Abstract
While some researchers have predicted a fertility transition in sub-Saharan Africa similar to that witnessed in Europe, Latin America, or Asia, where practices of fertility limitation and long-term contraception have played a major role, others believe that it will differ. The aim of this article is to contribute to this debate, testing Caldwell's prediction of comparable declines in fertility in Africa at all ages through the adoption of modern contraceptives. We examine the cases of Dakar, Ouagadougou, and Nairobi, three capital cities where fertility is now relatively low (around three children per woman in 2010–2015), drawing on the various available data sources going as far back as the early 1970s. The data for these three cities confirm the hypothesis of a similar fertility reduction across all ages. However, Nairobi is the only city where contraception is the main strategy adopted by all age groups. Sexual non-exposure outside of the postpartum period played a key role in attaining low family sizes at younger and older reproductive ages in Ouagadougou and Dakar, as well as postpartum insusceptibility in Dakar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While all other world regions have already completed their fertility transition, the decline in sub-Saharan Africa began only in the late 1980s. Since then, the rate of decrease has been slow, and the total fertility rate (TFR) remains high (5.1 in 2015) (Casterline, 2001; Bongaarts & Casterline, 2013; UN, 2015). The relative universality of the socioeconomic factors behind fertility decline (Kravdal, 2002; Bongaarts, 2003; Gurmu& Macer, 2008; Shapiro & Gebreselassie, 2009; Assefa & Semahegn, 2016) (the question of why) makes it all the more interesting to investigate variations in their mode of transmission (the question of how) in different regions and countries in response to the cultural, technological, and institutional characteristics specific to each context (Doyle, 2013; Johnson-Hanks et al., 2011; Mason, 1997).
Fertility reduction in developing regions has been mainly attributed to stopping and the use of modern contraception, often long-acting methods such as IUDs and sterilization later in reproductive life (Grace & Sweeney, 2013; Hirschman & Young, 2000; Hosseni et al., 2014; Ishida et al., 2009; Jadhav & Vala-Haynes, 2018; Joshi et al., 2015; Mason, 1997). However, analyzing the first signs of decline at the national level in Africa, Caldwell et al. (1992) argued that African fertility transitions would be different. They posited that Africa would experience a new type of demographic transition, marked by modern contraceptive as well, but fertility decline at all ages. Recently, Caldwell's intuitions have been substantiated: Johnson-Hanks (2007), Timaeus & Moultrie (2008, 2013, 2020), Lerch & Sporenberg (2020), and Millogo (2020) have underscored the main role played by lengthening birth intervals in African fertility transitions; they highlighted the adoption of modern contraception to this effect particularly in Eastern and Southern Africa.
Despite this consensus on the role of birth spacing, Bongaarts & Casterline (2013) found that in Africa, older women's contribution to the overall number of children was no different from elsewhere. This result seems to invalidate Caldwell's hypothesis of equal fertility reductions at all ages in Africa, in contrast to the concentrated reductions toward the end of reproductive life seen in other regions. Moreover, the uptake of modern contraception has perhaps been less successful than Caldwell anticipated, especially among younger women (Alkema et al., 2013; Garenne, 2017a, 2017b): African women wishing to avoid pregnancy may use other methods. Bongaarts (1978) identified three other proximate factors which can have massive effects on the level of general fertility: sexual non-exposure, induced abortion, and postpartum insusceptibility. One of these factors, or all of them, may be additional drivers of the transition in sub-Saharan Africa at certain ages.
Contraceptives are the main driver of fertility reduction but only among women aged 25–34 years (Jolly & Gribble, 1993). At the entry of the reproductive life course, age at young women's sexual debut, union formation, and first childbirth have been increasingly delayed in African countries in recent decades (Clark et al., 2017; Mensch et al., 2006); sexual non-exposure may thus play a great role in inhibiting fertility at young ages as observed in North Africa (Kateb & Ouadah-Bedidi, 2002; Zagaglia, 2013). Moreover, contrary to other regions, women in Africa appear to use abortion mainly at the beginning of their reproductive life (Guillaume & Rossier, 2018; Shah & Åhman, 2012). At the other end of the life course, DHS data show that the median duration of insusceptibility remains long among women aged 30 years and above in sub-Saharan Africa, although it has been decreasing. Moreover, a relatively large share of women in union are also sexually inactive after age 40 (Peytrignet, 2019).
The first objective of this paper is to formally test the hypothesis of a similar fertility decline at all ages. To do so, we study three African capitals. As fertility is now relatively low in these cities, tracing fertility over time since the 1970s will allow us to observe nearly complete fertility transitions. Focusing on a few cities enabled us to reconstitute the historic series. Our second objective is to measure the impact of the various proximate determinants of fertility at different ages and to identify those which played the greatest role over the period 1990–2010 in these three cities. We will test whether, as predicted by Caldwell, fertility declined at all ages similarly in these three cities, and this is due to the use of modern contraception at all ages.
Study Populations and Context
We will examine the cases of Dakar, the capital of Senegal; Ouagadougou, the capital of Burkina Faso; and Nairobi, the capital of Kenya. All three cities are more affluent than the rest of their respective countries, and all currently have relatively low fertility. The choice of these contexts is linked not only to the availability of data, but also to our familiarity with their economic and demographic situations. In 2013, Dakar had 3 million inhabitants lived in urban areas (ANSD, 2014), and fertility in the city was 3.0 children per woman in 2015–17 (ANSD and MI Inc. 2018). Ouagadougou (the capital of Burkina Faso) had 2 million inhabitants in 2012, (according to the 2012 Population enumeration of Ouagadougou and Bobo Dioulasso), and fertility was 3.4 children per woman in 2008–10 (INSD and MI Inc. 1994, 2012). The last of the three cities in the study, Nairobi (capital of Kenya), had 3 million inhabitants in 2009 (KNBS, 2010a, 2010b), with a mean of 2.7 children per woman in 2012–14 (KNBS and MI Inc., 2010, 2015).
We choose these three capitals because their situations are quite diverse. Kenya and Senegal have an economic advantage over Burkina Faso: the three countries' GDP per capita in 2017 was 1595, 1329, and 642 USD, respectively (World Bank, 2019). In terms of schooling, the populations of francophone African countries remain among the least educated in the world. In contrast, in 1980, primary school enrollment in Kenya was close to 100% (Omwami & Omwami, 2010).
Furthermore, the first private family planning clinics were established in Kenya (Nairobi and Mombasa) in 1952 (Fendall & Gill, 1970). By the early 1980s, the country had achieved some success in introducing family planning practices: the country's first population policy had been adopted in 1969 (Population Council, 1969; National Research Council, 1993). After the 1994 Cairo conference, Kenya established a sexual and reproductive health strategy to combat HIV-AIDS, and in 2003, it adopted a sexual health policy for teenage girls (Oranje & Zulu, 2015). The prevalence of modern contraception among women in union in Nairobi grew from 46.8% in 1998 to 49.0% in 2008 and 58.3% in 2014, with a mean of 2.7 children per woman in 2012–14 (KNBS and MI Inc., 2010, 2015).
