The effect of TiB2 additions on the structure, phase composition, and mechanical and tribological properties of materials in the Fe–FKh800 system was studied. The introduction of titanium boride additions activated compaction of the iron-based composites through sintering with participation of the liquid phase that emerged with the formation of low-melting Fe–C–B (Tm ~ 1050°C) and γ-Fe–Fe2B–TiB2 (Tm ~ 1162) eutectics and led to a 50–70°C decrease in the sintering temperature of the compacts. Titanium boride additions between 0.38 to 0.74 wt.% provided 20–25% higher bending strength of the 65 wt.% Fe–35 wt.% FKh800 composite with a slight increase in hardness. Metallographic studies, X-ray diffraction, and electron microprobe analysis of the Fe–35 wt.% FKh800–TiB2 materials showed that titanium boride additions promoted a multiphase, microheterogeneous matrix-reinforced composite, consisting of Kh17 chromium steel, double M7C3 and M3C iron–chromium carbides, and complex Me3(CB) carboborides. The influence of TiB2 additions on the wear resistance of the composites subjected to dry friction against fixed diamond wheel particles and ShKh15 steel was studied. The study showed that the abrasive mass wear (Im) of carbide steels decreased from 36.94 to 14.8 mg/km and their linear wear (Il) decreased from 0.197 to 0.079 mm/km with TiB2 additions increasing from 0.38 to 1.48 wt.%. Titanium boride additions ranging from 0.38 to 2.2 wt.% reduced the mass wear rate from 4.9 to 1.9 mg/km in dry friction of the composite against a ShKh15 steel counterface with 50–55 HRC hardness and decreased the friction coefficient from 0.49 to 0.38.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing need for materials possessing excellent mechanical and tribological properties and high corrosion resistance in virtually all industrial applications. Articles made of such materials perform effectively when simultaneously subjected to friction, abrasive wear, and corrosive environments. High-alloy steels and chromium cast irons are used in modern mechanical engineering to fabricate such articles. Powdered carbide steels, particularly iron–chromium–carbon alloy composites, should also be regarded as promising materials. Despite longer than a 100-year research history, the Fe–Cr–C system remains the focus of scholars and applied scientists in the development of wear- and corrosion-resistant materials. This is ascertained by a great number of publications, whose overview as of 2005 inclusive is provided in the fundamental paper [1].
The Fe–Cr–C system is known to contain complex M23C6, M7C3, and M3C2 carbides, which are in equilibrium with both austenite and ferrite.
The hypereutectic Fe–Cr–C alloy is a characteristic wear-resistant material. Its high wear resistance is due to the formation of complex transition metal carbides of M7C3 type. The weight content of primary M7C3 carbides increases with carbon amount. The wear resistance increases accordingly. The orientation of primary M7C3 carbide can also influence the wear resistance of the eutectic Fe–Cr–C alloy. In rapid cooling conditions, M7C3 carbides increase the eutectic alloy hardness [2, 3].
Modern Fe–Cr–C materials such as carbide steels and associated articles are successfully produced by powder metallurgy techniques: solid-phase and liquid-phase sintering or hot forging of powder composites [4].
Our previous papers [5, 6] show that small additions of nickel boride Ni3B to 65 wt. Fe–35 wt.% FKh800 chromium carbide steel lead to a 50–70°C decrease in sintering temperature and a significant increase in the abrasion and corrosion resistance in alkaline and acid solutions. In this connection, the effect of other borides (more thermodynamically stable and less scarce) on the structure, mechanical, and tribological properties of chromium carbide steels should be studied. Such borides include TiB2, having high microhardness, wear resistance, elevatedtemperature oxidation resistance, and adequate ductility. This boride is added in amounts smaller than 1% for refining the grains and improving the hardness of classical carbide steels [7].
The objective is to examine the effect of titanium diboride additions varying from 0.38 to 2.2 wt.% on the sintering temperature, structurization, phase composition, and mechanical and tribological properties of the powdered 65 wt.% Fe–35 wt.% FKh800 chromium carbide steel.
Experimental Procedure
The starting materials were iron powder of PZhR 3-200-28-30 grade (as per GOST 9849–86), high-carbon ferrochrome FKh800 (GOST 4757–79), and titanium boride powder (TU 6-09-03-7–78). The high-carbon ferrochrome powder was produced from lump FKh800 ferrochrome in a jaw crusher and further ground in a planetary-ball mill.
