Abstract
Plant height (PH) is one of the most important traits related to plant architecture in wheat. Together, the lengths of individual internodes determine plant height and have a great influence on lodging resistance. To specify the genetic basis of wheat internode characteristics, we identified quantitative trait loci (QTLs) for each internode component index (ICI) and plant height component index (PHCI) using a recombinant inbred line (RIL) mapping population derived from ‘Kenong 9204’ (‘KN9204’) × ‘Jing 411’ (‘J411’). Up to 57 putative additive QTLs for the four ICIs and PHCI were detected, which together covered 20 of the 21 wheat chromosomes, with the exception of chromosome 1B. Among them, eight QTLs were major, stable QTLs with a logarithm-of-odds (LOD) score of ≥ 3.0 and a phenotypic variance explained (PVE) of ≥ 7.0%. In the epistatic analysis, only one pair of epistatic QTLs was identified for the first internode component index (FIITCI) and three pairs of epistatic QTLs for the third internode component index (TITCI). A total of 20 of the 57 detected QTLs (35.1%) were co-localized QTLs for PH, spike length, and internode lengths, indicating that those traits have their own individual genetic basis in most cases. Moreover, 12 QTL clusters for PHCI/ICIs and yield-related traits were identified, indicating that plant architecture plays a potential role in the formation of yield in wheat. The plant architecture with gradually bottom-up shortened internode lengths tends to be high-yielding potential, especially for the uppermost internode. This study may provide useful information for understanding the genetic basis of plant height components and to accelerate the genetic improvement of plant ideotypes designed to increase yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is one of the world’s most important food crops, providing approximately 20% of the food energy for global human consumption (Xia et al. 2017). Plant height (PH) is a complex trait that has great influence on yield potential as well as on yield stability in wheat (Peng et al. 1999; Sourdille et al. 2000; Hedden 2003; Würschum et al. 2014). The introduction of Rht semi-dwarfing genes into wheat cultivars resulted in huge wheat yield increases, creating a new situation called the “Green Revolution” from the 1970s (Hedden 2003). The stem of a wheat plant consists of nodes and internodes. Internode lengths and internode component indices (ICIs) together determine the final PH and then affect plant architecture (Cui et al. 2011, 2012; Zhang et al. 2018a, b). ICI, a derivative trait of PH, is the ratio of internode length to the sum of the corresponding internode length and the next lower internode length (Cui et al. 2012). Wheats possessing ideotype especially with short basal internode length not only have characteristics of lodging resistance, but also have considerable yield potential (Pinthus and Levy 1983; Cui et al. 2011, 2012).
With the advances in wheat genomics and genetics, increasing numbers of QTLs for PH have been reported in wheat (Law et al. 1978; Cui et al. 2011; Liu et al. 2011; Li et al. 2013; Zhang et al. 2017; Chai et al. 2019). By now, more than 25 genes that control PH and related traits have been reported in wheat (Evans 1998; Worland et al. 1998; Tian et al.2017; Würschum et al. 2017). Previous studies indicated that different QTLs for final PH might be induced by variation of different PH components (PHCs) (Kato et al. 1999; Cui et al. 2011; Maria and Herman 2016; Zhang et al. 2017), including spike length and internode lengths. However, most previous QTL studies have focused on the impact on final PH without considering its component traits (Qiao et al. 2007; Singh et al. 2016; Zhang et al. 2018a, b; Ma et al. 2019; Xue et al. 2019). There have been few studies on QTL localization for ICIs and PHCI (Cui et al. 2012; Li et al. 2013; Ren et al. 2014; Zhang et al. 2017).
The objectives of the present study were (1) to detect QTLs with additive effects for ICIs and PHCI in multiple environments, (2) to characterize the genetic relationships between PH and PHC, and (3) to reveal the genetic relationships among ICIs, PHCI, and yield-related traits.
Materials and Methods
Experimental Populations and Trait Evaluation
A recombinant inbred line population (RIL) containing 187 lines (denoted as KJ-RILs) derived from the F8:9 generation of the cross ‘Kenong 9204’ (‘KN9204’) × ‘Jing 411’ (‘J411’) was used in this study. The KJ-RILs, together with the two parents, were planted in Yantai (37°53′N, 121°37′E, altitude 4 m) in Shandong Province, eastern China in four environments (2 years × 2 nitrogen treatments), namely high-nitrogen (HN) and low-nitrogen (LN) treatments over 2 years, i.e., 2016–2017 with HN, 2016–2017 with LN, 2017–2018 with HN, and 2017–2018 with LN, respectively (Zhao et al. 2019a, b), which were defined as E1, E2, E3, and E4, respectively. In the HN plot, 300 kg ha−1 of diammonium phosphate and 225 kg ha−1 of urea were applied to the seedbed before sowing, and 150 kg ha−1 of urea was applied as a top dressing at the elongation stage each year. In the LN plots, no nitrogen fertilizer was applied during the planting season. A randomized block design with two replications was used in each of the four environments, and each row was planted with 40 seeds by hand in two plots with a row spacing of 0.25 m and a length of 2 m. All of the locally recommended agronomic practices were followed in each of the trials except for the nitrogen fertilization treatment described above.
Five representative plants in the middle of each row were selected to measure plant height (PH) and the internode lengths from the first to the fifth internode counted from the top, i.e., the first internode length (FIRITL), the second internode length (SECITL), the third internode length (THITL), the fourth internode length (FOITL), and the fifth internode length (FIFITL).
