The effect of metallic nanocoatings on the wetting of silicon oxide with lead-based filler melts (Pb–15 wt.% In and Pb–2.5 wt.% Ag) was studied by the sessile drop method with capillary cleaning of the melt in 1 ∙ 10–3 Pa vacuum at 500°C. The dependence of the contact angle between the filler melt and single coatings (Ti, Nb, Cr, V, Mo) on their thickness δ is shown: the contact angle decreases linearly (from the angle for silicon oxide to the angle for ‘threshold’ coating thickness) with increasing coating thickness. The ‘threshold’ coating thickness for different metals depends on the chemical affinity of the coating metal to oxygen. The higher the chemical affinity, the greater the ‘threshold’ thickness coating. The immobilization (adhesive bonding) of metal coatings on the substrate surface is determined by the contact interaction of SiO2 and the coating metal. The choice of metal couples for Mo–Cu, Nb–Cu, V–Cu, Cr–Cu, and Ti–Cu double coatings deposited on SiO2 is due to various metal interactions. The dependences of contact angle on thickness, the second layer (Cu coating) having constant thickness δCu of 100 nm, are similar in nature to that for a single coating. In these systems, wetting improves linearly with increasing coating thickness in the region of small δ. The wetting of the metallic coating deposited on the oxide surface by metallic filler melt is determined by the coating thickness (amount of metal deposited), coating structure, affinity of the coating metal to oxygen (coating–substrate adhesive attraction) promoting the shape of ‘islands’ and the ease of dispersion, dissolution of the coating in the filler melt, and wettability of the adhesive metal oxide coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overview of national and foreign research efforts of recent years testifies that the development of new nanostructured materials is very promising [1,2,3,4,5,6]. There is a growing interest in the new class of materials in both fundamental applied sciences and in industry and business. This interest is due to a number of reasons, such as the demand for miniaturized parts, unique properties of nanostructured materials, need to design and introduce materials with brand new and better properties, development of new process approaches and methods, and implementation of up-to-date instrumentation for diagnostics and modification of nanomaterials.
The properties of nanomaterials and nanoparticles most strongly change when the crystallites are 10–100 nm in size [2,3,4,5]. In terms of shape and size, they may be bulk particles or films. Nevertheless, an important fact is that the transition from macroscopic and microscopic sizes to 1–10 nm leads to qualitative changes in the physicochemical properties (electrical conductivity, magnetism, light absorption and emission, optical refraction, thermal stability, strength) and to the manifestation of catalytic capacity or reactivity of compounds and properties of associated materials that are not observed in macroscopic and microscopic items of the same chemical nature [5]. The fraction of atoms in a thin (~1 nm) surface layer of nanoparticles is significantly higher than that of microscopic particles. Not all surface atoms are bonded with the neighboring atoms. The bonds of atoms on surface protrusions are even more unsaturated. This results in strong lattice distortions in the surface layer, leading up to changes in lattice type. Another aspect is that free surface is a sink for crystalline defects. Their concentration becomes much higher with particle refinement since most structural defects emerge on the surface, and the nanostructured material thus becomes free of structural defects and chemical impurities.
The nanomaterials science was predicted to rebuild all production industries, lead to a new scientific and technical revolution, and influence the development of social structure. On the other hand, the nanostructured research areas have almost completely changed from the production and study of nanocrystalline substances and materials to nanotechnology, i.e. to the development of parts, devices, and systems with nanosized elements [7].
The main applications of nanosized elements are electronics, medicine, chemical pharmaceuticals, and biology. The nanocomposite area is also actively developing. The development trends show that the refinement of structural elements and deeper insight into the physics of deformation processes that determine the ductility of nanostructured materials may prompt the design of new materials that combine high strength and ductility, nanostructured ceramic and composite articles of precise shape, nanostructured hardmetals for the production of cutting tools with increased wear resistance and fracture toughness, protective nanostructured heat- and corrosion-resistant coatings, and polymer composites with nanoparticle and nanotube reinforcements with high strength and low flammability [1].
The production and application of nanofilms represent a promising area in the development of nanotechnology. For example, the advances made in the technology and materials sciences of thin oxide films permit the deposition of high-quality layers of desired thickness onto various substrates, being as good as ‘ideal’ single crystals. At the same time, effects of a strong electrical field can be easily induced in the films, and studies over a wide temperature range can be conducted. In interaction with short-wave laser radiation or electron and iron beams, thin samples are appropriate for the uniform distribution of absorbed energy.
