It is shown that porous calcium phosphate ceramics can be produced from monetite and biogenic hydroxyapatite, the starting materials being in the ratios 25 : 75, 50 : 50, and 75 : 25 wt.%. It is established that phase transitions and solid-phase reactions take place during sintering to form polyphosphate ceramics consisting of hydroxyapatite (Ca5(PO4)3(OH)), β-pyrophosphate (β-Ca2P2O7), and β-tricalcium phosphate (β-Ca3(PO4)2), in which β-Ca2P2O7 and Ca5(PO4)3(OH) phases are predominant, depending on starting composition. When the biogenic hydroxyapatite content changes from 25 to 75 wt.%, the grain size decreases and the pore size increases. The ceramics have 40 to 42% porosity with predominant open porosity for all compositions. The ceramics show 32–55 MPa strength, which increases with the amount of biogenic hydroxyapatite in starting composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is believed that the human body’s response to a foreign material is largely dependent not only on its phase composition but also morphology. Mechanical properties are major criteria in selecting materials to replace bone tissue. Particularly, they include strength, which is to be close to that of bone tissue, and porous structure, especially open porosity needed for tissue ingrowth and circulation of body fluids.
The most common material for bone tissue replacement is hydroxyapatite (HA, Ca5(PO4)3(OH)), both synthetic and biogenic. Biogenic hydroxyapatite (BHA) is very promising because of its close similarity to the chemical composition of natural bone tissue. It is obtained from cattle bones [1, 2], mollusk shells [3], cuttlefish [4], sea corals and algae [5, 6], enamel and dentin [7, 8], and eggshell [9, 10].
To control the resistivity of hydroxyapatite, other phases are introduced into HA materials. Tricalcium phosphate (TCP, β-Ca3(PO4)2) and bioglass of various compositions [11–16] have been studied to the greatest extent. A series of research efforts have been made in recent years to make composites from tetracalcium phosphate (TTCP, Ca4(PO4)2O and calcium pyrophosphate (CPP, Ca2P2O7) [17]. However, the use of CPP is still to be understood, though pyrophosphate ions form the basis of many medications for osteoporosis and diabetes [18] and play a significant role in many biological processes, their highest concentration being observed in osteoblasts [19]. Moreover, CPP stimulates bone tissue formation better than HA [20]. To control the resorption rate of calcium phosphate composites with sodium borosilicate glass, in our previous research [21] we used a four-phase mixture of synthetic calcium phosphates as a phosphate component. Besides HA, this mixture contained TCP, CPP, and TTCP phases, which allowed us to increase the dissolution rate in vitro, contrastingly to composites from biogenic or synthetic HA.
Owing to its bioactive properties and ease of production, dicalcium phosphate CaHPO4 (monetite) has been of interest recently [22, 23]. The paper [24] describes the production of porous calcite and monetite cements to heal bone defects. The materials have up to 6 MPa strength, 30–70% porosity, and neutral pH (7.2–7.5). The literature testifies that monetite promotes better bone tissue regeneration (than hydroxyapatite) [25]. Monetite is known to form CPP [26] during heat treatment, which can be beneficial for producing polyphosphate ceramics with improved properties [27]. In addition, monetite is used in food and pharmaceutical industries as a food supplement in prepared breakfast cereals, flour, pasta, and toothpastes to remove odontolith.
Our objective is to study the production of calcium phosphate ceramics from monetite and biogenic hydroxyapatite (25, 50, and 75 wt.% BHA) and examine their composition, porous structure, strength, and solubility in media analogous to the body’s fluids in experiments in vitro.
Experimental Procedure
To produce calcium phosphate ceramics, we used monetite powders with particles 700 nm in size and BHA powders with 160 μm particles. Dicalcium phosphate (monetite) was synthesized chemically by precipitation from solutions of Ca(NO3)2 · 4H2O and (NH4)H2PO4. Biogenic hydroxyapatite was obtained from cattle bones by the process described in the patent [28]. The starting mixtures with 25, 50, and 75% BHA were prepared by mechanical mixing on a roller bed. The composite samples produced by uniaxial pressing with a hydraulic press at 100 MPa were sintered in a muffle furnace at 800°C in air for 2 h. Their shrinkage and weight loss during sintering as well as total and open porosity were determined.
