnitude of the benefits of elevated CO2 for non-CCM species (Maberly and Madsen 1998; Raven et al. 2005a). However, non-CCM macroalgae are more abundant in regions with elevated CO2 in freshwater environments (Maberly et al. 2014), which leads support to the hypothesis that non-CCM species will benefit from ocean acidification.
Research is clearly required to investigate the response of multiple non-CCM species to the effects of ocean acidification, given the greater role of non-CCM macroalgae in some communities. Communities of macroalgae already composed predominately of non-CCM species, such as those in deeper habitats and those exposed over long time periods to tannin-enriched seawater, could show net increases in productivity due to ocean acidification. In addition, the competitive ability of non-CCM species could likely increase due to ocean acidification (Hepburn et al. 2011). The viability of these low-light habitats for calcareous species (or even non-calcareous CCM species) could be reduced further in the future, if enhanced competition with non-CCM species does indeed occur. Alternatively, habitats dominated by CCM species could encounter reduced productivity, due to an increase in notably smaller non-CCM species, akin to the larger shifts from kelp to turf-dominated systems observed in more disturbed ecosystems (Connell et al. 2013).
References
Axelsson L (1988) Changes in pH as a measure of photosynthesis by marine macroalgae. Mar Biol 97:287–294
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Ciais P, Sabine CL, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quêrê RB, Myneni RB, Piao S, Thornton P (2013) Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner G-K et al (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Clementson LA, Parslow JS, Turnbull AR, Bonham PI (2004) Properties of light absorption in a highly coloured estuarine system in south-east Australia which is prone to blooms of the toxic dinoflagellate Gymondinium catenatum. Estuar Coast Shelf Sci 60:101–112
Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD (2013) The other ocean acidification problem: CO2 as a resource amongst competitors for ecosystem dominance. Phil Trans R Soc B 368:20120442. http://dx.doi.org/20120410.20121098/rstb.20122012.20120442
Cornelisen CD, Wing SR, Clark KL, Bowman MH, Frew RD, Hurd CL (2007) Patterns in the δ13C and δ15N signature of Ulva pertusa: interaction between physical gradients and nutrient source pools. Limnol Oceanogr 52:820–832
Cornwall CE, Hepburn CD, Pritchard DW, McGraw CM, Currie KI, Hunter KA, Hurd CL (2012) Carbon-use strategies in macroalgae: differential responses to lowered pH and implications for ocean acidification. J Phycol 48:137–144
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for Ocean CO2 measurements. North Pacific Marine Science Organization, Sidney
Dunton KH (2001) δ15N and δ13C measurements of Antarctic Peninsula fauna: trophic relationships and assimilation of benthic seaweeds. Am Zool 41(1):99–112
Fischer G, Wiencke C (1992) Stable carbon isotope composition, depth distribution and fate of macroalgae from the Antarctic Peninsula region. Polar Biol 12:341–348
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL (2011) Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob Change Biol 17:2488–2497
Kevekordes K, Holland D, Häbner N, Jenkins S, Kos R, Roberts S, Raven JA, Scrimgeour CM, Shelly K, Stojkovic S, Beardall J (2006) Inorganic carbon acquisition by eight species of Caulerpa (Caulerpaceae, Chlorophyta). Phycologia 45:442–449
Korb RE, Raven JA, Johnston AM, Leftley JW (1996) Effects of cell size and specific growth rate on stable carbon isotope discrimination by two species of marine diatom. Mar Ecol Prog Ser 143:283–288
Kroeker KJ, Kordas RL, Crim RN, Hendriks IE, Ramajo L, Singh GG, Duarte CM, Gattuso JP (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19(6):1884–1896
Kübler JE, Raven JA (1994) Consequences of light limitation for carbon acquisition in three rhodophytes. Mar Ecol Prog Ser 110:203–209
Kübler JE, Johnston AM, Raven JA (1999) The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant, Cell Environ 22:1303–1310
Lee D, Carpenter SJ (2001) Isotopic disequilibrium in marine calcareous algae. Chem Geol 172:307–329