The titanium boride content of the mixtures was varied between 0.38 and 2.2 wt.%, corresponding to 0.12–0.67 wt.% B in TiB2. Boron is known [8] to have a several times stronger effect on iron than carbon, promoting grain refinement and significantly improving the wear resistance in friction of white cast irons. Moreover, boron forms with iron a low-melting eutectic, Fe–Fe2B, to activate the sintering of powdered carbide steels that contain high-carbon ferrochrome.
The starting powder mixtures were prepared by wet grinding/blending in a planetary-ball mill in alcohol in conditions described in [9]. The samples were compacted in a closed die at 800 MPa and sintered in an electric vacuum furnace at 1100–1250°C.
The mechanical properties were measured in compliance with standard procedures used to test powder materials and hardmetals [10, 11]. The abrasive wear resistance was determined by shaft (counterface)–plane (sample) testing in dry friction against an ASV 160/125 diamond wheel and a ShKh15 counterface at room temperature (+30°C). The friction test was performed at sliding sped V = m/sec, load P = 0.5 MPa, and 1 km friction distance.
The microstructure and phase composition of the sintered samples were examined employing optical (Olympuis LX-70) and scanning (TESCAN VEGA) electron microscopes, and a DRON-3 diffractometer was used for X-ray diffraction. Electron microprobe analysis was carried out with a MS-46 microprobe.
Experimental Results and Discussion
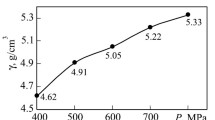
We examined how the sintering temperature influenced the bulk shrinkage, density, hardness, and bending strength of carbide steels with TiB2 0.38–2.2 wt.% additions introduced into the Fe–35 wt.% FKh800 mixture. When sintering temperature increased from 1100 to 1200°C, the bulk shrinkage and density of the samples grew monotonically (Fig. 1a, b). The bulk shrinkage of the material with 0.74 wt.% TiB2 was 24.9–32.8% even at solidphase sintering temperatures of 1100–1150°C; this was 1.8–2.0 times greater than that of the samples without TiB2 additions. When sintering temperature increased to 1250°C, the shrinkage and density of the composites with TiB2 additions reached the highest values (porosity lower than 2–3%). This indicates that titanium boride (boron) additions activate compaction by promoting sintering involving the liquid phase that may emerge with the formation of low-melting Fe–C–B (Tm ~ 1050°C) and γ-Fe–Fe2B–TiB2 (Tm ~ 1162°C) eutectics. That such eutectics exist in the Fe–C–B and Fe–Ti–B system is ascertained, respectively, by the research effort [12] and fundamental paper [13]. It is rather difficult to quantify the composition of these eutectics and indicate more accurate temperatures of their formation within this research effort because we deal with a multicomponent iron-based system (including, besides Ti and B, a significant amount of Cr and C) and are not aware of any data on this system in accessible sources.
Effect of the sintering temperature and amount of TiB2 additions on the bulk shrinkage (a), density (b), hardness (c), and bending strength (d) of the Fe–FKh800 samples, wt.%: 65 Fe–35 FKh800 (1); 64.8 Fe–34.8 FKh800–0.38 TiB2 (2); 64.6 Fe–34.6 FKh800–0.74 TiB2 (3); 64.4 Fe–34.4 FKh800–1.12 TiB2 (4); 64.2 Fe–34.2 FKh800–1.48 TiB2 (5); 63.9 Fe–33.9 FKh800–2.2 TiB2 (6)
At sintering temperatures above 1250°C, the bulk shrinkage and density increase at a slower rate, probably because the liquid phase amount becomes excessive and the samples partially deform and lose shape. This is especially noticeable at TiB2 content ranging from 1.48 to 2.2 wt.%.
When sintering temperature rises from 1100 to 1200°C, the hardness and bending strength of the basic material doped with titanium boride also increase according to near-parabolic dependences with gentle maxima at 1200°C (Fig. 1c, d). A further increase in the sintering temperature to 1250°C causes some decrease in hardness and bending strength of the composites. This may result from both grain growth in the hard component (from 8–13 μm to 15–25 μm) and shape loss of the samples caused by an excess amount of the liquid phase. The maximum bulk shrinkage and density (minimum porosity) and the best combinations of hardness and bending strength of the samples allow us to state that 1200°C is close to the optimum sintering temperature for most materials with TiB2 additions.
