Alloys of the ternary Cu–Ti–Zr system with 50–100 at.% Cu, annealed at 750°C, are studied by scanning electron microscopy, electron microprobe analysis, and X-ray diffraction. The isothermal section at 750°C is constructed in this composition range. A new hexagonal ternary phase of composition Cu63.5Ti14.5Zr22 is found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first paper [1] of the series provides a brief overview of the literature on phase equilibria in the ternary Cu–Ti–Zr system [2,3,4,5,6,7,8] and presents our own experimental results as the isothermal section of the phase diagram at 750°C with copper content from 0 to 50 at.% [1]. It is shown that the τ1 ternary phase (Laves phase of MgZn2 type) has an appreciable homogeneity range at 750°C. Its extension by copper is ~7 at.% (in a range from 42.6 to 49.8 at.% Cu) and by titanium is about 8 at.% (in a range from 20 to 28 at.% Ti). The copper content of the β-(Ti, Zr) bcc solid solution at 750°C becomes higher when Ti content increases from 1.5 at.% (at 11 at.% Ti) to ~4 at.% (at 81.5 at.% Ti). The continuous series of solid solutions [Cu(Ti, Zr)2, MoSi2 type], i.e., the γ phase, is linear by copper content (within the error of electron microprobe analysis). To construct the section, the boundary binary Cu–Ti [9], Cu–Zr, and Ti–Zr phase diagrams were used [10]. The data obtained on the constant (CuX2) copper content of the γ phase support the Cu–Ti phase diagram according to which CuTi2 compound has no homogeneity range [9].
Our objective is to study the Cu–Ti–Zr system at 750°C in the range 50–100 at.% Cu. Additional study of the system is necessitated by distinctions in the phase equilibria not only at different temperatures (703°C [5] or 750°C [6]), but also at the same temperature, 800°C, in [7, 8]. Table 1 briefly outlines the analysis methods and composition–temperature ranges of the phase diagram according to different researchers. Special attention is paid to the purity of starting materials and alloy production conditions (synthesis and heat treatment of the samples) since they can substantially influence the alloy structure and, accordingly, phase equilibria.
The crystallographic data on interim binary and ternary phases of the Cu–Ti–Zr system at copper content from 50 to 100 at.% are provided in Table 2.
Experimental Procedure
Ternary alloys of more than 20 compositions (Table 3, Fig. 1) were melted for the experimental study in this region. The starting materials were electrolytic copper (99.99 wt.%) and iodide-refined titanium (99.94 wt.%) and zirconium (99.96 wt.%). The ingots with a weight of 10 g were melted in an arc furnace with a nonconsumable tungsten electrode on a water-cooled copper hearth in an argon atmosphere purified by melting titanium–zirconium getter (argon pressure ~80 kPa) for 5 min. The ingots were cooled down at a rate of ~100°C/sec.
The ingots were flipped and remelted at least three times to avoid inhomogeneity of chemical composition. Since the weight loss after melting was no more than 0.2 wt.%, the alloy composition was accepted as in the starting charge. The presence of admixtures in the starting copper and, selectively, in the annealed alloys was checked with spectral (ternary alloys Cu55Ti25Zr20 (sample 32), Cu63.5Ti14.5Zr22 (sample 36), and Cu63.5Ti20Zr16.5 (sample 37)) and chemical (binary alloys Cu27.6Zr72.4 and Cu33.3Zr66.7) analyses. It is shown that the ternary alloys contain 10–2–10–3 wt.% and less of Fe admixtures, 10–1–10–2 wt.% and less of Ni, and 10–1 wt.% Zn; Al, Mo, Si, and W were not revealed. The oxygen content of binary alloys Cu27.6Zr72.4 and Cu33.3Zr66.7 is 0.052 and 0.014 wt.%, respectively, while N and H were not revealed.
