The contact and capillary processes that occur in the interaction of eutectic Nb melts containing Co, Ni, and Fe with Al2O3–SiO2 and AlN–TiN solid substrates at 1400–1450°C in 10–3 Pa vacuum have been considered. The microstructure and phase composition of the interaction products in the contact area have been examined, and thermodynamic analysis of potential reactions has been performed. The effect of the solvent metal for niobium on these processes has been determined, and explanation for the different wettabilities of the same substrates has been proposed in terms of the properties (composition and structure) of the solid phase formed at the metal–ceramic interface. An example of the potential application of these melts for brazing nonmetallic materials is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the important applications of niobium is brazing and welding with nonmetallic materials and, in particular, with aluminum oxide (single crystals and ceramics). Solid-phase welding is often performed with the use of pressure or in the presence of a thin layer of liquid phase. In the latter case, the literature defines such technologies as TLP (transient liquid phase), PTLP (partial transient liquid phase), etc. ‘Moderate’ chemical activity of niobium relative to nonmetallic substrate components (oxygen, nitrogen, and carbon) prevents intensive interaction with ceramics that can lead to rather thick and fragile layers of intermediate phases and erosion of the contact surfaces of both ceramic and metal parts. This moderate interaction contributes to better adhesion of the components (for example, in the niobium–oxide system). In this regard, the use of niobium should be considered promising for strong high-temperature brazed joints in the ceramic (or other nonmetal)–metal systems.
The literature offers a relatively small number of theoretical and experimental papers devoted to this problem [1,2,3,4,5,6]. Thus, the paper [1] describes solid-phase welding (without formation of a liquid film in the contact area) of niobium with alumina ceramics under pressure in a vacuum of 10–3 Pa (t = 1500°C, P = 15 MPa, τ = 15–20 min). The joint strength in this case is relatively small (80–100 MPa). In later papers [2, 3], the welding temperature and pressure were reduced and the length of isothermal holding was significantly increased (t = = 1400°C, P = 10 MPa, τ = 3–4 h and longer). However, even under such conditions, areas were revealed where the materials failed to bond in the junction zone. The joint strength is not increased significantly and is no more than 100 MPa.
To intensify contact processes, a thin layer of liquid copper (PTLP technology) was introduced into the junction zone. For this purpose, a copper layer 3 μm thick was preliminary deposited onto a niobium or oxide material. The liquid copper dissolved niobium and accelerated its interaction with the oxide. This method allowed the joint strength to be increased to 240 MPa [2, 3]. The paper [2] also specified that ceramics could be bonded in this way at lower temperatures as well (1150°C). It should also be noted that the above-mentioned research efforts focused on joining ceramic materials with each other and used niobium as an intermediate thin (up to 150 μm thick) layer.
Analysis of the contact areas in the above-mentioned compounds showed that copper collected in intergranular microscopic hollows on the surface of niobium foil [4]. Copper is chosen as a liquid layer because it does not form intermetallides with niobium nor does it make it brittle. Detailed studies on how niobium is wetted with copper showed that this process was very sensitive to the oxygen content on Nb surface [5]. The contact angle formed by copper on niobium is 35° in a vacuum of 10–4 Pa and 14° in hydrogen (i.e., even small oxygen content on the Nb surface significantly influences the contact angle) at 1200°C. Hence, the contact angle is 56° at 1.5 at.% O on niobium and increases to 79° at 6.5 at.% O.
According to [6], the contact angle decreases to 20° in the Cu–1.4 at.% Nb–Al2O3 system after significant (hours) holding at 1150°C. At the same time, these data have not been confirmed by other researchers (including us) and require further verification. This phenomenon is probably associated with the formation of nonequilibrium receding contact angles due to partial evaporation of copper.
Note also that no recommendations have been developed so far regarding the optimum composition of metal brazes for joining oxide and other nonmetallic materials in which niobium would be the capillary active (adhesively active) element. Research papers that would study in detail the capillary and contact processes in the ‘solid oxide–niobium-containing melt’ systems have not been found either (except for the efforts mentioned above and our paper [7]). It should also be noted that titanium is added to the metal melt [8] in most cases to ensure the adequate wetting of nonmetals and that, as our previous studies [7] showed, silicon dioxide SiO2 is very well wetted by Ni–Nb melts.