The study of thin films substantially expanded insights into the anisotropy peculiar to ferromagnetic materials and allowed various remagnetization processes to be revealed and examined and new physical phenomena to be found. Important applications of the films are magnetic media for recording and storing information on memory devices [8]. X-ray multilayer optics are used widely to filter and control polarization in synchrotron sources. The lenses of space telescopes comprising X-ray mirrors still regularly transmit Sun images in Fe IX-Fe XI (17.5 nm) and He II (30.4 nm) lines to the Earth [1].
Metallic films for different functional purposes are of special interest. Nanofilms represent highly developed surfaces with great surface energy. This determines the strong adhesion of nanofilms to the substrate surface and their high adsorption properties, including greater surface oxidation, affecting the wetting and brazing processes.
The use of adhesive transition metals to be deposited onto oxide materials improves wetting and adhesion of the metallic melt to the nonmetallic material. The second layer of copper, silver, and nickel film is applied to protect metals of the first layer against oxidation and improve melt spreading [9,10,11,12]. The functional application of nanofilms allowed the development of process for their brazing with metallic elements to produce braze units when this is not possible with other joining techniques.
The objective of this effort is to examine the surface modification of oxide materials in the absorption immobilization (bonding) of metallic nanostructured coatings, analyze the absorption immobilization of metals to activate the surface of oxide materials, and study the wetting of metallic films with filler melts to be further used for bonding of nonmetallic and metallic materials, particularly quartz glass and aluminum alloys.
Experimental Procedure
The wetting was studied by the sessile drop method with capillary cleaning of the melt in 2 ∙ 10–3 Pa vacuum at 500°C in the experimental process [13]. The solid phase was presented by silicon oxide substrates (quartz glass KB with 99.95% SiO2), which were preliminary polished to a surface roughness of 0.01 μm. Prior to the experiment (coating deposition), the substrates were cleaned with acetone and alcohol and annealed in vacuum at 1100°C for 60 min. The experiments used indium of 000 grade, lead of OVCh grade, and silver (99.999% purity). Silver and indium were preliminary melted in graphite crucibles in vacuum at 1000°C and lead at 650°C, and so were filler melts of lead with additions of 2.5 wt.% Ag and 15 wt.% In.
Molybdenum, iodide-refined titanium, and electrolytic vanadium, niobium, and chromium were deposited as nanocoatings. Metals for the first layer (Ti, Nb, Cr, V, Mo) were chosen so that mutual solubility of the filler melt and film was excluded. In addition, these metals had different affinities to oxygen; this contributed to their active adhesion to SiO2.
The coatings were deposited onto substrates by electron-beam evaporation of metals in vacuum. The thickness of reference films was determined employing different methods: a Micron Alpha contactless interference optical 3D profiler (Fig. 1), a special quartz thickness gauge located directly in the spraying area (sensor developed at the V.N. Karazin Kharkiv National University), and the technique described in [12, 14]. The film quality was checked with an XJL-17 metallographic microscope.
Copper of grade B3 (99.995% purity) was selected as the second metal applied to the surface with the primary metallic layer. The choice of metal couples for the first and second coating layers was due to their different chemical interaction [15, 16].
Experimental Results and Discussion
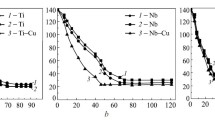
Data on the wetting of single and binary metallic films deposited on quartz glass with lead-based filler melts at 500°C are presented in Fig. 2. The dependences of contact angle δ on thickness of the single metallic films show that the curve slope differs from the angle corresponding to δ = 0 to the angle corresponding to the ‘threshold’ thickness. The threshold thickness is the minimum film thickness denoting wetting corresponding to the film contact angle. The dependence remains the same: the contact angle linearly decreases when film thickness increases from θ for SiO2 to the contact angle between the metallic film and lead-based filler melt. The threshold film thickness depends on both the chemical affinity of the film metal to oxygen and the solubility of the film metal in filler melt. The higher the chemical affinity, the greater the threshold film thickness: some part of the adhesive metal transforms to oxide, and oxides are wetted worse than metallic surfaces are. The Gibbs energy to form coating metal oxides (∆fG°298) [17, 18] referred to 1 g/at oxygen decreases in the TiO2 → NbO → V2O3 → Cr2O3 → MoO2 row (kJ/mole) as follows: 444.3 → 379.4 → 379.0 → 353.0 → 266.6. The threshold film thickness (nm) decreases in the same row: 70 → 65 → 62 → 60 → 50 in case of wetting of the films (Ti, Nb, V, Cr, Mo) on a SiO2 substrate with the lead–indium melt. The contact angles differ insignificantly (in the range 20–30°).