The phase composition was analyzed by X-ray diffraction (XRD) using a DRON-3 diffractometer (Burevestnik Enterprise, Russia) equipped with an X-ray tube with a copper anode and graphite monochromator. The fracture surface was examined by scanning electron microscopy with a JEOL Superprobe 733 microscope. The structure was analyzed employing the SIAMS PhotoLab software. In addition, differential thermal gravimetric analysis (DTGA) was used to analyze the starting monetite powder. Thermal curves were plotted in air with the Derivatograph System (F. Paulk, J. Paulk, L. Erdey, MOM, Budapest, Hungary). The samples were heated at a rate of 10 °C/min (the same as in the sintering of ceramics).
The uniaxial compression tests were performed in compliance with GOST 27034–86 Solid Sintered Alloys. Methods for Determining Compressive Strength and Yield Stress using a universal Ceram Test System machine.
Experiments in vitro were conducted in saline, synthetic blood plasma, and seawater. A ceramic sample was thermostatically kept in a test fluid for some time at 36–37°C and then its weight loss was determined.
Results and Discussion
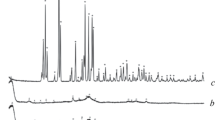
The XRD data (Fig. 1) indicate that sintering of the starting mixtures containing various ratios of monetite and BHA in air at 800°C induces phase transformations that result in polyphosphate ceramics with HA, β-CPP, and β-TCP phases; their amounts depend on the starting BHA/monetite ratio. After sintering of the starting mixture containing 25 wt.% monetite, the principal HA lines remain in the Debye powder pattern; it also shows β-TCP lines resulting from phase transformations of monetite and indicates monetite traces. Further increase of the starting monetite content to 50 wt.% causes the principle HA lines to disappear and become the secondary phase together with β-CPP, β-TCP being predominant. When starting monetite content increases to 75 wt.%, the HA amount decreases further and β-TCP and β-CPP become the main phases, β-TCP remaining predominant. X-ray diffraction also indicates that sintering of starting monetite (without BHA) leads to phase transformations resulting in a mixture of β-CPP and β-TCP, with CPP being the main phase.
These results agree well with the literature data [26, 29]. Hence, monetite undergoes phase transformations at 270–500°C to form γ-Ca2P2O7, further increase in temperature to 500–750°C leads to the β-Ca2P2O7 phase, and α-Ca2P2O7 appears at 1165°C. The CPP phase can interact with water vapors at 280°C to produce TCP. Moreover, HA can react with Ca2P2O7 to form TCP [27]. It is established in [16] that the mechanical mixtures consisting of thermally stable stoichiometric powders of synthetic HA and β-TCP do not retain their phase composition during sintering (900–1100°C): under the action of TCP, HA transforms into β-TCP, and this secondary tricalcium phosphate increases the total TCP amount in the composite to 100%. Hydroxyapatite transforms into β-TCP by dehydration through interface activation, which causes the limiting nucleation stage to disappear in the HA–β-TCP mechanical mixtures since the product phase (β-TCP) is in close contact with the hydrate phase, distributing its nuclei over the material, from the very beginning. Phase boundaries are more imperfect in the two-phase HA–TCP mixture than single-phase ones in pure HA and thus are more permeable for water vapors and remove more efficiently the gaseous reaction product.
Therefore, in case of 25 wt.% monetite in the starting mixture, it almost completely transforms to TCP through phase transformations during sintering, HA remaining the main phase. Although biogenic hydroxyapatite is more thermally stable than synthetic one and retains its composition up to 1300°C [30, 31], it can be assumed that phase transitions of monetite promote BHA transformation under the action of formed CPP and TCP phases if starting monetite content increases to 25–50 wt.% (BHA content decreases accordingly).
The starting monetite behavior with temperature increasing to 1000°C was also examined with DTGA, whose results confirm phase transformations. Absorbed water is removed at ~100°C, phase transformations involving considerable weight loss (to 21%) proceed with increasing temperature and finish by 500°C, and then the material densifies (Fig. 2). Therefore, analysis of the DTGA curves shows that the monetite powder can transform during heat treatment to produce a mixture of phases.
