The wetting of compact materials made of pure copper and copper bronzes (tin, beryllium, aluminum) by low-temperature tin-based solders is studied with the sessile drop method with drop melt capillary purification in the experiment. The Sn–Ag–Cu solders wet the bronzes more actively than Sn–Pb (POS-61) and Sn–Bi alloys. The wetting of vacuum-annealed copper and bronze substrates by POS-61, O-2, Sn–Bi, CASTIN, and SAC alloys show that copper and beryllium bronze are wetted by solder alloys better. The Sn–Ag–Cu solders and Sn–Bi alloys are used for metallization and soldering of copper mesh structures. Soldered copper wire 0.1 mm in diameter is obtained to produce mesh representing a knitted soldered structure. The resistivity of O-2, SAC, Sn–Bi, and POS-61 alloys and the surface resistivity of the knitted soldered copper mesh are determined. The metallization of copper wire and soldering of mesh knots substantially influence the mesh surface resistivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mesh structures have found applications along with compact copper materials in electrical engineering. These structures use low-temperature tin-based solder melts for soldering and metallization of wire mesh conductors in the lightning protection systems for polymer composites (carbon fiber reinforced polymers) in aircraft and wind power engineering [1, 2]. The lightning conductor effect allows the rate and direction of thermal and electrical energy transfer from the lightning-affected carbon fiber area to be controlled depending on the condition and resistivity of soldered mesh knots. The presence of solder on wires and at mesh knots also promotes energy dissipation as lightning-affected areas heat up and low-melting solder locally evaporates [3]. Moreover, the metallization of copper wire protects it against corrosion in the carbon fiber structure.
In the technology of conducting wire compounds, wetting of the wire with metallic solder melt is important since it ensures high-quality metallization of the copper wire and reliable soldering of knitted wire and mesh knots. In addition, the solder melt should not significantly increase the resistivity of the conducting mesh structure.
Lead-based alloys have been used until recently as low-temperature solders for copper soldering. These solders show high fluidity, good performance, high corrosion resistance, and ease of operation. However, the use of lead in solders has been prohibited on the European market since 2006 because of its toxicity.
Research efforts are currently underway to develop soldering processes applying various low-temperature lead-free solders. The following major groups of lead-free solders are promising.
The Sn–Cu System. The disadvantage of these copper-containing eutectic alloys is an increased melting point (compared to the operating temperature of lead-containing solders) and lower mechanical properties compared to other lead-free solders [4].
The Sn–Ag System. The silver-containing solders possess good mechanical properties and are soldered better than copper-containing ones. These solders are eutectic (melting point 221°C). Comparative soldering tests using this solder and conventional lead-containing one show that the lead-free solder is much more advantageous in terms of soldering reliability [4].
The Sn–Ag–Cu System. The eutectic alloy of tin, silver, and copper had long been in use before the Sn–Ag solder appeared. The advantage of this solder is a lower melting point (217°C). Conventional SAC solders of composition (wt.%) 95.5Sn + 3.2Ag + 0.7Cu are more reliable and show better performance than silver- or copper-containing lead-free solders. An addition of antimony (0.5 wt.%) resulted in even greater performance of this solder (commercially called CASTIN) and allowed its use in industry [4].
The Sn–Ag–Bi (Cu, Ge) System. The melting point of this alloy is 200–210°C, it shows the best performance among the lead-free solders, and an addition of Cu and/or Ge improves both the wettability of surfaces being soldered and the strength of soldered compound as a whole [4].
The objective of this research is to examine the wetting of copper-based compact materials by some practically important lead-free solders, compare their wettability with that of the POS-61 solder, select the conditions for metallization and soldering of copper-based materials, determine the resistivity of solder alloys and surface resistivity of knitted mesh samples, and develop recommendations for soldering using the wire mesh melts proposed in this paper.
Experimental Procedure
The wettability was studied with the sessile drop method using melt capillary purification in the experiment [5] conducted in a vacuum of 2 · 10–3 Pa in the range 250–450°C. Melt dripping through a graphite dropper (Fig. 1) with a hole diameter to ~10–3 m promoted separate heating of the drop and substrate, as well as capillary and vacuum heat treatment of the melt. The sessile drop method with capillary purification allows temperature and kinetic dependences of the contact angles to be plotted, the results being highly reproducible.