In the above regard, the objective of this effort was to study contact and capillary processes in the systems consisting of eutectic niobium melts containing cobalt, iron, and alloys of cobalt and nickel, on the one hand, and solid oxides of the binary Al2O3–SiO2 system and nitride ceramic materials (AlN, TiN, Si3N4, etc.), on the other.
Experimental Procedure
We used electrolytic cobalt, nickel, and iron (99.99%) and vacuum-melted niobium (99.9%). Cobalt, nickel, and iron were subjected to additional melting in a vacuum of 10–3 Pa. The Co–13.9 at.% Nb (20.3 wt.% Co, tmelt = 1237°C), Co–43.2 at.% Nb (55.0 wt.% Co, tmelt = 1378°C), and Co–61 at.% Nb (71 wt.% Co, tmelt = 1374°C) eutectic alloys [9, 10] were preliminary fused from the respective components in alundum crucibles. The Fe–Nb alloys were fused from Fe and Nb foils in Al2O3 crucibles in vacuum. In some instances, the foils were fused in the wetting process.
The substrates to be wetted were polished (Rz ≈ 0.01 μm) leucosapphire (unoriented) plates, KI quartz glass, VK-94-1 ceramics (Al2O3–2.8 wt.% SiO2–2.4 wt.% MnO–0.5 wt.% Cr2O3 [1]), and specially synthesized ceramic materials such as mullite (3Al2O3–2SiO2) and hard porcelain (Al2O3–3.8 wt.% SiO2). The Al2O3–SiO2 phase diagram and substrate compositions are shown in Fig. 1.
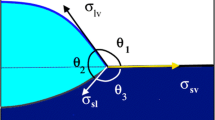
We used advanced equipment [7] that allowed us to partially immerse a 20 mm × 5 mm × 0.5 mm plate into the melt and extract it directly at the experimental temperature. The wetting process was monitored through the side window, and the sample appearance was fixed using digital instrumentation. The method involving immersion and partial extraction of the plate from the melt is used to measure both the advancing angles formed by the melt on the pure test sample and the receding and advancing angles formed on the layers of products resulting from interaction of the melt with the substrate (this, in turn, allowed us to assess the role of chemical reaction at the interface in the melt spreading process and the degree of hysteresis in the wetting process). This procedure permitted us to define the structural composition of the interaction products that formed directly at the experimental temperature.
The microstructure of the contact area between the metal melts and nonmetals was examined using optical light image (OLI) microscopy and electron microprobe analysis with a Superprobe 733 microanalyzer in SEI (secondary electron image) and X-ray (characteristic radiation of elements) modes. The phase composition of the interaction products was analyzed using DRON-3 diffractometers. X-ray diffraction patterns were measured from the surface of samples.
Experimental Results and Discussion
Wetting and Contact Interaction in the Nb–Co, Nb–Fe, and Nb–Co–Ni Eutectic Systems with Al2O3–SiO2Oxide Materials. The experimental results on the wetting of oxide systems by Co–Nb eutectic melts at 1400°C are presented in Fig. 2.
The contact angles formed by the Co–43.2 at.% Nb and Co–61.0 at.% Nb eutectics on sapphire at t = = 1400°C are 90–93°. The advancing and receding angles measured in partial immersion of the sapphire plate into the melt and its extraction are virtually the same: ~90°. Hence, no significant hysteresis is observed in the wetting of sapphire. The contact angle formed by the eutectic melt on quartz glass is small, 25–30°, and decreases to 20–15° with increasing temperature (at a rate of ~5 K/min) to 1450°C and after isothermal holding for 30 min. Note also that increase in the niobium content of the melt from 43.2 to 61 at.% decreases the contact angles on SiO2 by no more than 10°, while the contact angle on sapphire does not change at all (this is also pointed out in [11]).
The wetting of oxide materials with an intermediate amount of SiO2 improves with increase in its concentration in the substrate. The degree of wetting mainly changes within the first 7–10 min in the isothermal holding process at 1400°C, whereas subsequent (up to 30 min) holding causes only a slight decrease in the contact angle. Increase in the experimental temperature to 1450°C hardly influences the contact angle.
Additions of Ni–Nb eutectic alloys to the Co–Nb melts slightly reduce the contact angle (to 84–86°) on Al2O3. We have not revealed any effect of these additions in amounts of 50 mol.% on the melt solidification temperatures, nor have we found any Co–Nb–Ni phase diagrams.