In Burkina Faso, despite the promotion of birth spacing and the fight against the exclusion of students who became pregnant in schools were started in 1979 by the NGO ABBF,Footnote 1 population policies got off to a much later and slower start. The National Population Council, tasked with conceiving and proposing a population policy, was set up in 1983. The first family planning policy was adopted in 1986, but due to a lack of resources and the low priority given to the issue, these efforts bore little fruit (Conseil National de Population 2000). Notable progress was observed only in the 2010 decade, after the Ouagadougou Conference in 2011 re-positioning of contraception. The prevalence of modern contraception among women in union in Ouagadougou grew from 19.9% in 1993 to 32.6% in 2010.
In Senegal, in 1970, family planning was first offered in the private Clinic “Clinique Croix Bleue” but the national family planning program was created in 1990, even later than in Burkina Faso (Senegalese Ministry of Health and Social Action 1990, cited by Diallo, 2014; Petit & O’Deye, 2001). Family planning in Senegal nonetheless received new impetus at the same time and in the same context as in Burkina Faso. In 1992–93, contraceptive prevalence was 11.8% among city-dwelling women in union in Senegal; in Dakar, it was estimated at 21.4% in 2010–11 and at 42.3% in 2017 (ANSD and MI Inc., 1994, 2012, 2018).
Data and Sample
The data used to answer the research questions are drawn from the Demographic and Health Surveys (DHS), along with complementary data from population censuses and from a demographic surveillance site. The DHS surveys began in 1984 in Kenya, in 1986 in Senegal, and in 1993 in Burkina Faso. The most recent DHS was carried out in 2017 in Senegal (DHS Continuous), in 2014 in Kenya, and in 2010 in Burkina Faso. In order to extend the series for Burkina Faso over the recent period, notably for the first research question, we used data from the Ouagadougou Health and Demographic Surveillance siteFootnote 2 (Ouaga HDSSFootnote 3) (Rossier et al., 2012). The HDSS is located in northern Ouagadougou and is not representative of the entire city, but fertility measured in 2009–10 (a total fertility rate-TFR of 3.1) and contraceptive prevalence were close to the levels observed by the DHS in Ouagadougou as a whole. To extend the series further into the past, for Dakar we use Senegal 1970–71 National Demographic Survey (EDN) (Ferry, 1976) and the 1978 Senegal Fertility Survey. For Ouagadougou, we use Burkina Faso 1976 post-census survey and Burkina Faso 1985 census (INSD, 1984 and 1989). For Nairobi, the Kenya 1979 census (CBS 1996) and the 1977–78 Kenyan Fertility Survey (KFS) (Cross et al., 1991) provide past data series for the first research question.
Before estimating the factors that contributed to fertility reduction by age over the period 1990–2010, the size of the different samples must be established (Table 1). That table presents the sub-samples of weighted Age-specific fertility rates and births from the two DHS surveys carried out in the early 1990s and the late 2000s in the three cities. The Nairobi samples are the smallest (in 1993, the three oldest age groups had to be combined to calculate the duration of insusceptibility). The total sample sizes are large enough to calculate all the aggregate indices for women aged 15–49 years.
Methods
Age-Specific Fertility
Using the DHS data, we calculated age-specific fertility rates by dividing the number of live births in the last five years by exposure in 5-year age groups (Schoumaker, 2013). Confidence intervals are calculated directly in DHS and WFS data, by stata fr2 command (Ibid.) or by using normal approximation of the binomial if total births in each age group is 100 or more else Poisson approximation (Taylor & Morrell, 2015). The mean rates were estimated over five-year periods in order to remedy the problem of the small sample sizes in the DHS data which could potentially lead to an underestimation of TFR. Due to a severe underestimation of births, the directly estimated TFRs between the late 1990s and the early 2000s were re-calculated by reconstructing them on the basis of the (following) DHS of the late 2000s (Schoumaker, 2009). Recent rates in Ouagadougou were estimated using data from the Ouaga HDSS but for 1970s and 1980s, TFR was indirectly estimated by Gompertz relational model. The fertility rates for the 1970s in Nairobi were drawn from the 1979 census, which clearly underestimated fertility in the city: the TFR calculated from the Kenya Fertility Survey in 1975–78 was 6.1, whereas the census-based rate calculated for the period 1969–1979 was only 4.9. But assuming that the underestimation of births in the census was randomly distributed across ages, this should not affect results on the regularity of fertility reduction.
Contributions of Different Age Groups to the Mean Number of Children
To answer our first question on the contributions of the different age groups to fertility reduction, we adapted the method of Bongaarts & Casterline (2013). This entails calculating the contribution to the TFR of each age group in successive surveys, and dividing it by that of a reference year, using the following equation:
where \({T}_{xi}^{j}\) is the fertility rate of age group x in year i and city j; i = 0 indicates the reference year, and \({TFR}_{i}^{j}\)—the total fertility rate. The value of α is 1 in a situation where all the curves are exactly proportional. We consider the open interval 0.5–2.0 as a good approximation of proportionality to deal with the fluctuations in the samples; outside this interval the ratio of rates is more than double or less than half. In addition, a statistical test of proportionality ratios was calculated in each city on a matrix whose lines are the age groups, whose columns are years and whose values are derived from Eq. (1).
Stover's Model
Estimation of Stover's Model
The second part of the paper measures the impact of proximate determinants on fertility at different ages in the early 1990s and the late 2000s. These analyses were performed using Stover's (1998) model, with five component indices: sexual exposure, postpartum insusceptibility, contraception, abortion, and infertility. The model uses total potential fertility (Pf) and multiplies it by the above indices in order to obtain the mean number of children. We chose this model over that of Bongaarts (2015) because we believe it deals better with the question of exposure to pregnancy.Footnote 4
Stover's model is a multiplicative model which uses five proximate determinants, whose fertility-inhibiting effects are estimated by the five indices defined below. The number of children per woman is estimated as (6) \( \widehat{TFR}={C}_{x}\times {C}_{f}\times {C}_{a}\times {C}_{i}\times {C}_{u}\times Pf\). This model, like that of Bongaarts, can be applied to specific age ranges. In this case, sexual activity (Cx), infertility (Cf), and contraceptive use (Cu) are identical to those in the aggregate model. The equation for age-specific postpartum insusceptibility (Ci) is also identical to the aggregate equation, but age-specific values are used for the mean duration of insusceptibility; the same applies to age-specific values for potential fertility (Stover, 1998). Until now, the model version with age-specific parameters has rarely been used, notably because relatively large sample sizes are needed (Casterline et al., 1984; Singh et al., 1985).