Table 1 shows data on the volume shrinkage and weight loss of ceramic samples during sintering. It should be noted that sintering of the samples produced from both pure monetite and its mixtures with BHA is accompanied by substantial weight loss (compared to pure BHA) resulting from the above phase transformations, which in turn compensate volume shrinkage, being no higher than 2.2%. Porosity of the polyphosphate ceramics hardly depends on starting composition and is 40–42%. The fraction of open porosity in the samples of all compositions is more than 0.97. This is a favorable characteristic of bioactive materials in view of bone ingrowth and circulation of body fluids.
Figure 3 shows fracture surface of the polyphosphate ceramics versus starting composition. The structure substantially depends on phase composition of the material; this is especially evident from structural differences between the materials produced, as seen at great magnification. Hence, higher BHA content leads to polyphosphate ceramics with rounded grains.
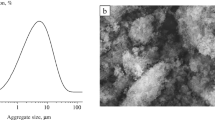
Table 2 shows structural analysis data processed with the Siams PhotoLab software package. With higher starting BHA content, the minimum and maximum grain sizes decrease, the values of root-mean-square deviation being close. This may be associated with phase processes and formation of polyphosphate ceramics, with the prevailing TCP phase. According to grain-size distribution (Fig. 4), the most probable grain size decreases from 0.4 to 0.25 μm with increasing BHA content of the starting mixture. In the starting ceramics containing 25 wt.% BHA, there are 62% of 0.2–0.5 μm grains. When BHA content increases to 50 wt.%, 65% of grains reach 0.2–0.4 μm; for 75 wt.% BHA, 67% are 0.2–0.35 μm grains. Therefore, with greater BHA content, the main grain size range narrows but the grains become less homogeneous by size, which is evidenced by variation coefficient.
With higher BHA content, the minimum and maximum pore sizes in ceramics increase, while the average pore size remains unchanged (Table 2). The most probable pore size for the samples produced from the mixtures with 25 and 50 wt.% BHA is 0.25 μm; when BHA content increases to 75 wt.%, this size decreases to 0.13 μm (Fig. 4). In addition, with 25 wt.% BHA in the starting mixture, the majority of pores in the ceramics (about 70%) correspond to the range 0.2–0.5 μm. When starting BHA content reaches 50%, 84% of pores in the ceramics fall into the 0.1–0.4 μm range. If the HA phase prevails in the ceramics, there are 83% of 0.1–0.5 μm pores. Hence, the main pore size range widens under phase transformations in the monetite and BHA mixtures and becomes narrower with increasing BHA content.
The calcium phosphate ceramics were tested by uniaxial compression. According to visual observations, a macrocrack does not form immediately, and cracking proceeds gradually between individual particles. Figure 5 shows compressive loading curves versus starting composition; it is seen that all composites are characterized by brittle fracture. The compressive strength (breaking stress σb) of polyphosphate ceramics increases from 32 to 55 MPa with greater starting BHA content and is higher than that of the ceramics produced from pure monetite or BHA (Table 1). The fracture pattern and high strength of the ceramics are due to structure and composition; particularly, they are associated with finer grains and polyphosphate composition resulting from polymorphic transformations. Fine grains accumulate high excessive energy on their boundaries and thus allow the strength to be increased, and polymorphic transformations lead to additional grain bonding and material hardening. Hence, the polyphosphate ceramics satisfy requirements for strength of human spongy bone [32].
Experiments in vitro have shown that the solubility of samples in model environments simulating the living organism’s media depends not only on the phase composition of polyphosphate ceramics but also on the type of model fluid. Studies in saline (0.9% NaCl aqueous solution), which is contained in blood plasma and tissue fluids and is an important inorganic component maintaining osmotic pressure of blood plasma and extracellular fluid, testify that the polyphosphate ceramic samples dissolve only on the 14th day in the solution (Table 1). When starting BHA reaches 50–75 wt.%, there is no substantial difference in solubility, while it is 1.3 times higher for the composition with prevailing starting content of monetite (25 wt.% BHA), compared to the above compositions. This may be associated with the phase composition of ceramics, particularly with predominant calcium phosphate phases that have greater solubility than HA (for example, β-TCP); the dissolution rate of ceramics reaches 0.014–0.019 wt.%/day. If the samples are held in synthetic blood plasma and seawater, there is no dissolution even on the 30th day. Since the dissolution rate of resulting ceramics is not high, they can be promising for filling spongy bone tissues when low resorption rate is required.