Lepoint G, Nyssen F, Gobert S, Dauby P, Bouquegneau JM (2000) Relative impact of a seagrass bed and its adjacent epilithic algal community in consumer diets. Mar Biol 136:513–518
Lüning K, Dring MJ (1979) Continuous underwater light measurements near Helgoland (North Sea) and its significance for characteristic light limits in the sublittoral region. Helgol Mar Res 32:403–424
Maberly SC (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. Phycologia 26:439–449
Maberly SC, Madsen TV (1998) Affinity for CO2 in relation to the ability go freshwater macrophytes to use HCO3 −. Funct Ecol 12:99–106
Maberly SC, Berthelot SA, Stott AW, Gontero B (2014) Adaptation by macrophytes to inorganic carbon down river with naturally variable concentrations of CO2. J Plant Phys 172:120–127
Marconi M, Giordano M, Raven JA (2011) Impact of taxonomy, geography, and depth on δ13C and δ15N variation in a large collection of macroalgae. J Phycol 47:1023–1035
Murru M, Sandgren CD (2004) Habitat matters for inorganic carbon acquisition in 38 species of red macroalgae (Rhodophyta) from Puget Sound, Washington, USA. J Phycol 40:837–845
Nelson W, Neill K, D’Archino R, Anderson T, Beaumont J, Dalen J (2014) Beyond diving depths: deepwater macroalgae in the New Zealand region. Mar Biodiv. doi:10.1007/s1256-014-0293-5
R Core Development Team (2008) R: a language and environment for statistical computing. 2.7.0 edn. R Foundation for Statistical Computing, Vienna
Raven JA (1991) Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature. Plant, Cell Environ 14:779–794
Raven JA (1997) Putting the C in phycology. Eur J Phycol 32:319–333
Raven JA (2010) Inorganic carbon acquisition by eukaryotic algae: four current questions. Photosynth Res 106:123–134
Raven JA, Beardall J (2014) CO2 concentrating mechanisms and environmental change. Aquat Bot 118:24–37
Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Clayton MN, Vanderklift MA, Fredriksen S, Dunton KH (2002a) Seaweeds in cold seas: evolution and carbon acquisition. Ann Bot 90:525–536
Raven JA, Johnston AM, Kübler JE, Korb RE, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Vanderklift MA, Fredriksen S, Dunton KH (2002b) Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct Plant Biol 29:355–378
Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC (2005a) Algae lacking carbon-concentrating mechanisms. Can J Bot 83:879–890
Raven JA, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A (2005b) Ocean acidification due to increasing atmospheric carbon dioxide. The Royal Society, London
Raven JA, Giodarno M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109:281–296
Raven JA, Beardall J, Giordano M (2014) Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth Res 121:111–124
Runcie JW, Gurgel CFD, McDermid KJ (2008) In situ photosynthetic rates of tropical marine macroalgae at their lower depth limit. Eur J Phycol 43(4):377–388
Scott FJ (2012) Rare marine macroalgae of southern Australia. University of Tasmania, Hobart
Silberfeld T, Leight JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F (2010) A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the “brown algal crown radiation”. Mol Phylogenet Evol 56:659–674
Steneck RS, Graham MH, Bourque BJ, Corbett P, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Tegner MJ, Dayton PK (2000) Ecosystem effects of fishing in kelp forest communities. ICES J Mar Sci 57:579–589
Wiencke C, Fischer G (1990) Growth and stable carbon isotop composition of cold-water macroalgae in relation to light and temperature. Mar Ecol Prog Ser 65:283–292
Acknowledgments
We thank S. Ling, A. Fowles, F. Scott, D. Britton, E. Flukes, C. Layton, M. Cameron, M. Taksumi, J. Kean and T. Baulch for assistance in the field, laboratory or with identification of species. We also thank E. Flukes and C. Johnson for providing the irradiance estimates for Fortescue Bay. Samples were collected using the Department of Primary Industries, Parks, Water & Environment Tasmania permit number 13120.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cornwall, C.E., Revill, A.T. & Hurd, C.L. High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynth Res 124, 181–190 (2015). https://doi.org/10.1007/s11120-015-0114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0114-0