Wetting of Al2O3–SiO2Oxide Materials by the Fe–Nb Eutectic Melts. According to the phase diagram, the Fe–Nb system [10] has three eutectics: 1) Fe–10.4 at.% Nb (tmelt = 1373°C), 2) Fe–40.2 at.% Nb (tmelt = 1535°C), and 3) Fe–65.6 at.% Nb (tmelt = 1400°C).
The Fe–10.4 at.% Nb and Fe–65.6 at.% Nb eutectic melts formed contact angles θ = 84 ± 3° on leucosapphire and alumina ceramics at 1450°C after 15 min holding; i.e., no explicit composition dependence of the contact angles was observed (however, note that the wettability of Al2O3 slightly improves when the solvent for niobium was changed from Ni to Fe).
The cold droplets were broken off at the interfaces with the substrate. No significant dissolution of leucosapphire in the melt after isothermal holding at 1450°C for 15 min was observed. These alloys only partially fused on quartz glass at 1450°C (this is probably due to the intensive interaction at the interface with the subsequent formation of refractory niobium silicides). The Fe–65.6 at.% Nb eutectic alloy is silvery at the interface and, hence, the formation of NbOx≥1 oxide compounds is unlikely.
X-Ray Diffraction of the Contact Areas between the Solidified Co–Nb Droplets and Quartz Glass. X-ray patterns were measured from the surface of a metal droplet broken off from the oxide substrate. The experimental results are summarized in Table 1.
Thermodynamic calculations of interaction in the Nb–SiO2 system show that lower niobium oxides and Nb5Si3 and NbSi2 silicides [9] are most likely to form:
The structural composition of these interaction products (selected among isostructural phases) was refined upon microradiography analysis of the interfaces between the Co–Nb alloy and quartz glass.
Microstructure of the Contact Areas between the Co–Nb Alloys and Oxide Substrates. Figures 3 and 4 show the microstructures of the contact areas between quartz glass and Co–13.9 at.% Nb and Co–61.0 at.% Nb melts. The contact areas between the glass and Co–13.9 at.% Nb melt remained almost intact, and only slight niobium absorption is observed at interfaces.
As seen, increase in the niobium content of the melt from 13.9 to 61.0 at.% enhances the interaction of niobium with glass: new Nb phases that contain neither cobalt nor silicon are formed (they can be considered to be NbOx phases). No significant accumulations that would contain silicon are found in Fig. 4. Therefore, we believe that silicon did not form any silicides, but dissolved in the melt. Accordingly, we can refine the X-ray diffraction results for quartz glass with the Co–61.0 at.% Nb melt (t = 1400°C, τ = 30 min), where oxygen-containing compounds Co6Nb6O and NbO are the main components of solid-phase interaction at interfaces.
Figure 5 shows the microstructure of contact areas between mullite and Co–43.2 at.% Nb melt. There is a wide zone of intensive interaction between the melt and substrate. The interaction in this system is also peculiar in that there are fine aluminum-containing particles. Hence, silicon dioxide is reduced from the mullite and aluminum oxide remains in the form of fine disperse aggregates.
There is also a silicon cluster in the contact area of the system in question, which may be due to obstacles in the removal of Si from the contact area to the melt. In addition, when wetted with the Co–Nb melts, mullite and hard porcelain become blue and quartz glass does not change its color. This may indicate that there is weakly (more weakly than SiO2) bound oxygen that oxidizes both niobium and cobalt in the mullite and hard porcelain (for example, in closed pores). The degree of coloration (blue) depends on the amount of weakly bound oxygen in the mullite contributing to the oxidation of cobalt. The vacuum melting of mullite significantly reduces, but does not eliminate completely, the blue color of the sample.
Wetting and Contact Interaction in the ‘Co–Nb Eutectic Melt–Nitride Material’ Systems. The Si3N4 ceramics are wetted by the Co–Nb melts in the way similar to the Ni–Nb melts: the melt spreads over a long time (to 40 min and longer) and completely covers the ceramic sample like a film. Thermodynamic calculations show that niobium silicide (Nb5Si3), which is well wetted by the metal melt, is most likely to form at interfaces.
The wetting behavior of Co–Nb melts on AlN and TiN + 10AlN nitride materials at 1420°C is shown in Figs. 6 and 7. Titanium nitride is wetted with the cobalt–niobium melt much better than aluminum nitride. Our long-term kinetic dependence of the AlN wetting process indicates that the contacting phases also change their composition at the interface, which agrees with the thermodynamic calculations. Aluminum nitride begins to evaporate substantially at 1500°C (–logpN2 = 3.918 at.%; –logpAl = +3.617 at.% [12]).