The immobilization (adhesion bonding) of metallic films on the silicon oxide surface is determined by the formation of chemical compounds, particularly oxides [19]. The potential to form chemical reaction products on the SiO2 surface is confirmed by the thermodynamic calculation of the heat released in the interaction for Ti, Nb, V, Cr, and Mo films (formation enthalpies of compounds (∆fH°298) are taken from [17, 18, 20, 21]):
Equations (1)–(4) show that film metal oxides form on the silicon oxide surface.
Spectrophotometric studies of the optical transmittance of thin metal films (Mo and V) were conducted in [12] to prove that the metal deposited on SiO2 formed oxides. Metals have the highest possible absorption coefficient in a very wide spectral range, while oxides are characterized by a much lower absorption coefficient in wide spectral ranges [22]. The study of optical properties of thin Mo films on SiO2, freshly deposited and annealed at 600°C, did not confirm the formation of molybdenum oxides, which is ascertained by thermodynamic calculation (Eq. (5)). Annealing at 900°C changed the dependence of optical transmittance on wavelength, being evident of chemical interaction at the oxide–film interface to form molybdenum oxide or silicide. Analysis of the spectral transmittance curves for thin vanadium films (δ = 20 nm) deposited on SiO2 shows that a substantial oxide layer develops after annealing at 600°C. This is determined by a general increase in transmittance and characteristic change in transmittance in the shortwave range [12]. This fact is confirmed by Eq. (3).
Therefore, the threshold film thickness for different metals depends on the affinity of the film metal to oxygen and on the solubility of the film metal in the filler melt.
For wetting of binary films deposited on SiO2, the choice of metal couples for the coating (Mo–Cu, Nb–Cu, V–Cu, Cr–Cu, and Ti–Cu) is due to differences in their interaction [15, 16]. Hence, the Ti–Cu system is characterized by the strongest interaction between the components (there are four intermetallics), the Nb–Cu, V–Cu, and Cr–Cu systems by weaker interaction (there is insignificant solid-state solubility of components, no intermetallides being present), and the Mo–Cu system by the weakest interaction (solid-state solubility is zero and liquid-state immiscibility is observed).
When the second layer (copper coating) has constant thickness (δCu = 100 nm), the dependences of the contact angle on the thickness of binary coatings are of the same nature as those for the single films. In these systems, the contact angle increases linearly with higher δ to θ values corresponding to the contact angle of the film metal in these systems at small thickness.
There is no difference in the wettability of single (Mo) or binary (Mo–Cu) film with lead melts (Fig. 2a) in the SiO2/Mo–Cu system. This is explained by weak interaction between Cu and Mo, resulting in complete dissolution of copper in the filler melt. In the Nb–Cu, V–Cu, and Cr–Cu systems, the binary film is wetted better than the single one (Fig. 2b–d). The binary film has greater thickness and acquires its integrity earlier. The binary films in the Ti–Cu system (Fig. 2e) are wetted much better than the single titanium film does. There is almost linear dependence of θ on film thickness δ. Although intermetallics formed in the Ti–Cu system are more poorly wetted than the copper film is, the binary film is wetted better because of greater total coating thickness.
Therefore, the wetting of the metallic film deposited onto the oxide surface with the filler melt is determined by the following factors: film thickness (amount of deposited metal), film structure (continuous or insular), affinity of the film metal to oxygen (adhesive film–substrate bonding, determining the ease of dispersion and shape of ‘islands’), dissolution of the film in the filler melt, and wettability of the adhesive metal oxide film.
Conclusions
For single films, the contact angle linearly decreases with increasing film thickness δ from the contact angle θ for SiO2 to the contact angle for compact film metal wetted with the filler melt. The threshold film thickness for different metals depends on the chemical affinity of the film metal to oxygen and the solubility of the film metal in the filler melt. The higher the chemical affinity, the greater the threshold film thickness.
The immobilization (adhesion bonding) of metallic films on the substrate is determined by contact interaction between silicon oxide and film metal. The wetting of the metallic film deposited onto the oxide surface with the filler melt is determined by the film thickness (amount of deposited metal), film structure, affinity of the film metal to oxygen (adhesive film–substrate bonding), dissolution of the film in the filler melt, and wettability of the adhesive metal oxide film.
References
Zh.I. Alferov, P.S. Kopiev, R.A. Suris, A.L. Aseev, S.V. Gaponov, V.I. Panov, E.A. Poltoratskii, and N.N. Sibeldin, “Nanomaterials and nanotechnologies,” Mikrosyst. Tekh., Issue 8, 3–13 (2003).