Conclusions
The sintering of mixtures consisting of biogenic hydroxyapatite and more than 25 wt.% monetite leads to partial decomposition of BHA under the action of phase transformations and solid-phase reactions induced by monetite, and also to the formation of polyphosphate ceramics with 40–42% porosity, containing hydroxyapatite, β-tricalcium phosphate, and β-calcium pyrophosphate (in different ratios depending on starting composition). When BHA content of the composite increases, the grain and pore size ranges become narrower. Monetite additions strengthen calcium phosphate ceramics through phase transformations and grain bonding, thus increasing the strength of polyphosphate ceramics by 1.5 times.
The starting ceramics containing 25 wt.% BHA are considered to be the most optimum among the studied materials. They show the widest pore and grain size ranges (required for appropriate bone tissue ingrowth and circulation of body fluids), the highest solubility, and strength comparable with that of human bone tissue.
References
K. Haberko, M. M. Bucko, J. Brzezinska-Miecznik, et al., “Natural hydroxyapatite—its behavior during heat treatment,” J. Eur. Ceram. Soc., 26, No. 4–5, 537–542 (2006).
C. Faucheux, R. Bareille, F. Rouais, et al., “Biocompatibility testing of a bovine hydroxyapatite ceramic material with the use of osteo-progenitor cells isolated from human bone marrow,” J. Mater. Sci. Mater. Med., 5, 635–639 (1994).
K. S. Vecchio, X. Zhang, J. B. Massie, et al., “Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants,” Acta Biomater., 3, No. 6, 910–918 (2007).
J. H. G. Rocha, A. F. Lemos, S. Agathopoulos, et al., “Scaffolds for bone restoration from cuttlefish,” Bone, 37, No. 6, 850–857 (2005).
U. Ripamonti, J. Crooks, L. Khoali, and L. Roden, “The induction of bone formation by coral-derived calcium carbonate / hydroxyapatite constructs,” Biomaterials, 30, No. 7, 1428–1439 (2009).
P. J. Walsh, F. J. Buchanan, M. Dring, et al., “Low-pressure synthesis and characterization of hydroxyapatite derived from mineral red algae,” Chem. Eng. J., 137, No. 1, 173–179 (2008).
D. S. Seo and J. K. Lee, “Dissolution of human teeth-derived hydroxyapatite,” Ann. Biomed. Eng., 36, No. 1, 132–140 (2008).
F. N. Oktar, “Microstructure and mechanical properties of sintered enamel hydroxyapatite,” Ceram. Int., 33, No. 7, 1309–1314 (2007).
D. S. R. Krishna, A. Siddharthan, S. K. Seshadri, and T. S. S. Kumar, “A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste,” J. Mater. Sci. Mater. Med., 18, 1735–1743 (2007).
G. Gergely, F. Wéber, I. Lukács, et al., “Preparation and characterization of hydroxyapatite from eggshell,” Ceram. Int., 36, No. 2, 803–806 (2010).
R. F. Ellinger, E. B. Nery, and K. L. Lynch, “Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report,” J. Periodont. Restor. Dent., 3, 223–233 (1986).
L. Cheng, F. Ye, R. Yang, et al., “Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula,” Acta Biomater., 6, No. 4, 1569–1574 (2010).
S. Padilla, J. Roman, S. Sanchez-Salcedo, and M. Vallet-Regi, “Hydroxyapatite/SiO2–CaO–P2O5 glass materials: in vitro bioactivity and biocompatibility,” Acta Biomater., 2, No. 3, 331–342 (2006).
A. Yao, F. Ai, X. Liu, et al., “Preparation of hollow hydroxyapatite microspheres by the conversion of borate glass at near room temperature,” Mater. Res. Bull., 45, No. 1, 25–28 (2010).
A. Yu. Malysheva, B. I. Beletskii, and E. B. Vlasova, “Structure and properties of composites for medical applications,” Steklo Keram., No. 2, 28–31 (2001).