To estimate the likelihood of new solids to form at the melt–solid interfaces, influencing both wetting and joint strength, we calculated changes in the Gibbs energy, ∆G°, for potential reactions between niobium and wetted substrate:
The calculations show that interaction between niobium and Si3N4 by Eq. (4) is the most probable. The small positive value in Eq. (5), such as ∆G°1500K = +28.1 kJ/mol-at. N, allows us to assume that AlN can dissolve in the presence of Nb in vacuum conditions.
Some improvement in the wettability of TiN relative to AlN cannot be explained by the results of these calculations since, according to Eq. (6), Gibbs energy ∆G°1500K has a high positive value: +70.8 kJ/mol-at. N. This effect may be attributed to a wide homogeneity range of TiN. Hence, the following reaction can occur:
where [N]Nb is a solution of nitrogen in niobium. This equation indicates that TiN can lose the nonmetal (nitrogen), and the metal–metal bonding contribution can increase on the substrate surface, which will improve the substrate wetting with the metal melt.
In addition, according to Samsonov, TiN can lose nitrogen up to the formation of TiN0.52 under vacuum alone at high temperatures [13, 14]. This, in turn, increases the metallic properties of the nitride formed and, consequently, improves the degree of wetting.
The contact area between AlN and TiN brazed with a mixture of Co–43.2 at.% Nb and Ni–40.5 at.% Nb eutectic powders in a 1 : 1 ratio is shown in Fig. 8 as a sample application of niobium melts. Figure 8 indicates that nickel and cobalt are evenly distributed in the brazed joint, which has dense structure. Under mechanical loading, such a joint fails in the ceramic part in most cases.
Comparison of Wetting Behavior of the Co–Nb and Ni–Nb Melts on Al2O3–SiO2Oxide System and AlNBased Ceramics. The comparison results, in particular, for Al2O3–SiO2 oxides at 1400°C are presented in Fig. 9.
In general, the results indicate that the Ni–Nb melts wet these materials better than Co–Nb. At the same time, these results do not agree with the general thermodynamic considerations relating to wetting: the bonding energy of Ni–Nb is greater than that of Co–Nb, as shown in Table 2, so Co–Nb melts should ensure better wettability.
The answer, most likely, should be sought in terms of the properties, composition, and state of the products resulting from the interaction between the droplet melt and solid wetted surface.
We previously found that Ni–Nb melts formed solids with lower oxygen content at interfaces with oxides [7] than Co–Nb melts, which may lead to better wetting. At the same time, aluminum nitride ceramics are wetted by the Co–Nb melts not worse than by Ni–Nb: the contact angles formed by the Ni–40.5 at.% Nb melt on the AlN + (3–5%) Y2O3 surface are 95–105° (t = 1400°C, τ = 60 min) [18]. We performed refinement experiments to show that the Co–43.2 at.% Nb melt formed contact angles of 95–110° in these conditions. Therefore, the solvent (Ni or Co) has no effect on the wetting of AlN substrates.
Considering the appearance of blue color on the mullite and porcelain when they are wetted by the Co–Nb melts, being indicative of cobalt oxide, we analyzed the NiO–SiO2 and CoO–SiO2 phase diagrams [9]. We established that the oxide melt appeared in the NiO systems only at 1680°C, which is much higher than the wetting temperature. In the systems containing CoO, the oxide melt shows up already at 1380°C, i.e., at temperatures below the wetting temperature (t = 1400–1450°C). It is this factor (the appearance of oxide melt in the interaction products in the contact area) that influences the composition, structure, and adhesion properties of the systems in question. From an applied viewpoint, this result is important in choosing the composition of brazes, crucibles for metal
melting, etc.
Conclusions
In general, the experimental results indicate that niobium can be used as an adhesively active element in metal brazes to improve the degree of wetting and adhesion of the melts to oxide, nitride, and many other materials.
We have examined the production of a brazed joint for ceramic materials (in particular, AlN and TiN) using niobium-containing brazes.
References
I.I. Metelkin, M.A. Pavlova, and N.V. Pozdeev, Welding of Ceramics with Metals [in Russian], Moscow (1997), p. 159.