Metallographic studies, X-ray diffraction, and electron microprobe analysis of the Fe–35 wt.% FKh800 materials showed (Fig. 2, Table 1) that the introduction of even 0.38% TiB2 resulted in a microheterogeneous, multiphase composite microstructure, generally consisting of an iron–chromium metallic matrix containing 14–17% Cr (spectrum 3), complex Me7C3 and Me23C6 chromium–iron carbides (spectra 5 and 6), and complex Me3(CB) carboboride (spectrum 4). Figure 2 indicates that additions of 0.38–1.48 wt.% TiB2 promote homogeneous distribution of the metallic (spectra 3, 7, 9, 11) and hard (spectra 4, 8, 10, 12) phases and refine the Me3(CB) grains to 4–6 μm (Fig. 2b, c, e) compared to the cementite grains (8–23 μm) in the material without TiB2 (Fig. 2a). This can be attributed to boron that forms low-melting Fe–B–C (Tm ~ 1050°C) and Fe–Fe2B–TiB2 (Tm ~ 1162°C) eutectics and thus decreases the liquid-phase sintering temperature by 50°C, which inhibits the growth of carbide and carboboride grains in recrystallization through the liquid phase.
Electron microprobe analysis (Table 1) indicates that TiB2 additions also promote the transformation of chromium–iron cementite of Me3C type into complex iron–chromium carboboride (boron cementite) of Me3(CB) type. Note that boron cementite of Me3(CB) type is a predominant conducting phase in all composites with TiB2 additions, though X-ray diffraction (Fig. 3) identified also a small amount of Me7C3, Me23C6, and Fe3C complex carbides.
That the X-ray diffraction patterns do not show intensive independent lines of boron cementite may be because these line overlap with the γ-Fe line, complicating the identification of carboboride phases in such systems. These complications were pointed out in the paper [12] focusing on the phase formation and structurization mechanism in liquid-phase sintering of Fe–C–B composites.
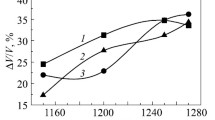
Considering that wear-resistant structural components and friction parts that perform without lubrication are fabricated from carbide steels, an important task is to examine the effect exerted by titanium boride additions ranging from 0.38 to 2.2 wt.% on the abrasive wear resistance and dry friction of Fe–35% FKh800 carbide steel samples against a ShKh15 steel counterface. The research results demonstrated (Table 2) that the TiB2 addition increasing from 0.38 to 1.48 wt.% reduced the carbide steel mass wear Im from 36.94 to 14.8 mg/km and linear wear Il from 0.197 to 0.079 mm/km in friction against diamond wheel particles.
This may be attributed to the formation of complex carboborides of Me3(CB) type, improving hardness and wear resistance of the composites. However, a further increase in the doping content to 2.2 wt.% intensifies both the mass wear (from 14.8 to 20.8 mg/km) and linear wear (from 0.079 to 0.141 mm/km), which may be associated with the development of a coarser structure and the precipitation of eutectic layers at Me3(CB) grain boundaries (Fig. 2f).
In dry friction against ShKh15 steel, titanium boride additions reduce the mass wear (from 4 to 1.9 mg/km) and linear wear (from 0.0397 to 0.022 mm/km) of the composites.
The tribological tests also found that the mass wear of the ShKh15 steel counterface was 6.2–7.1 mg/km. Moreover, 0.38–2.2 wt.% titanium boride additions decreased the dry friction coefficient of the composite with ShKh15 steel from 0.49 to 0.38 (Fig. 4) through the formation of complex iron–chromium carboboride of Me3(CB) type.
Conclusions
We have studied the effect of small TiB2 additions on the structure, phase composition, and mechanical and tribological properties of Fe–FKh800 materials. The introduction of TiB2 additions to the starting Fe–FKh800 mixture leads to a 50°C decrease in the sintering temperature of all composites through the formation of lowmelting iron-based eutectics. Titanium boride additions ranging from 0.38 to 0.74 wt.% in the 65 wt.% Fe–35 wt.% FKh800 material improve its hardness and bending strength (by 20–25%) and promote a multiphase, microheterogeneous matrix-reinforced composite consisting of Kh17 chromium steel, complex Me3(CB) carboborides, and a small amount of residual Me7C3 iron–chromium carbide.