The alloy samples (from 6 to 13 pieces), each being wrapped in titanium foil, were placed in a quartz capsule 25 mm in diameter and 170 mm in length, considering extension of the hot area (100–110 mm). The capsule walls and lower and upper parts were additionally insulated with titanium foil and shavings. Then the capsule was evacuated and filled with argon (excess pressure ~30,397 Pa). Three to six capsules were placed into a ceramic crucible. The space between the capsules, as well as between the capsules and crucible wall, was filled with sand to decrease temperature gradient in the furnace during annealing. The samples, except for alloys Cu53Ti11Zr36 (sample 30), Cu63.5Ti14.5Zr22 (sample 36), Cu63.5Ti20Zr16.5 (sample 37), Cu63.5Ti29.5Zr7 (sample 38), and Cu70Ti2Zr28 (sample 39), were annealed in an electric muffle furnace at 750°C for 536 h. Alloys 30 and 36–39 were annealed in two steps—200 h and 523 h at the same temperature. Following annealing, the capsules with the samples were placed into cold water without breaking because of high activity of the metal components.
The alloys were examined by XRD, OM, SEM, and EMPA. The alloy microstructures are represented by well-faceted phase grains with clear boundaries. This indicates that the annealing time was sufficient to reach the equilibrium state. The X-ray diffraction patterns were taken with a DRON-3 diffractometer for powders or sections in filtered copper radiation (Cu ‐ K α, λ = 0.1542nm) and processed using the PowderCell 2.2 full-profile analysis software.
In addition, sample 36 was studied in a Guinier camera in monochromatic copper radiation (λ = = 0.154051 nm). Scanning electron microscopy and EMPA were carried out employing Superprobe JXA-8200 (JEOL Ltd., Japan), INCA Penta FETx3 (Oxford Instrument, United Kingdom), and Zeiss SUPRA55VP (Carl Zeiss Group, Germany).
Results and Discussion
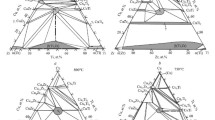
The chemical and phase compositions of the alloys annealed at 750°C are provided in Table 3. An isothermal section has been constructed in the CuZr2–Cu–CuTi2 region (Fig. 1).
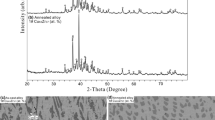
In the alloys with 52.7 to 63.5 at.% Cu (Table 3), a new ternary phase was revealed (τ2); its composition was determined as Cu63.5Ti14.5Zr22 according to averaged EMPA data for alloys in adjacent phase regions. The alloy of the same composition that was produced additionally (sample 36) turned out to be single-phase after annealing at 750°C for 200 + 523 h (Fig. 2 a), thus confirming the existence of the ternary compound. The homogeneity range of the new phase is not extensive and lies within the measurement error. The diffraction pattern of annealed alloy Cu63.5Ti14.5Zr22 was taken in a Guinier camera in the range 0–100° at increment 2θ = 0.005° (Fig. 3 a). Table 4 shows angular positions and intensities of the strongest reflections (2θ).
Comparison of the X-ray diffraction pattern for the new τ2 phase (Fig. 3 a) and theoretical diffraction patterns for the known ternary Laves phase, Cu2TiZr (τ1), and binary orthorhombic Cu10Zr7 and hexagonal Cu51Zr14 compounds (Fig. 3 b, c), which have extensive homogeneity ranges and coexist with the τ2 phase at 750°C in the ternary system (Fig. 1), shows that they substantially differ in angular positions and intensity of reflections.
Hence, the crystal structure of the τ2 phase is not identical to these compounds. We did not manage to obtain an isolated single crystal for crystallographic analysis. According to study of eight fragments of the annealed sample, the τ2 phase has a 1200 pm × 980 pm hexagonal cell.
The microstructure of two-phase alloy Cu55Ti20.7Zr24.3 (sample 31), which has two ternary phases (τ1 + τ2) according to EMPA and XRD, is shown in Fig. 2 b and a fragment of its experimental diffraction pattern in Fig. 4 a. These ternary compounds have very close microhardness, differing within the measurement error and constituting ~6.0 GPa. These two phases also differ only little in contrast in backscattered electrons. Therefore, only EMPA can be used for reliable identification (and differentiation) of these ternary compounds in metallographic analysis of the alloys.