Sexual Exposure Index (Cx)
The sexual exposure index expresses the fertility reduction attributable to the fact that women (whether married or not) are not always sexually active. The idea is to measure the fertility-inhibiting effect of sexual inactivity through the proportion of women who have recently had sexual relations, adding those who have recently been pregnant. Among the latter, recent (or less recent) sexual activity (through pregnancy or postpartum abstinence) evidently does not influence the probability of having recently been pregnant. The same issue has been raised about the marital fertility of subgroups of Iranian married women who are sexually inactive due to the seasonal migration of their husbands or some married couple who do not live together due to socioeconomic or cultural reasons (Erfani & McQuillan, 2008). A woman (among those who are not pregnant nor postpartum abstinent) is sexually active if she has had sexual relations at least once in the last month (here, the last 30 days). The idea behind this index is to offset the overestimation of sexual activity produced by Bongaarts's (1978) marriage index. Cx = p is the proportion of women of reproductive age who are sexually active (or were recently became pregnant or postpartum abstinent); when commenting on this index, we use the term "sexual exposure".
Postpartum Insusceptibility Index (Ci)
This index measures the fertility-inhibiting effects of postpartum abstinence and amenorrhea. It is expressed, for women aged 15–49 years, by (2) \({C}_{i}=20/(18.5+i)\) where i is the mean duration of postpartum insusceptibility in months, calculated using a lifetime method on women who have had live births within the last 36 months (Rutstein and Rojas, 2006). Stover (1998) defines the values and parameters in (2) in the same way as in the age-specific version (2b) \({C}_{i}=BI/(BI-1.5+i)\). BI is the mean interval between two births in the absence of breastfeeding, contraception, and postpartum abstinence. When the weighted sample for a given age group of women is small, it is combined with an adjacent group to obtain a weighted sample of at least 30 individuals. We combined groups for this reason mainly in the case of Nairobi, merging two to three age groups to estimate the mean duration of postpartum insusceptibility, a component of the corresponding index.
Contraception Index (Cu)
This index measures the number of children avoided through the use of contraception other than sterilization (which is uncommon in Africa). It is expressed as (3) \({C}_{u}=1-u\times e\), where u is the proportion of sexually active, fertile women who are not amenorrheic (or postpartum abstinent) and who are using contraceptives. In the present article, we take the same approach as Stover, excluding only women who use contraceptives and are amenorrheic during the first 5 months after childbirth.Footnote 5 In (3), e represents the efficacy of the method used; the values used are those given by Bongaarts (1992).
Infecundity/Sterility Index (Cf) and Abortion Index (Ca)
The infecundity/sterility index is expressed by (4) Cf = 1–f, where f represents the proportion of women who are sexually active but infertile. The abortion index is calculated as \({C}_{a}=TFR/(TFR+0.4\times \left(1+u\times e\right)\times TAR)\) where u is contraceptive prevalence, e is contraceptive efficacy, and TAR is the abortion rate. The abortion index is not calculated here due to the lack of (sufficiently robust) data by age for African contexts, and the infecundity/sterility index, which has contributed only marginally to fertility reduction in the contexts studied here, will be discussed only briefly.
Potential Fertility (Pf)
Potential fertility (Pf) is the total number of births that a woman can have in the absence of all limiting factors (including intrauterine mortality). Stover estimates a figure of 21 births per woman. Given that almost all the factors with the potential to reduce the number of children are taken into account through the different indices, we use Stover's estimate. Bongaarts, in his model, uses the total fecundity rate (TF), which he estimated at 15.3 children per woman on the basis of historical populations; this value takes into account intrauterine mortality.
All of the calculated indices were weighted, and the Stata software package was used to analyze the data.
Results
Did the Fertility Reduction Observed in Dakar, Ouagadougou, and Nairobi Result from Equivalent Reductions in Fertility at All Ages?
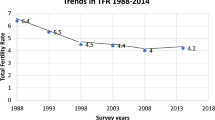
Fertility decreased in different ways over time in the three capitals, Dakar (Senegal), Ouagadougou (Burkina Faso), and Nairobi (Kenya), beginning earlier in Nairobi, and later in Dakar and Ouagadougou. In the 1970s, the TFR in the three cities was above 6.0; by 1988–92, it had fallen to 5.0 in Dakar, and to 4.7 in Ouagadougou, but had already dropped to 3.4 in Nairobi (Fig. 1). The pre-transition increase in Dakar has often been observed elsewhere (Pison et al., 1995). In the early 1990s, the fertility levels in Dakar and Ouagadougou were similar to those of Nairobi (estimated at 4.5) in 1984–88. While fertility in Nairobi fell below three children per woman in the early 2000s, this only happened in 2014 in Ouagadougou, when, according to HDSS Ouaga data, the TFR fell to 2.9. The TFR in Dakar is still above 3.0 (3.3 for the period 2010–15 as estimated from the continuous DHS). Fertility in Nairobi seems to have increased slightly in the early 2010s. There was virtually no underestimation of births (verified by our calculations) five years earlier, so this stagnation can be considered as real (Bongaarts & Casterline, 2013; Casterline, 2001; Schoumaker, 2009, 2019).
Fertility before age 20 was higher in Nairobi than in Ouagadougou and Dakar over the whole period but in 1970s, there is not difference across cities (crossing of confident intervals) (Fig. 2 and Appendix Table 3): due to small sample sizes, confidence intervals for Nairobi are large. However, as fertility decreased more quickly among adolescents in Nairobi than in the two other cities, the gap narrowed over time. Between the mid-1980s and the 2010s, fertility fell most sharply among women aged 20–24 in Ouagadougou and Nairobi, and those aged 30–34 in Dakar. The number of children per 1000 women aged 20–24 years fell by 60 and 90 in Ouagadougou and Nairobi, respectively, and by around 80 per 1000 among women aged 30–34 years in Dakar. With respect to the 1970s, while the age group with the fastest fertility reduction has remained the same in Ouagadougou and Nairobi (with Kenya 1979 population census), it has shifted from women aged 30–34 to those aged 25–29 in Dakar and Nairobi when we refer to KFS.
These data on age-specific fertility reduction confirm the absence of a large drop at more advanced ages (crossing of confidence intervals since mid-1980s in Dakar and Ouagadougou and mid-1970s in Nairobi), the principal characteristic of fertility reduction in historical Europe, Asia, and Latin America. Birth stopping nonetheless seems to have played a more important role in Nairobi than in the two other cities, with a larger decline in fertility rates after age 30. But at the end of fertile life, as expected, the level of fertility is similar in all three cities. Generally speaking, the curves of fertility by period seem to have shifted downwards quite evenly. Does this mean that the contributions of women of each age to general fertility have remained the same over time?