Compact bronze substrates of various grades (tin BrO10, aluminum BrA7, and beryllium BrB2) and M1 copper substrate were wetted by lead-free solders. The liquid phase was presented by Sn–8 wt.% Bi melts, SAC (95.5 wt.% Sn–3.8 wt.% Ag–0.7 wt.% Cu) solder, and standard SAC (Sn–3.2 wt.% Ag–0.7 wt.% Cu), O-2 (~99.95 wt.% Sn), and CASTIN (Sn–2.5 wt.% Ag–0.7 wt.% Cu–0.5 wt.% Sb) solders, standard POS-61 (61 wt.% Sn, ≤0.8 wt.% Sb, the rest is Pb) solder being used as reference. The nonstandard solders were prepared by preliminary melting of different metals (with up to 99.999 wt.% major component) in graphite crucibles in vacuum at 350°C.
The resistivity of the solders and mesh was determined with the four-probe method at room temperature. Special rod samples were made to measure solder resistivity (rods were melted and further drawn to reach the required diameter and length).
Experimental Results and Discussion
The wettability of bronzes and copper in the range 250–450°C is compared in Fig. 2. The contact angles decrease with increasing temperature. The wetting of vacuum-annealed copper [4, 6] and bronze substrates by O-2 (Fig. 2 a), SAC (Sn–3.2 wt.% Ag–0.7 wt.% Cu) (Fig. 2 b), Sn–8 wt.% Bi (Fig. 2 c), CASTIN (Sn–2.5 wt.% Ag–0.7 wt.% Cu–0.5 wt. % Sb) (Fig. 2 d), SAC (Sn–3.8 wt.% Ag–0.7 wt.% Cu) (Fig. 2 e), and POS-61 (Fig. 2 f) melts shows that copper and beryllium bronze are wetted well by the solder melts and the lowest wettability is exhibited by aluminum bronze; its contact angles exceed 90° since the substrate surface is oxidized. The wetting with lead-free solders is somewhat better than that with the POS-61 solder.
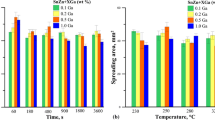
The contact angles decrease in the solder series as follows: POS-61 → O-2 → Sn–Bi → SAC (Sn–3.2 wt.% Ag–0.7 wt.% Cu) → CASTIN → SAC (95.5 wt.% Sn–3.8 wt.% Ag–0.7 wt.% Cu). The Sn–Ag–Cu solders wet bronzes more actively than Sn–Pb and Sn–Bi alloys: the contact angles are 19–25° at 400°C when beryllium bronze is wetted by the SAC and CASTIN alloys, 27° for the Sn–Bi alloy, and 37° for the POS-61 solder. The contact angle for the standard SAC solder alloy differs from that prepared before the experiment apparently because we used high-purity metals. The solder spread over the beryllium and tin bronze substrates within 15–20 min (Fig. 3).
The results demonstrate that tin-based lead-free solders can be used for soldering and metallization of copper wires. However, it should be borne in mind that we obtained these contact angles in vacuum with additional melt purification. These conditions differ from the metallization of copper wire and soldering of mesh knots with a flux. Therefore, it was needed to examine the wetting of copper materials by lead-free solders in air with a flux.
We studied the wetting of compact copper and beryllium bronze by the SAC (Sn–3.8 wt.% Ag–0.7 wt.% Cu) solder at 250°C with the LK-2 flux (28 wt.% rosin, 3 wt.% zinc chloride, 1 wt.% ammonium chloride, 68 wt.% ethyl alcohol).
The spreading in this case is rather fast, but the contact angles after 5–10 min are somewhat lower than those obtained in vacuum: 21° (in vacuum) and 18° (under flux) for copper and 42° (in vacuum) and 38° (under flux) for beryllium bronze.
The experimental results demonstrate that lead-free solders can be used for soldering and metallization of copper wire materials in process conditions. This allowed us to select the solders and parameters for metallization of wire mesh (knitted copper lightning-protection mesh structure is shown in Fig. 4).
In aviation and wind power engineering applications, lightning-protection mesh is commonly placed on the outer surfaces of carbon fiber reinforced polymer parts. When lightning strikes knitted wire mesh, representing soldered knots along with metallized wire, the total resistivity of this structure (mesh surface resistivity) and resistivity of soldered knots are important.
he resistivity of knitted mesh can be reduced through reliable electrical contacts at mesh knots. For this purpose, we used knitted copper wire mesh with knots metallized by the low-melting lead-free solder. The current-conducting capability of lightning-protection mesh can be characterized by solder resistivity; the influence of soldering on the resistivity of copper wire and mesh is also indicative. Therefore, we measured the resistivity of the solder alloys and knitted mesh.