M.L. Shalz, B.J. Dalgleish, A.P. Tomsia, R.M. Cannon, and A.M. Glaeser, “Ceramic joining: III. Bonding of alumina via Cu/Nb/Cu interlayers,” J. Mater. Sci., 29, No. 14, 3678–3690 (1994).
J.D. Sugar, J.T. McKeown, and R.A. Marks, “Liquid-film-assisted formation of alumina/niobium interfaces,” J. Am. Ceram. Soc., 85, No. 10, 2523–2530 (2002).
J.T. McKeown, J.D. Sugar, R. Gronsky, and A.M. Glaeser, “Effects of impurities on alumina–niobium interfacial microstructures,” Mater. Charact., 57, 50–57 (2006).
O.F. De Lima, M. Krehl, and M. Schuzek, “Wetting characteristics of copper on niobium,” J. Mater. Sci., 20, 2464–2470 (1985).
A.P. Tomsia, E. Saiz, S. Foppiano, and R.M. Cannon, “Reactive wetting: Ridging, adsorption, and compound formation,” in: Proc. 2nd Int. Conf. High Temperature Capillarity HTC–97 (Cracow, Poland, 1997), Cracow (1998), pp. 59–80.
V.S. Zhuravlev, N.Yu. Taranets, A.Yu. Koval, M.V. Karpets, and Yu.V. Naidich, “Wetting and interface microstructure in the system of Al2O3–SiO2 based ceramics/Nb-containing melts,” Open Ceram. Sci. J., 2, 8–14 (2012).
Yu.V. Naidich, V.S. Zhuravlev, V.G. Chuprina, and L.V. Strashinskaya, “Adhesion, wetting, and formation of intermediate phases in systems composed of a titanium-containing melt and an oxide,” Powder Metall. Met. Ceram., 12, No. 11, 895–899 (1973).
FACT, Fact-Web, Reaction-Web-properties of a species or chemical reaction, URL: http://www.crct.polymtlca/fact.
T.B. Massalski and H. Okamoto, Binary Alloy Phase Diagrams, 2nd ed., Ohio, USA (1990), p. 3589.
F. Valenza, M.L. Muolo, A. Passerone, and A.M. Glaeser, “Wetting and phenomena in joining of alumina via Co/Nb/Co interlaуers,” J. Eur. Ceram. Soc., 33, 539–547 (2013).
I.S. Kulikov, Thermal Dissociation of Compounds [in Russian], Moscow (1969), p. 574.
G.V. Samsonov, Nitrides [in Russian], Kyiv (1969), p. 377.
Y. Nakao, K. Nishimoto, and K. Saida, “Reaction layer in nitride ceramics (Si3N4 and AlN) to metal joints bonded with active filler metals,” ISIJ Int., 30, No. 12, 1142–1150 (1991).
T.N. Rezukhina, L.I. Kravchenko, and B.S. Pokarev, “Thermodynamic properties of Laves phases,” in: Metal Physics (Republican Interagency Collection of Papers) [in Russian], Kyiv (1973), Issue 46, pp. 21–28.
V.I. Alekseev, G.B. Petrov, and G.V. Shcherbediiskii, “Study of thermodynamic properties of Ni–Nb alloys,” Izv. Akad. Nauk SSSR. Met., No. 5, 59–62 (1978).
V.N. Drobyshev, Thermodynamic Study of Some Alloys of Molybdenum and Niobium with Iron Group Metals [in Russian], Author’s Abstract of PhD Thesis in Chemical Sciences, Moscow (1965), p. 14.
V.S. Zhuravlev, N.Yu. Taranets, A.Yu. Koval, M.V. Karpets, and Yu.V. Naidich, “Scientific and technical fundamentals for use of niobium of an adhesive component in metallic brazes for joining oxide and nitride materials,” Adhez. Rasp. Paika Mater., No. 43, 78–90 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 57, Nos. 9–10 (523), pp. 94–104, 2018.
Rights and permissions
About this article
Cite this article
Zhuravlev, V.S., Sydorenko, T.V., Karpets, M.V. et al. Contact and Capillary Processes in Systems Promising for Brazing: Eutectic Melts of Niobium with Cobalt, Nickel, and Iron–Nonmetallic Al2O3–SiO2 and AlN–TiN Materials. Powder Metall Met Ceram 57, 573–581 (2019). https://doi.org/10.1007/s11106-019-00018-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-019-00018-7