We checked the constancy of the contributions of different age groups to fertility using Eq. (1) presented in the Methods section above. As previously mentioned, ratios close to 1.0 would confirm Caldwell et al.'s (1992) hypothesis (Bongaarts & Casterline, 2013). The ratios were between 0.62 and 1.25 in Dakar, and between 0.55 and 1.29 in Ouagadougou; in both contexts, they were highly concentrated between 0.9 and 1.1 (Fig. 3). In Nairobi, however, in both the early and late 1990s, the ratio was very different from 1.0 at ages 40–44 (Fig. 3). One of these two values was below 0.5 in the early 1990s (the contribution seems to have decreased by half in the late 1990s with respect to 1984–1988), and the other above 2.0 in the late 1990s (the contribution seems to have doubled in the late 1990s with respect to 1984–1988). But these seem to be age-linked errors, as the situation later returned to more moderate levels. This hypothesis is more visible in 1988–1993, when the shift in the ratio beyond the upper end of the range for women aged 40–44 seems to have led to the opposite shift for women aged 35–39. Assuming this is correct, we may thus conclude that fertility reductions were of equal amplitude across ages in these three relatively lower-fertility African contexts. In addition, with a probability close to 1 in Dakar and Ouagadougou and of 0.99 in Nairobi, the chi2 test on equal proportions for age groups confirms that the decline is of equal magnitude at each age. These data thus confirm Caldwell's hypothesis of equivalent fertility reductions in Africa at all ages, rather than a pattern mainly reflecting family size limitation (with the largest reductions at advanced ages), for these three capital cities, as can be expected from the diffusion on long births intervals observed in the region (Timaeus & Moultrie, 2008, 2013, 2020, Lerch & Spoorenberg, 2020; Millogo, 2020).

Source: DHS for Senegal, Kenya and Burkina Faso, EDN, Kenyan population census, Burkina 1976 post-census survey and Ouaga DHSS; own calculations. * Reference for the calculation of ratios: Dakar 1971–72, Ouagadougou: 1988–1992, Nairobi: 1984–88
Ratio of each age group's contribution to the TFR in different periods to their contribution to the TFR for a reference date.
Low Fertility in the Three Capitals: The Impact of Proximate Determinants, 1990–2010
Was the spread of contraception responsible for the fertility reductions observed at different ages in these three capital cities? In other words, is contraception the main contributor to fertility inhibition (all ages combined), and to the same extent at each age? This part of the analysis is limited to the timespan for which data are available for the three capitals, i.e., the two decades between the early 1990s and the late 2000s, a period when the TFR decreased from 5.0 to 3.6 in Dakar, from 4.7 to 3.3 in Ouagadougou, and from 3.4 to 2.8 in Nairobi.
We began by estimating the number of children avoided due to each proximate determinant in each of the three capitals in two different periods (early 1990s and late 2000s). Note that the effect of abortion, which is also an important factor, is not measured here due to the lack of reliable data (we will get back to the question of its impact in the discussion). In Stover's model, estimation of overall TFR (15–49 years) takes priority over estimation by age group (before summation), in order to avoid overestimation due to the smaller numbers mentioned above. The estimation of the model for each period yields a difference of one child at most with respect to the TFR calculated for the 3 years preceding the survey. In the late 2000s, the gap between the estimated and measured TFR was + 0.3 for Dakar and Ouagadougou, and + 0.9 for Nairobi (Table 2). In the early 1990s, the difference was –0.8 for Dakar, –0.4 for Ouagadougou, and + 1.0 for Nairobi. Overall, the model estimated constant fertility between 1990 and 2010 in the three capitals, which fails to reflect the observed reductions. However, as the differences between observed and estimated TFRs remain modest, Stover's model offers workable approximation of the contribution of each proximate factor for the period taken as a whole. To test whether contraception is the main fertility-inhibiting factor at all ages, we nevertheless focus on the 2010 period only.
The same calculation (Table 2) indicates that the factors avoiding at least two children per woman in Dakar in 2010–11 were sexual non-exposure (4.5 children avoided), followed by postpartum insusceptibility (2.1), and last, contraception (1.8). For 1992–93, the order of these factors in Dakar was the same, the corresponding figures being 3.9, 2.2, and 2.0 children avoided. In Ouagadougou in 2010, sexual non-exposure reduced TFR by 3.0, contraception by 2.8, and postpartum insusceptibility by 2.0. In 1993, insusceptibility was the most important factor (3.1), followed by contraception (2.8) and then sexual inactivity (2.4). In 2008–09 in Nairobi, the factors that decreased fertility by at least 2.0 children were contraception (4.1) and sexual inactivity (3.0). These two factors were also the most important in 1993, with associated reductions in the TFR of 4.5. (this number is slightly lower in 2008–2009 although the prevalence of contraception increased because the model estimates a "constant" fertility, and because sexual inactivity increased faster than contraception) and 2.5, respectively.
This first analysis of the number of children avoided in 1990–2010 in the three cities for all age groups combined shows that sexual non-exposure played a very important role in Dakar and an important role in the two other cities; that postpartum insusceptibility played a moderate role in Ouagadougou and a moderate, but slightly smaller role in Dakar; and finally, that contraception played an important role in fertility reduction in Nairobi, a moderate role in Ouagadougou, and a moderate, but smaller role in Dakar.
The Effect of Proximate Determinants by Age in the Three Capitals in the Late 2000s
This raises the question of how the effects of proximate factors are distributed across different ages. Is the effect of sexual non-exposure limited to younger women, and does it primarily represent non-exposure to marriage, which occurs relatively late? Individuals of which age groups have adopted contraception? Are all ages equally affected by postpartum insusceptibility? Here, we examine the situation in the late 2000s.
Sexual Exposure Index
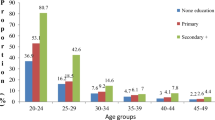
Figure 4 indicates the proportions of women who were sexually active (including women who were pregnant or postpartum abstinent, because they had been recently active) in the three cities in the late 2000s, by age. As seen above for the number of children avoided for all ages combined, this index was lower (i.e., the effect was stronger) for Dakar in the late 2000s in comparison with the other two cities. In this period, its value was 0.45 for Dakar and 0.54 for Ouagadougou and Nairobi, i.e., the proportion of women aged 15–49 who were sexually inactive (and who were neither pregnant nor postpartum abstinent) in these cities was 55% and 46%, respectively. Looking at the figures by age group in the late 2000s, sexual inactivity was greatest before age 25 in all three cases, followed by women aged 40 and above in Dakar and Ouagadougou, and those aged 45 and above in Nairobi.
Postpartum Insusceptibility Index
Figure 5 shows the effect of postpartum insusceptibility on age-specific fertility in the late 2000s in Dakar, Ouagadougou, and Nairobi. As seen with the number of children avoided, the strongest effect of this factor (and thus the lowest index) in the late 2000s was observed in Dakar and Ouagadougou. The protection offered by insusceptibility was quite evenly distributed across ages in Ouagadougou, while in Dakar and Nairobi, this factor was most protective at early and intermediate ages (before age 35 or 40, depending on the case).
Contraception Index
Regardless of age, the effect of this index was substantially larger (i.e., it had a lower value) in Nairobi, followed by Ouagadougou, and finally Dakar. Its value in the late 2000s was estimated at 0.46, 0.55, and 0.68 for the three cities, respectively. In Ouagadougou and Nairobi, the effect of contraception on fertility was practically the same at all ages, with the exception of women aged 30–34 in Ouagadougou, where it was largest. In Dakar, the effect was largest among women of intermediate ages, between 25 and 40 years.