Table 1 shows the resistivity of solder alloys at room temperature. The results are highly reproducible.
We established that the solders had much higher resistivity than copper. The solder high resistivity contributes to the total resistivity of soldered mesh knots, where contact resistance is essential. It is knitted mesh knots where intensive heating from Joule heat occurs and the low-melting solder starts evaporating (lightning energy dissipation effect), leading to the loosening of knots, decrease in mechanical load (relaxation of mesh mechanical stresses), and expenditure of energy for drawing wire from the binder layer. All these effects decrease the destructive lightning action on carbon fiber reinforced polymers.
The surface resistivity of knotted mesh structures is important for aircraft lightning protection since the integrity of carbon fiber reinforced polymers depends on this quantity.
Table 2 compares the surface resistivity of tin-coated copper mesh with POS-61 and some lead-free solders. Apparently, metallized copper wire and soldered knots reduce the mesh surface resistivity to almost one forth compared to the initial mesh, thus promoting high-quality lightning protection.
The resistivity of solders and the surface resistivity of knitted mesh structure metallized and soldered with POS-61 and lead-free solders are close. The lead-free solders are advantageous since they are environmentally friendly.
Hence, the rational choice of a lead-free solder and knitted mesh structure in the lightning-protection systems for carbon fiber reinforced polymers is beneficial for the production of mesh lightning conductors using more environmentally friendly solder alloys.
Conclusions
The temperature dependences of the contact angle for lead-free solder melts show that it decreases with increasing temperature. The wettability of beryllium bronze with solder melts is almost the same as that of compact copper.
Environmentally friendly lead-free solders, such as SAC (Sn–3.2 wt.% Ag–0.7 wt.% Cu) and CASTIN (Sn–2.5 wt.% Ag–0.7 wt.% Cu–0.5 wt.% Sb), can be used for metallization and soldering of copper and bronze wire mesh structures, in particular, for the production of knotted mesh. These solders are promising for replacement of lead solders in knitted lightning-protection mesh.
Metallized copper wire and soldered mesh knots should ensure high-quality aircraft lightning protection.
References
L. R. Vishnyakov, “Knitted wire mesh reinforced powder composite materials produced by rolling,” Powder Metall. Met. Ceram., 52, Nos. 9–10, 498–504 (2014).
L. R. Vishnyakov, I. N. Kokhanaya, and V. A. Kokhanyi, “Knitted wire mesh technologies for lightning protection of polymer composite materials,” Tekhnol. Syst., No. 4, 30–33 (2008).
L. R. Vishnyakov, I. I. Chernyavskii, and O. V. Zubkov, “Studying the lightning protection of polymer composite materials reinforced with knitted copper mesh,” Visn. Inzh. Akad. Ukr., No. 2, 143–148 (2012).
V. P. Krasovskii, L. R. Vishnyakov, N. A. Krasovskaya, and E. G. Ivanov, “Lead-free solders for metallization and soldering of copper materials,” Adgez. Raspl. Paika Mater., Issue 41, 53–62 (2008).
Yu. V. Naidich, Yu. N. Chuvashov, N. F. Ishchuk, and V. P. Krasovskii, “Wetting of some nonmetallic materials by aluminum,” Powder Metall. Met. Ceram., 22, No. 6, 481–483 (1983).
V. P. Krasovskii, L. R. Vishnyakov, N. A. Krasovskaya, and V. A. Kokhanyi, “Metallization and soldering of copper-based wire mesh materials with lead-free solders,” in: Proc. 1st Int. Sci. Conf. New Technologies in Materials Science [in Russian], Izd. Red. Izd. Tsentr Bashkir Gos. Univ., Ufa (2015), pp. 28–29.
I. P. Bogoroditskii, V. V. Pasynkov, and B. N. Tarev, Electrical Materials [in Russian], Energoatomizdat, Leningrad (1985), p. 304.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 56, Nos. 1–2 (513), pp. 132–140, 2017.
Rights and permissions
About this article
Cite this article
Krasovskii, V.P., Vishnyakov, L.R., Kokhanyi, V.A. et al. Lead-Free Solders for Copper Alloy Wire Mesh. Powder Metall Met Ceram 56, 102–107 (2017). https://doi.org/10.1007/s11106-017-9868-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9868-6