Phase analysis of experimental X-ray diffraction spectra of the alloys: 31 (a), 1—reflections of the τ2 phase, 2—reflections of the τ1 phase; 34 (b), 1—reflections of the τ2 phase, 2—reflections of the CuTi phase; 37 (c), 1—reflections of the τ2 phase, 2—reflections of the CuTi phase, 3—reflections of the Cu51Zr14 phase
At 750°C, the τ2 phase forms a number of two-phase equilibria—τ2 + τ1 (Figs. 2 b and 4 a), τ2 + (Cu10Zr7), τ2 + (Cu51Zr14), and τ2 + (CuTi) (Fig. 4 b)––and three-phase equilibria—τ2 + τ1 + (CuTi) (Fig. 2 c, d), τ2 + τ1 + (Cu10Zr7) (Fig. 2 e), and τ2 + (CuTi) + (Cu51Zr14) (Fig. 2 f). Three-phase alloys τ2 + (Cu51Zr14) + (Cu10Zr7) were not obtained. Nevertheless, the three-phase τ2 + (Cu51Zr14) + (Cu10Zr7) equilibrium in the isothermal section is clearly evidenced by the existence of boundary two-phase regions, such as (Cu51Zr14) + (Cu10Zr7), τ2 + (Cu51Zr14), and τ2 + (Cu10Zr7), established experimentally (Fig. 1).
The existence of phase equilibria involving the τ2 phase and three-phase equilibria, such as τ1 + (CuZr) + (Cu10Zr7) (Fig. 5 a; Table 3) and τ1 + (CuZr) + γ[1], significantly distinguishes our isothermal section at 750°C from the isothermal section at the same temperature provided in [6] Fig. 6. The existence of ternary τ1 + (CuZr) (Cu10Zr7) and τ1 + (CuZr) + γ equilibria is consistent with the data at 703°C [5] and 800°C [7, 8].
Isothermal section of the Cu–Ti–Zr phase diagram in the CuZr2–Cu–CuTi2 region at 750°C [6]; our data are dashed
Figure 1 shows that the Cu51Zr14-based phase dominates in the region under study. It participates in all expected three-phase equilibria: (Cu51Zr14) + (Cu10Zr7) + (Cu8Zr3) (Fig. 5 b), (Cu51Zr14) + (Cu4Ti3) + (CuTi) (Fig. 5 c), (Cu51Zr14) + (Cu4Ti) + (Cu3Ti2) (Fig. 5 e), (Cu51Zr14) + (Cu3Ti2) + (Cu4Ti3), (Cu51Zr14) + (Cu4Ti) + (Cu) (Fig. 5 f), and (Cu51Zr14) + (Cu5Zr) + (Cu). This agrees with the previous studies [6, 7].
Note that the paper [8] does not consider two three-phase equilibria: (Cu51Zr14) + (Cu10Zr7) + (Cu8Zr3) and (Cu51Zr14) + (Cu5Zr) + (Cu).
The maximum titanium content of the (Cu51Zr14) phase reaches 10 at.%. Significant titanium solubility is also observed in the (Cu10Zr7) phase, 12 at.%, and (CuZr) phase, 7.3 at.%, which is not the case for the Cu5Zr and Cu8Zr3 phases (Fig. 1). The solubility of zirconium in binary compounds in the Cu–Ti system is insignificant: it is the highest in (CuTi), about 3 at.%. Data on the solubility of the third component agree well with the results reported in [6,7,8]. The only exception is [6], according to which titanium is hardly dissolved by CuZr.
Conclusions
In the ternary Cu–Ti–Zr system, a new ternary phase of hexagonal syngony has been found at 750°C and denoted by τ2; its crystal structure has not been ascertained. This phase coexists in two-phase and three-phase equilibria with the Cu2TiZr (τ1), CuTi, Cu10Zr7, and Cu51Zr14 phases. The microhardness of the τ2 phase is 6.0 ± ± 0.1 GPa.