A comparison of the negative effect of these factors on age-specific fertility shows that in Dakar in 2010–11 the most important factor for all ages (combined or separate) was sexual inactivity (Figs. 4, 5, 6). Below this factor, postpartum insusceptibility and contraception had comparable effects at all ages, except among women aged 30–44, among whom contraception was the most important factor after abstinence. In Ouagadougou in 2010, the principal factor for women aged 15–24 was sexual inactivity, followed by contraception. The main factor among women aged 25–49 years in this city was contraceptive use; the two other factors had similar effects at other ages, with the exception of postpartum insusceptibility which was the second most important factor at ages 35–39, and sexual inactivity from age 40 onward. In Nairobi, in 2008–09, before age 20, sexual inactivity was the most important factor, followed by contraception; at ages 20–24 years, contraception and sexual inactivity had the same effect; and after age 25, contraception was the most important factor, followed by sexual inactivity. In the late 2000s, postpartum abstinence and amenorrhea still represented the second most important strategy for regulating fertility at certain ages in Dakar (among women below 30) and in Ouagadougou (at ages 35–39), whereas in Nairobi, it was the last resort.
In sum, this second part reveals different situations in the three cities in the late 2000s. Notably, in Dakar and Ouagadougou, contraception played only a limited role at certain ages; Nairobi is the only city that confirmed the hypothesis of the leading role of contraception in fertility avoidance (first for all ages combined, second at each age). In Nairobi, contraception was the main fertility inhibitor; its effect was the same at all ages, and in comparison to other strategies its effect was the largest at all ages, except among women in the earliest phase of sexual activity (aged 15–19 years), for whom the principal factor was abstinence or infrequent sexual relations. In Ouagadougou, the most important strategies were contraception and infrequent sexual relations, with similar effects. Comparing ages, the effect of contraception was largest at ages 30–34, but identical and lower at other ages; comparing strategies, it was the most often used at ages 25–49. Infrequent sexual relations had a greater effect among women under 25. In Dakar, infrequent sexual relations contributed the most to avoiding fertility. Contraception was practically the lowest-ranked strategy; only among women aged 30–44 years did it rank second, ahead of insusceptibility.
Discussion
We have shown that the contributions of women in different 5-year age groups to the total fertility rate remained constant over more than 3 decades of decreasing fertility in three African capital cities (Dakar, Ouagadougou, and Nairobi). It remains to be determined whether other large African cities have followed the same pattern. This result, if it were to prove general, would offer strong indications of future trends on the continent as a whole. Cities, where populations are more educated and more affluent than in rural areas, are precursors on the path of demographic transition (Coale, 1974; Oris, 1995) in Africa and elsewhere. In historical Europe, decreasing fertility in rural areas followed the trend initiated in the cities (Oris, 1988; Perrenoud, 1989; Beauvalet-Boutouyrie, 1990).
In a test of Caldwell's hypothesis with two age categories (below 30 and 30 or above), Bongaarts & Casterline (2013) failed to find any difference between Latin America, Asia, and sub-Saharan Africa in patterns of fertility reduction by age. What might explain the discrepancy between their findings and ours? One hypothesis is that this division into two age groups may have obscured the patterns of disparities revealed by the finer-grained division into seven age groups used in the present study; the figure in Appendix Table 4 shows that division into two age classes has the effect of bringing all indices closer to 1.0 (values between 0.8 and 1.3, with a concentration around 1.0). Alternatively, our results may mean that Bongaarts & Casterline's, 2013 article showed that fertility reductions in Latin America and Asia were also distributed across all age groups, contrary to the consensus in the literature that long prevailed. In line with this suggestion, Casterline & Odden (2016) and Timaeus & Moultrie (2020) showed that longer birth spacing seems to have played a pronounced role in reducing fertility in Asia and Latin America, as in Africa. However, verifying this point is beyond the scope of the present study.
We also examined whether the low fertility levels attained in these cities around 2010 could be attributed to modern contraceptive uptake at all ages. Our results show that the situation is not only highly diverse, but that fertility seems to have declined in certain cities and at certain ages with little contribution from contraceptive use. In 2010, the avoidance of births in Dakar still followed a traditional pattern: even in a nearly low fertility regime, the most important fertility inhibitor was sexual non-exposure (low frequency of sexual relations) at all ages, followed by postpartum insusceptibility. In Ouagadougou in 2010, the factors with the largest (and equal) negative impact on fertility were sexual inactivity (most prevalent among the youngest women, but also the oldest) and contraception (practiced mainly by women at ages 30–34, and less, but equally, at all other ages). Postpartum insusceptibility (equally prevalent at all ages) played a moderate role. In Nairobi in 2008–09, contraception (practiced “equally” at all ages) and sexual non-exposure (greater at ages 15–24) were the strongest fertility inhibitors.
The possible role of sexual inactivity in fertility reduction in the three cities is easily understood in light of the shift toward later entry into union for young women. In all African contexts, the frequency of sexual activity has decreased considerably among girls and young women, a trend closely linked to union postponement (Clark et al., 2017; Delaunay & Guillaume, 2007). This trend is explained not only by increasing levels of education and duration of schooling, but also by men's greater reluctance to marry, as revealed in Senegal by Antoine (2006). It seems that growing unemployment and men's increasingly late labor market entry have led to tension on the marriage market for women. Given the strong stigma attached to sexuality outside marriage in Senegal (and to a lesser extent also in Ouagadougou and Nairobi) levels of abstinence are high among girls and young women (Bajos et al., 2013; Diallo, 2014).
The prevalence of sexual inactivity is also high at intermediate and advanced ages in Dakar and Ouagadougou, however. One explanation relates to divorce, which is frequent in Dakar. While one in three unions among the 1942–56 female birth cohorts ended in divorce, the estimated rate had increased to one in two for the cohorts born in 1967–76 (Antoine, 2006). While divorce is less common in Ouagadougou (one in 10 unions ends in divorce within 20 years in Ouagadougou and Bobo Dioulasso, the two largest cities in Burkina Faso) than in Dakar, it has also been increasing across birth cohorts (Thiombiano & LeGrand, 2014). Divorce apart, the volume of male migration largely explains the high prevalence of sexual inactivity within marriage in the region, and its frequency at intermediate ages (Peytrignet, 2019). Moreover, Peytrignet (2019) recently showed that among cohabiting couples, half of the remaining sexually inactive (outside periods of pregnancy/postpartum abstinence) are at ages 40 and above. These older women have likely long marriage durations and are passed the family formation phase. Regarding postpartum insusceptibility, its continuing importance in Dakar and Ouagadougou can be explained by the various efforts by the authorities and NGOs to encourage breastfeeding to ensure high-quality infant nutrition (Garenne, 2018).