M.C. Roco, R.S. Williams, and P. Alivisatos (eds.), IWGN Workshop Report: Nanotechnology Research Directions. Vision for Nanotechnology in the Next Decade, Kluwer, Boston (1999).
M.J. Pitkethly, Nanotoday, December (2004) pp. 20–29.
N. Kobayashi, Introduction to Nanotechnology [Russian translation], BINOM, Moscow (2005), p. 134.
A.I. Gusev, Nanomaterials, Nanostructures, and Nanotechnologies [in Russian], Fizmatlit, Moscow (2005).
S.N. Shtykov and T.Yu. Rusanov, “Nanomaterials and nanotechnologies in chemical and biochemical sensors: capabilities and applications,” Ros. Khim. Z., LII, No. 2, 92–100 (2008).
Ch. Poole and F. Owens, Introduction to Nanotechnology, John Wiley, New York (2003).
N.G. Vnukov and G.N. Churinov, Nanomaterials and Nanotechnologies [in Russian], Izd. Sib. Federl. Univ., Krasnoyarsk (2007), p. 103.
B.D. Kostyuk, Yu.V. Naidich, G.A. Kolesnichenko, and S.S. Shaikevich, “Wetting of bimetal Mo–Cu, Ti–Cu, and V–Cu coatings deposited on SiO2 with tin melt,” Adgez. Raspl. Paika Mater., No. 12, 11–13 (1982).
Yu.V. Naidich, I.I. Gab, B.D. Kostyuk, and T.V. Stetsyuk, “Study of joining (brazing) of ceramic materials using metal nanocoatings,” Dop. Nats. Akad. Nauk Ukrainy, No. 5, 97–104 (2007).
V.P. Krasovskii, I.I. Gab, B.D. Kostyuk, N.O. Krasovska, and T.V. Stetsyuk, “Development of brazing process for materials with a great difference in the thermal expansion coefficients,” Mizhvuz. Zb. Naukovi Notatki, Issue 66, 172–179 (2019).
Yu.V. Naidich, B.D. Kostyuk, G.A. Kolesnichenko, and S.S. Shaikevich, “Wettability in the metallic melt–thin metallic film–nonmetallic substrate system,” in: Physical Chemistry of Condensed Phases, Superhard Materials, and Their Interfaces [in Russian], Naukova Dumka, Kyiv (1975), pp. 15–27.
Yu.V. Naidich, Yu.N. Chuvashov, N.F. Ishchuk, and V.P. Krasovskii, “Wetting of some nonmetallic materials by aluminum,”Powder Metall. Met. Ceram., 22, No. 6, 481–486 (1983).
Yu.V. Naidich, I.I. Gab, T.V. Stetsyuk, and B.D. Kostyuk, “Atomization kinetics of chromium nanofilms deposited onto oxide materials in vacuum annealing,” Adgez. Raspl. Paika Mater., Issue 51, 54–61 (2018).
M. Hansen and K. Anderko, Constitution of Binary Alloys, McGraw-Hill, New York (1958).
F.A. Shunk, Constitution of Binary Alloys, McGraw-Hill, New York (1970).
V.A. Rabinovich and Z.Ya. Khavin, Concise Chemical Handbook [in Russian], Khimiya, Leningrad (1978). A.I. Efimov, L.P. Belorukova, I.V. Vasilkova, and V.P. Chechev, Properties of Inorganic Compounds: Handbook [in Russian], Khimiya, Leningrad (1983), p. 392.
Yu.V. Naidich, “The wettability of solids by liquid metals,” Prog. Surf. Membr. Sci., 14, 353–484 (1981).
A.G. Ryabukhin and O.N. Gruba, “Formation enthalpies for silicides of 3-d elements in the Periodic Table,” Vest. Yuzh. Ural. Gos. Univ., No. 3, 74–82 (2007).
G.V. Samsonov, L.A. Dvorina, and B.M. Rud, Silicides [in Russian], Metallurgiya, Moscow (1979), p. 272.
V.P. Tolstykh, Introduction to Optical Absorption Spectroscopy of Nanosized Materials [in Russian], Solo, Saint Petersburg (2014), p. 187.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 59, Nos. 1–2 (531), pp. 42–50, 2020.
Rights and permissions
About this article
Cite this article
Krasovskyy, V.P., Kostyuk, B.D., Gab, I.I. et al. Effect of Metallic Nanocoatings Deposited on Silicon Oxide on Wetting by Filler Melts I. Wetting of Ti, Nb, Cr, V, and Mo Nanocoatings Deposited on SiO2 with Filler Melts. Powder Metall Met Ceram 59, 29–34 (2020). https://doi.org/10.1007/s11106-020-00135-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-020-00135-8