There are 11 tie-line triangles in the isothermal section at 750°C in the CuZr–Cu–CuTi region. The Cu51Zr14 phase is in equilibria with most phases of the ternary system at 750°C. Its dominant role in the region reflects relatively high thermodynamic stability, which is consistent with its congruent formation in the binary Cu–Zr system.
References
A. M. Storchak-Fedyuk, L. V. Artyukh, L. A. Duma, et al., “Phase equilibria in the Cu–Ti–Zr system at 750°C. I. The isothermal section with copper content from 0 to 50 at.%,” Powder Metall. Met. Ceram., 56, No. 1–2, 78–87 (2017).
E. Ence and H. A. Margolin, “A study of the Ti–Cu–Zr system and the structure of Ti2Cu,” Trans. Metal. Soc. AIME, 221, 320–322 (1961).
V. N. Chebotnikov and V. V. Molokanov, “Structure and properties of Ti2Cu–Zr2Cu alloys in the Ti–Zr–Cu system in amorphous and crystalline states,” Izv. Akad. Nauk SSSR. Neorg. Mater., 26, No. 5, 960-964 (1990).
Yu. K. Kovneristyi and A. G. Pashkovskaya, “Bulk amorphization of alloys in the intermetallic Ti–Cu–Zr system,” in: Amorphous (Glassy) Metallic Materials [in Russian], Inst. Metall. RAN, Moscow (1992), pp. 153-157.
C. G. Woychik and T. B. Massalski, “Phase diagram relationships in the system copper–titanium–zirconium,” Z. Metallkd., 79, No. 3, 149-153 (1988).
P. G. Qin, H. Wang, H. S. Zhang, et al., “The isothermal section of the Cu–Ti–Zr system at 1023 K measured with diffusion-triple approach,” Mater. Sci. Eng. A, 476, 83-88 (2008).
U. E. Klotz, C. Liu, P. J. Uggowitzer, et al., “Experimental investigation of the Cu–Ti–Zr system at 800°C,” Intermetallics, 15, 1666-1671 (2007).
W.-R. Chiang, K.-C. Hsieh, Y. A. Chang, et al., “Phase equilibrium in the Cu–Ti–Zr system at 800°C,” Mater. Trans., 48, No. 7, 1631-1634 (2007).
I. Ansara and V. Ivanchenko, “Cu–Ti (copper–titanium),” in: G. Effenberg (ed.), MSIT Binary Evaluation Program, MSIT Workplace, Materials, MSI (2002).
T. V. Massalski (ed.), P. R. Subramanian, H. Okomoto, et al., Binary Alloy Phase Diagrams, 2nd ed., 3 vols., ASM International, Materials Park, Ohio (1990), p. 3589.
T. Velikanova and M. Turchanin, “Copper–titanium–zirconium,” in: Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, W. Martiensen (ed.), New Series. Group IV: Physical Chemistry. Ternary Alloy Systems. Phase Diagrams, Crystallographic and Thermodynamic Data, Vol. 11C3, Springer-Verlag, Berlin, Heidelberg (2006), pp. 436–464.
M. Yu. Teslyuk, Intermetallic Compounds with Laves Phase Structure [in Russian], Nauka, Moscow (1969), p. 138.
X. Yan, X.-Q. Chen, A. Gritsiv, et al., “Crystal structure, phase stability, and elastic properties of the Laves phase ZrTiCu2,” Intermetallics, 16, 651-657 (2008).
Acknowledgements
The authors are grateful to L. A. Duma (Frantsevich Institute for Problems of Materials Science, National Academy of Sciences of Ukraine) and V. B. Sobolev (Technical Center under National Academy of Sciences of Ukraine) for technical support in the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 56, Nos. 3–4 (514), pp. 131–142, 2017.
Rights and permissions
About this article
Cite this article
Storchak-Fedyuk, A.M., Artyukh, L.V., Grytsiv, A.V. et al. Phase Equilibria in the Cu–Ti–Zr System at 750°C. II. The Isothermal Section with Copper Content from 50 to 100 at.%. Powder Metall Met Ceram 56, 220–230 (2017). https://doi.org/10.1007/s11106-017-9889-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9889-1