In Nairobi, Wamukoya et al. (2016)—who used an adapted Stover model that was slightly different from ours—also showed that contraception and sexual non-exposure are the main drivers of fertility avoidance. While divorce rates in Kenya are comparable to those in Burkina Faso (Clark & Brauner-Otto, 2015), delayed entry into union is more marked in the former. In Nairobi, in the mid-2000s, 61% of women aged 25–34 were married for the first time before age 25, versus 70% among women aged 45–54—a decrease of nearly 10% between these two cohorts (Bocquier & Khasakhala, 2009). The relatively greater role of contraception in Nairobi in comparison to the other two cities can be attributed to the history of the population program in the country, as described in the introduction.
It is important, despite the possible underestimation of abortion cases, to discuss the order of magnitude of their effect in contexts where the information is available. Indeed, Rossier et al (2006) and Mohamed et al (2015) estimated in Ouagadougou (in 2001) and Nairobi (in 2012 in a higher abortion scenario) abortion rates of 40 and 41 per 1000 for women of 15–49, respectively. The highest rates by age are 60 per 1000 for 15–19 year olds in Ouagadougou and 79 per 1000 in Kenya (not Nairobi). Based on the estimated fertility during these periods, the reducing effect of abortion (abortion index) can be estimated at 0.99 (1% reduction effect on fertility) in both cities for women aged 15–49: it is not significant for women taken together. However, it would be important for adolescents where it is estimated abortion index at 0.89 (11% reduction effect) for 15–19-year-olds in Ouagadougou and 0.77 (23% reduction effect) for 20–24 year olds in Kenya.
Fertility has indeed decreased more quickly in Nairobi than in the other cities, in conjunction with the rapid spread of modern contraception. Between 1978 and 1998, fertility fell by 0.15 per year on average, compared with an average decrease in of 0.08 in Ouagadougou and 0.07 in Dakar between 1993 and 2014. But as fertility stalled in Nairobi from 1998, while continuing to decline in the other two cities at half the speed witnessed in Nairobi, all three cities are now at about the same fertility level. Recent expansion of contraceptive use in Dakar and Ouagadougou may bring the two cities closer to the model of Nairobi in the coming years, with a decreasing role for traditional fertility-reducing factors. However, the non-use of contraception when breastfeeding, when avoiding sexual activity before marriage, when the husband is away, or after two decades of marriage, i.e., the practices underlying the traditional factors of fertility reduction, could well persist, even in the presence of vigorous family planning programs. Future progress in the diffusion of modern contraception will doubtless depend upon the large-scale adoption of long-acting methods.
To summarize, in the case of Nairobi, the data seem to confirm Caldwell et al.'s (1992) idea that fertility reduction would be associated with the spread of contraceptive use at all ages. In Ouagadougou, in 2010, however, the effect of contraceptive use on fertility at different ages was no greater than that of sexual inactivity, and in Dakar, it was smaller. The pattern in the two other cities thus does not confirm Caldwell et al.'s (1992) second hypothesis, although fertility has fallen to nearly the same levels as in Nairobi. These authors neglected the traditional drivers of fertility reduction, which, as we have seen, persisted at all ages in Ouagadougou, and to an even greater extent in Dakar (in addition to modern contraception).
The main limitations of this study are methodological. Firstly, the underestimation of fertility (poor data quality) has conducted to the use indirect methods, in particular the Gompertz method, to re-estimate the level of fertility. In addition, the Stover’s model adapted to this study requires a large sample size to use the version applicable to age groups. This, for example, necessitated age-grouping to compute average duration of postpartum insusceptibility.
Notes
Demographic and health surveillance sites track the population of a geographically defined area (a collection of villages, a city) over a relatively long period, collecting vital statistics (births, deaths, marriages, migration, etc.) through multi-round surveys.
Bongaarts (2015) suggests continuing to work with all women in union, adding those who are not married but who have recently been sexually active or who use contraceptives. This modification does not allow sexual inactivity within union outside of postpartum periods to be taken into account: according to Peytrignet (2019), around 15% of women in union in sub-Saharan Africa are sexually inactive outside the postpartum period (a proportion constant over time).
In the contraception index, Stover only excludes overlap between amenorrhea (but not postpartum abstinence) and contraceptive use, but Bongaarts (2015), accepting Stover's proposal, excludes overlap with both amenorrhea and abstinence. We believe that the latter choice is debatable: use of contraceptives by a “postpartum abstinent” woman suggests that she may be exposed to a risk of sexual relations, and thus of closely spaced pregnancies.
References
Antoine, P. (2006). Analyse biographique de la transformation des modèles matrimoniaux dans quatre capitales africaines : Antananarivo, Dakar, Lomé and Yaoundé. Cahiers Québécois De Démographie, 35(2), 5–37.
Alkema, L., Kantorova, V., Menozzi, C., & Biddlecom, A. (2013). National, regional and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: A systematic and comprehensive analysis. Lancet, 381(9878), 1642–1652.
ANSD and Macro International Inc. (1994). “Enquête démographique and de santé ”, Sénégal 1992–93. MA, ANSD, Macro International Inc.
ANSD and Macro International Inc. (2012). “Enquête démographique and de santé ”, Sénégal 2010–11. MA, ANSD, Macro International Inc.
ANSD, (2014) "RAPPORT DEFINITIF RGPHAE 2013". Dakar, Division de la documentation, de la diffusion et des relations avec les usagers.
ANSD and Macro International Inc. (2018). “Enquête démographique and de santé”, Sénégal 2017. MA, INSD, Macro International Inc.
Assefa, N., & Semahegn, A. (2016). Fertility is below replacement in Harar health and demographic surveillance system (Harar HDSS), Harar town, Eastern Ethiopia. Fertility Research and Practice, 2(1), 10.
Bajos, N., Teixeira, M., Adjamagbo, A., Ferrand, M., Guillaume, A., & Rossier, C. (2013). Tensions normatives and rapport des femmes à la contraception dans 4 pays africains. Population, 68(1), 17–39.
Beauvalet-Boutouyrie S, (1990). “La limitation des naissances : l'exemple de Verdun dans la deuxième moitié du XVIIIe siècle”. In : Annales de démographie historique. Société de démographie historique, p. 199–215.
Bongaarts J, (1978). "A framework for analyzing the proximate determinants of fertility". Population and development review, p. 105–132.
Bongaarts, J. (2003). Completing the fertility transition in the developing world: The role of educational differences and fertility preferences. Population Studies, 57(3), 321–335.
Bongaarts, J. (2015). Modeling the fertility impact of the proximate determinants: Time for a tune-up. Demographic Research, 33, 535.
Bongaarts, J., & Casterline, J. (2013). Fertility transition: Is sub-Saharan Africa different? Population and Development Review, 38(s1), 153–168.
Bocquier, P., & Khasakhala, A. (2009). Factors influencing union formation in Nairobi, Kenya. Journal of Biosocial Science, 41(4), 433–455.
Caldwell, J. C., Orubuloye, I. O., & Caldwell, P. (1992). Fertility decline in Africa: A new type of transition. Population and Development Review, 18(2), 211–242.