The influence of TiB2 additions on the wear resistance of the chromium carbide steel subjected to dry friction against fixed diamond wheel particles and ShKh15 steel has been studied. An increase in the TiB2 addition between 0.38 and 1.48 wt.% reduces the abrasive mass wear rate from 37 to 14.8 mg/km and improves the wear resistance of the carbide steel by a factor of 2 to 2.5. Titanium boride additions ranging from 0.38 to 2.2 wt.% decrease the mass wear rate of the chromium carbide steel in dry friction against a ShKh15 steel counterface (50–55 HRC hardness) from 4.9 to 1.9 mg/km and decrease the friction coefficient from 0.49 to 0.38.
References
A. Bondar, V. Ivanchenko, A. Kozlov A, and I.-C. Tedenac, “Carbon–chromium–iron,” in: Landolt–Börnstein, Numerical Data and Functional Relationships in Science and Technology, W. Martienssen (ed.), New Series. Group IV: Physical Chemistry, Ternary Alloy Systems, Phase Diagrams, Crystallographic and Thermodynamic Data Critically Evaluated by MSIT, Vol. 11D2, Springer–Verlag, Berlin, Heidelberg (2007), pp. 1–55.
D.N. Hanlon, W.M. Rainforth, and C.M. Sellars, “The rolling/sliding wear response of conventionally processed and spray formed high chromium content cast iron at ambient and elevated temperature,” Wear, 225–229, 587–599 (1999).
X.H. Tang, “Microstructure of high (45 wt.%) chromium cast irons and their resistances to wear and corrosion,” Wear, 271, 1426–1431 (2011).
O.M. Shevchenko, “Carbide steels. Grades and production methods (overview),” Sovr. Probl. Fiz. Materialoved. Ser. Fiz. Khim. Osn. Tekhnol. Poroshk. Mater., Issue 20, 51–64 (2011).
V.A. Maslyuk, E.S. Karaimchuk, M.I. Podoprygora, V.T. Varchenko, and Ya.A. Sytnyk, “Structure and mechanical and tribotechnical properties of powder iron–high-carbon ferrochrome doped with Ni3B additions,” Powder Metall. Met. Ceram., 57, No. 3–4, 175–181 (2018).
V.A. Maslyuk, G.A. Baglyuk, E.S. Karaimchuk, and M.I. Podoprygora, “Effect of nickel boride additions on the structure and properties of sintered iron–high-carbon ferrochrome alloy,” in: Scientific Papers. Interuniversity Collection [in Ukrainian], Lutsk (2016), Issue 54, pp. 203–208.
J. Kübarsepp, Hardmetals with a Steel Matrix [in Russian], Valgus, Tallinn (1991), p. 164.
S.M. Zunirov (ed.), Boron, Calcium, Niobium, and Zirconium in Cast Irons and Steels [Russian translation], Metallurgizdat, Moscow (1971), p. 459.
V.A. Maslyuk, Ya.A. Sytnyk, M.I. Pidoprygora, and R.V. Yakovenko, “The effect of chromium steels and nickel boride additives on the structure and properties of iron–high-carbon ferrochrome FKh800 powder composites,” Powder Metall. Met. Ceram., 54, No. 5–6, 292–297 (2015).
ISO 4498-1:1990, Sintered Metal Materials, Excluding Hardmetals—Determination of Apparent Hardness, Part 1: Materials of Essentially Uniform Section Hardness, ed. 1990-08-2, ISO (1990).
ISO 3327:2009, Hardmetals—Determination of Transverse Rupture Strength, ed. 2009-05-15, ISO (2009).
Yu.V. Turov, B.M. Khusid, L.G. Voroshin, and B.B. Khina “Structure formation in sintering iron–boron carbide powder composite,” Powder Metall. Met. Ceram., 30, No. 6, 465–470 (1991).
V.T. Witusiewicz, A.A. Bondar, U. Hecht, A. Theofilatos, N.I. Tsyganenko, S.V. Utkin, and I.B. Tikhonova, “Experimental study and thermodynamic re-modelling of the constituent binaries and ternary B–Fe–Ti system,” J. Alloys Compd., 800, 419–449 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 59, Nos. 9–10 (535), pp. 128–136, 2020.
Rights and permissions
About this article
Cite this article
Kyryliuk, Y., Maslyuk, V., Mamonova, A. et al. The Structure and Properties of 65 wt.% Fe–35 wt.% FKh800 Chromium Carbide Steel Doped with Titanium Boride Additions. Powder Metall Met Ceram 59, 585–591 (2021). https://doi.org/10.1007/s11106-021-00184-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00184-7