V. V. Skorokhod, S. M. Solonin, V. A. Dubok, et al., “Decomposition activation of hydroxyapatite in contact with β-tricalcium phosphate,” Powder Metall. Met. Ceram., 49, No. 5–6, 324–329 (2010).
A. G. Veresov, V. I. Putlyaev, and Yu. D. Tretiakov, “Advances in calcium phosphate materials,” Ros. Khim. Zh., 44, No. 6, Part II, 32–46 (2000).
Jr. L. Mattano, “Strategic approaches to osteoporosis in transplantation,” Pediatr. Transplantation, 8, Suppl. 5, 51–55 (2004).
V. M. Vagabov, I. S. Kulaev, and T. V. Kulakovskaya, High-Molecular Inorganic Polyphosphates: Biochemistry, Cell Biology, Biotechnology [in Russian], Nauchnyi Mir, Moscow (2005), p. 216.
J.-S. Sun, Y.-H. Tsuang, C.-J. Liao, et al., “The effect of sintered β-dicalcium pyrophosphate particle size on newborn wistar rat osteoblasts,” Artif. Organs, 23, No. 4, 331–338 (1999).
O. E. Sych, N. D. Pinchuk, L. A. Ivanchenko, and O. R. Parkhomei, Calcium Phosphate Composite Material [in Ukrainian], Ukrainian Utility Model Patent 43042, IPC (2009) A61K 33/42, A61P 19/00, Inst. Probl. Materialoved. NANU (applicant and patent holder), 2009 02943, appl. March 2009, publ. July (2009), Bulletin No. 14, p. 3.
T. Albrektsson and C. Johansson, “Osteoinduction, osteoconduction, and osseointegration,” Eur. Spine J., 10, No. 2, 96–101 (2001).
C. H. Haemerle and T. Karring,” Guided bone regeneration at oral implant sites,” Periodontol., 17, 151–175 (2000).
V. V. Smirnov, “Porous cements for filling bone tissue defects,” Materialovedenie, No. 8, 16–19 (2009).
F. Tamimi, J. Torres, D. Bassett, et al., “Resorption of monetite granules in alveolar bone defects in human patients,” Biomaterials, 31, No. 10, 2762–2769 (2010).
T. Kanazawa, Inorganic Phosphate Materials, Elsevier, Amsterdam (1989).
T. V. Safronova, V. I. Putlyaev, M. A. Shekhirev, et al., “Composite ceramics containing a bioresorptive phase,” Steklo Keram., No. 3, 31–35 (2007).
E. P. Podrushnyak, L. A. Ivanchenko, V. L. Ivanchenko, and N. D. Pinchuk, Hydroxyapatite and Method for Its Production (Options) [in Ukrainian], Ukrainian Patent 61938, IPC A61K35/32, A61K33/00, A61K6/02, A61P19/00, No. 99095233, appl. September 1999, publ. December (2003), Bulletin No. 12, p. 7.
T. V. Safronova, V. I. Putlyaev, A. V. Kuznetsov, et al., “Properties of calcium phosphate powders synthesized from calcium acetate and sodium hydrogen phosphate,” Steklo Keram., No. 4, 30–34 (2011).
W. I. Abdel-Fattah and M. M. Selim, “Thermal behavior and structural variations of both chemically precipitated and biological hydroxyapatites,” Ceram. Acta, 4–5, 65–76 (1991).
A. L. Giraldo-Betancur, D. G. Espinosa-Arbelaez, A. del Real-López, et al., “Comparison of physicochemical properties of bio- and commercial hydroxyapatite,” Curr. Appl. Phys., 13, No. 7, 1383–1390 (2013).
S. A. Goldstein, “The mechanical properties of trabecular bone: dependence on anatomic location and function,” J. Biomech., 20, No. 11–12, 1055–1061 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 53, Nos. 7–8 (498), pp. 58–68, 2014.
Rights and permissions
About this article
Cite this article
Sych, E.E., Pinchuk, N.D., Tovstonog, A.B. et al. The Structure and Properties of Calcium Phosphate Ceramics Produced from Monetite and Biogenic Hydroxyapatite. Powder Metall Met Ceram 53, 423–430 (2014). https://doi.org/10.1007/s11106-014-9634-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-014-9634-y