Casterline JB, Singh S, Cleland J. (1984) "The proximate determinants of fertility". WFS Comparative Studies no. 39. Voorburg, Netherlands: ISI.
Casterline, J. B. (2001). The pace of fertility transition: National patterns in the second half of the twentieth century. Population and Development Review, 27(Supplement: Global Fertility Transition), 17–52.
Casterline, J. B., & Odden, C. (2016). Trends in inter-birth intervals in developing countries 1965–2014. Population and Development Review, 42(2), 173–194.
Central Bureau of Statistics (CBS) [Kenya], 1996. “Kenya Population Census, 1989. Analytical Report volume iv : fertility and nuptiality”. Nairobi, Kenya.
Clark, S., & Brauner-Otto, S. (2015). Divorce in sub-Saharan Africa: Are unions becoming less stable? Population and Development Review, 41(4), 583–605.
Clark, S., Koski, A., & Smith-Greenaway, E. (2017). Recent trends in premarital fertility across sub-Saharan Africa. Studies in Family Planning, 48(1), 3–22.
Coale, A. J. (1974). The history of the human population. Scientific American, 231(3), 40–51.
Conseil National de Population, (2000). ”Politique Nationale de Population Burkina Faso”. Ministere de l’Economie and des Finances, Ouagadougou, Burkina Faso.
Cross A. R, Obungu W. and Kizito P, (1991). “Evidence of a transition to lower fertility in Kenya”. International Family Planning Perspectives, 4–7.
Delaunay V. and Guillaume A, (2007). "Sexualité and mode de contrôle de la fécondité chez les jeunes en Afrique subsaharienne". Santé de la reproduction and fécondité dans les pays du Sud. Nouveaux contextes and nouveaux comportements, Louvain-la-Neuve, Academia-Bruylant, 215–267.
Diallo, J. (2014). La politique de planification familiale au Sénégal : Approche sanitaire and conflits de norme. Autrepart, 2, 41–55.
Doyle, S. (2013). Before HIV: Sexuality, fertility and mortality in East Africa, 1900–1980. British Academy and Oxford University Press.
Erfani and McQuillan. (2008). Rapid fertility decline in Iran: Analysis of intermediate variables. Journal of Biosocial Science, 40, 459–478.
Fendall, N. R., & Gill, J. (1970). Establishing family planning services in Kenya. Public Health Reports, 85(2), 131.
Ferry B, (1976). "Données récentes sur la fécondité à Dakar (Sénégal)”. Population (french edition), 717–722.
Garenne M, (2017a) "Planning familial et fécondité en Afrique: Évolutions de 1950 à 2010”, hal-01578080.
Garenne M, (2017b). "Family planning and fertility decline in Africa: from 1950 to 2010”. Chapter 7, In: Zouhair Amarin (editor), Family Planning. London: InTechOpen book. DOI: https://doi.org/10.5772/intechopen.68266
Garenne M, (2018). "Tendances de l'État Nutritionnel des Jeunes Enfants dans les Pays Francophones du Sahel: 1990–2015”. (No. P245). FERDI.
Grace, K., & Sweeney, S. H. (2013). Understanding stalling demographic transition in high-fertility countries: A case study of Guatemala. Journal of Population Research, 30(1), 19–37.
Guillaume, A., & Rossier, C. (2018). L’avortement dans le monde. État des lieux des législations, mesures, tendances and conséquences. Population, 73(2), 225–322.
Gurmu, E., & Mace, R. (2008). Fertility decline driven by poverty: The case of addis ababa, ethiopia. Journal of Biosocial Science, 40(3), 339.
Hirschman, C., & Young, Y. J. (2000). Social context and fertility decline in Southeast Asia: 1968–70 to 1988–90. Population and Development Review, 26, 11–39.
Hosseni, H., Torabi, F., & Bagi, B. (2014). Demand for long-acting and permanent contraceptive methods among Kurdish women in Mahabad, Iran. Journal of Biosocial Science, 46(6), 772–785.
Ishida, K., Stupp, P., & Melian, M. (2009). Fertility decline in Paraguay. Studies in Family Planning, 40(3), 227–234.
INSD, (1984). “Fécondité des femmes voltaïques. Enquête post-censitaire 1976”. Ouagadougou, Burkina Faso.
INSD, (1989). “Analyse des résultats définitifs. Recensement General de la Population de 1985”. Ouagadougou, Burkina Faso.
INSD and Macro International Inc., 1994. “Enquête démographique et de santé”. Burkina Faso. (1993). Calverton. INSD, Macro International Inc.
INSD and Macro International Inc. (2012). “Enquête démographique and de santé and à indicateurs multiples (EDSBF-MICS IV)”, Burkina Faso 2010. MA, INSD, Macro International Inc.
Jadhav, A., & Vala-Haynes, E. (2018). Informed choice and female sterilization in South Asia and Latin America. Journal of Biosocial Science, 50(6), 823–839.
Johnson-Hanks, J. (2007). Natural intentions: Fertility decline in the African Demographic and Health Surveys. American Journal of Sociology, 112(4), 1008–1043.
Johnson-Hanks, J., Bachrach, C. A., Morgan, S. P., & Kohler, H. P. (2011). The theory of conjunctural action”. Understanding family change and variation (pp. 1–22). Dordrecht: Springer.
Jolly L. C. and Gribble J. N, (1993) “The proximate determinants of fertility: Demographic change in Sub-Saharan Africa”. Demographic change in sub-Saharan Africa, pp. 68–116.
Joshi, R., Khadilkar, S., & Patel, M. (2015). Global trends in use of long-acting reversible and permanent methods of contraception: Seeking a balance. International Journal of Gynecology & Obstetrics, 131(Supplement 1), S60–S63.
Kateb K and Ouadah-Bedidi Z, (2002).”L'actualité démographique du Maghreb”. Actes de la DESCO Université d’été.
KNBS and Macro International Inc. (2010). “Kenya demographic and health survey”, Kenya 2008–09. MA, KNBS, Macro International Inc.
KNBS, (2010a). “The 2009 Kenya Population and Housing Census. Counting Our People for the Implementation of Vision 2030, VOLUME I, Population distribution by age, sex and administrative units”. Nairobi, Kenya
KNBS, (2010b). “The 2009 Kenya population and housing census. Counting our people for the implementation of vision 2030, VOLUME II, Population and household distribution by socio-economic characteristics”. Nairobi, Kenya.
KNBS and Macro International Inc. (2015). “Kenya demographic and health survey”, Kenya 2014. MA, KNBS, Macro International Inc.
Kravdal, Ø. (2002). Education and fertility in sub-Saharan Africa: Individual and community effects. Demography, 39(2), 233–250.
Lerch, M., & Spoorenberg, T. (2020). The emergence of birth limitation as a new stage in the fertility transition in sub-Saharan Africa. Demographic Research, 42, 827–858.
Mason, K. O. (1997). Explaining fertility transitions. Demography, 34(4), 443–454.
Mensch, B. S., Grant, M. J., & Blanc, A. K. (2006). The changing context of sexual initiation in sub-Saharan Africa. Population and Development Review, 32(4), 699–727.
Millogo, R. M. (2020). “Transition de la fécondité à Dakar, Ouagadougou et Nairobi: similitudes et divergences avec les schémas classiques” (Doctoral dissertation, University of Geneva).
Mohamed, S. F., Izugbara, C., Moore, A. M., Mutua, M., Kimani-Murage, E. W., Ziraba, A. K., Bankole, A., Singh, S. D., & Egesa, C. (2015). The estimated incidence of induced abortion in Kenya: A cross-sectional study. BMC Pregnancy and Childbirth, 15(1), 185.
National Research Council. (1993). Factors affecting contraceptive use in Sub-Saharan Africa. Washington: National Academies.
Omwami, E. M., & Omwami, R. K. (2010). Public investment and the goal of providing universal access to primary education by 2015 in Kenya. International Journal of Educational Development, 30(3), 243–253.
Oranje Rose N. and Zulu Eliya M, (2015). “Understanding Power in Sexual and Reproductive Health policy and Legislative reform Processes in Kenya”. Paper presented at the PAA conference.
Oris M, (1988). “Fécondité légitime and illégitime: les indigents de Huy (Belgique) de 1815 à 1875”. In Annales de Démographie historique (pp. 141–155). Société de démographie historique.
Oris M, (1995). “La transition de la fécondité légitime dans le milieu urbain wallon and bruxellois”. Transitions démographiques and sociétés. Louvain-la-Neuve: Académia, L'Harmattan, 359, 379.
Perrenoud A, (1989), Les transitions démographiques, In Annales de démographie historique (pp. 7–11), Société de démographie historique.
Petit V. and O’Deye A, (2001). “Changement socio-économique, communautés villageoises et diffusion de la planification familiale en milieu rural sénégalais”. In F. Sandron et B. Gastineau: Dynamiques familiales et innovations socio-démographiques. Etudes de cas dans les pays du Sud, Paris, L'Harmattan collection Populations, 71–97.
Peytrignet M. C, (2019). “Fertility Regulation in Sub-Saharan Africa: The Role of Marital Sexual Inactivity”. Doctoral dissertation, University of Geneva, 92–95.
Pison, G., Hill, K. H., Cohen, B., & Foote, K. A. (1995). Population dynamics of Senegal. National Academy Press.
The Population Council. (1969). GHANA: Official Policy Statement. Studies in Family Planning, 1(44), 1–7.
Rossier, C., Guiella, G., Ouédraogo, A., & Thiéba, B. (2006). Estimating clandestine abortion with the confidants method—results from Ouagadougou, Burkina Faso. Social Science & Medicine, 62(1), 254–266.
Rossier, C., Soura, A., Baya, B., Compaore, G., Dabire, B., Dos Santos, S., Duthe, G., Gnoumou, B., Kobiane, J. F., Kouanda, S., Lankoandé, B., Legrand, T., Mbacke, C., Millogo, R., Mondain, N., Montgomery, M., Nikiema, A., … Zourkaleini, Y. (2012). The Ouagadougou health and demographic surveillance system international. Journal of Epidemiology, 41(3), 658–666.
Rutstein S. O. and Rojas G, (2006). “Guide to DHS statistics”. Calverton, MD: ORC Macro.
Schoumaker B, (2009) “Stalls in fertility transitions in sub-Saharan Africa: Real or spurious?” Louvain-la-Neuve: Département des sciences de la population and du développement, Université catholique de Louvain.
Schoumaker, B. (2013). A Stata module for computing fertility rates and TFRs from birth histories: Tfr2. Demographic Research, 28(38), 1093–1144.
Schoumaker, B. (2019). Stalls in Fertility Transitions in sub-Saharan Africa: Revisiting the Evidence. Studies in Family Planning, 50(3), 257–278.
Shapiro, D., & Gebreselassie, T. (2009). Fertility transition in Sub-Saharan Africa: Falling and stalling. African Population Studies. https://doi.org/10.11564/23-1-310
Shah, I. H., & Åhman, E. (2012). Unsafe abortion differentials in 2008 by age and developing country region: High burden among young women. Reproductive Health Matters, 20(39), 169–173.
Singh, S., Casterline, J. B., & Cleland, J. G. (1985). The Proximate Determinants of Fertility: Sub-national Variations. Population Studies, 39(1), 113–135.
Stover, J. (1998). Revising the proximate determinants of fertility framework: What have we learned in the past 20 years? Studies in family planning, 29, 255–267.
Taylor R and Morrell S, (2015) “Fertility Calculation Tool”. School of Public Health and Community Medicine ( SPHCM), University of New South Wales (UNSW), Sydney, NSW 2052, Australia.
Thiombiano, B. G., & Legrand, T. K. (2014). Niveau and facteurs de ruptures des premières unions conjugales au Burkina Faso. African Population Studies, 28(3), 1432–1446.
Timæus, I. M., & Moultrie, T. A. (2008). On postponement and birth intervals. Population and Development Review, 34(3), 483–510.
Timæus, I. M., & Moultrie, T. A. (2013). Distinguishing the impact of postponement, spacing and stopping on birth intervals: Evidence from a model with heterogeneous fecundity. Journal of Biosocial Science, 45(3), 311–330.
Timæus, I. M., & Moultrie, T. A. (2020). Pathways to low fertility: 50 Years of limitation, curtailment, and postponement of childbearing. Demography, 57(1), 267–296.
United Nations (UN). (2015). World population prospects: The 2015 revision. United Nations.
Wamukoya M, Beguy D, Muindi K, Mumah J. and Mberu B. U, (2016). “The effect of changing proximate determinants on fertility levels among urban poor women in Kenya: Evidence from Nairobi's informal settlements, 2000–2012”. African Population Studies, 30(3).
World Bank, (2019). Retrieved May 09, 2019 from https://databank.banquemondiale.org/data/source/world-development-indicators/preview/on.
Zagaglia, B. (2013). Demographic transitions and social changes in the mediterranean region. IEMed: Mediterranean yearbook, 2013, 285–287.
Acknowledgements
This research received funding from the DEMOSTAF project as part of the Horizon 2020 Framework Programme for Research and Innovation, under the Marie Sklodowska-Curie grant agreement no. 690984. Roch Millogo acknowledges support from the Federal Commission for Scholarships for Foreign Students for the Swiss Government Excellence Scholarship (ESKAS No. 2016.0534) for the academic years 2016-2020.
Author information
Authors and Affiliations
Contributions
RM analyzed the data and wrote the first draft of the paper. RM and CR read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
(See Figs. 7 and 8, Tables 3 and 4).
Rights and permissions
About this article
Cite this article
Millogo, R., Rossier, C. Fertility Transition in Dakar, Nairobi, and Ouagadougou Since the 1970s: An Identical Reduction at All Ages Through Modern Contraception?. Popul Res Policy Rev 41, 2115–2142 (2022). https://doi.org/10.1007/s11113-022-09717-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11113-022-09717-3