The internode component indices (ICIs) from the first internode (top internode) to the fourth internode were referred to as the first internode component index (FIITCI), the second internode component index (SITCI), the third internode component index (TITCI), and the fourth internode component index (FOITCI). The plant height component index (PHCI) and the internode component indices (ICIs) were calculated as follows (Cui et al. 2012):
PHCI = (FIRITL + SECITL)/ PH
FIITCI = FIRITL/ (FIRITL + SECITL)
SITCI = SECITL/ (SECITL + THITL)
TITCI = THITL/ (THITL + FOITL)
FOITCI = FOITL/ (FOITL + FIFITL)
The yield-related traits (YRTs) were thousand-kernel weight (TKW), kernel number per spike (KNPS), yield per plant (YPP), and spike number per plant (SNPP) were determined as described by Cui et al. (2014, 2016) and Fan et al. (2019).
Data Analysis and QTL Mapping
Basic descriptive statistical analysis was performed on the experimental data, using EXCEL 2016 and SPSS 24.0 (Chicago, IL, USA). A high-density wheat genetic map with 119,566 loci spanning 4424.4 cM has been published by Cui et al. (2017). We used the Basic Local Alignment Search Tool (BLAST) (ftp://ftp.ncbi.nlm.nih.gov/blast/executables/release/) to align the single-nucleotide polymorphism (SNP) probes to the KN9204 genome assembly (unpublished data) to locate the physical positions of these SNPs. The physical positions of the SNPs of KN9204 rather than their genetic locations were used for QTL mapping analysis in this study. The best linear unbiased estimate (BLUE) values of each of the 187 KJ-RILs were calculated by the QGAStation 2.0 based on the phenotypic data from the four environments. The data from each of the four environments, as well as the BLUE data, were used for QTL analysis in the current study. QTL detection was conducted using inclusive composite interval mapping by QTL IciMapping 4.1 software (https://isbreeding.caas.cn/rj/index.htm), based on stepwise regression of simultaneous consideration of all marker information (http://www.isbreeding.net/). For QTLs with additive effects, the walking speed chosen for all QTLs was 0.01 Mb, and the P value inclusion threshold was 0.001. The threshold of log-of-odds (LOD) scores was evaluated using 1000 permutations with a type I error of 0.05. For QTLs with epistatic effects, the walking speed chosen for all QTLs was 5.0 Mb, and the P value inclusion threshold was 0.001. The LOD scores were set at no less than 5. Only the datasets of the BLUE values for PHCI and ICIs were used for epistatic QTL detection.
Quantitative Trait Loci Nomenclature
The name of each QTL was designated as follows: the first letter ‘Q’ meant ‘QTL’; the letters between ‘Q’ and ‘-’ (dash) represented the abbreviation of the corresponding trait; the letter ‘KJ’ stood for the mapping population of KJ-RILs; the letters and numbers following the second dash represent the wheat chromosomes where the corresponding QTL was located; and the last number referred to the sequence number that the QTL was detected in the same wheat chromosome, from the short arm to the long arm. When two or more QTLs associated with the same trait with overlapping confidence intervals were detected in different environments, they were considered to be congruent QTLs.
Results
Phenotypic Performance for Internode Component Indices in the KJ-RIL Population
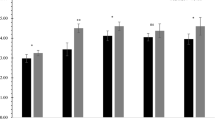
For the four environments, all the ICIs along with PHCI in the 187 kJ-RILs showed continuous variation with the absolute values of skewness and kurtosis being less than 1.0 in most cases, with the traits approximating to a normal distribution, indicating that they were typical quantitative traits and suitable for QTL analysis (Fig. 1; Table 1). The correlation coefficients of ICIs and PHCI among the four environments were significant (P ≤ 0.01) (Table 2). The broad heritability scores for the five traits ranged from 75.25 (FIITCI) to 19.86% (TITCI). These results indicated that genetic factors played important roles in determining the phenotypic variation of ICIs and PHCI in the 187 KJ-RILs.
Phenotypic distribution of wheat internode and plant height component indices in KJ-RILs under different environments. Parent value of KN9204 and J411 are shown by black and gray arrows, respectively. FIITCI, the first internode component index; SITCI, the second internode component index; TITCI, the third internode component index; FOITCI, the fourth internode component index; PHCI, the plant height component index
Correlation Analysis Between Plant Height/Internode Component Index and Individual Internode Lengths in the KJ-RIL Population
Correlation analysis results showed that PH was significantly negatively correlated with TITCI and FOITCI under both LN and HN conditions as well as with SITCI and PHCI under HN conditions (Table 3). On the other hand, PH was significantly positively correlated with FIITCI under both LN and HN conditions. FIRITL was significantly positively correlated with PHCI and FIITCI under both LN and HN conditions as well as with SITCI, but with the latter under only LN conditions. FIRITL was significantly negatively correlated with TITCI and FOITCI under both LN and HN conditions. SECITL and SITCI were significantly positively correlated with each other under both LN and HN conditions. Furthermore, SECITL was significantly negatively correlated with FIITCI and TITCI, although only under HN conditions. THITL was significantly negatively correlated with PHCI, FIITCI, SEITCI, TITCI, and FOITCI simultaneously under both LN and HN conditions, except for FIITCI and TITCI, which were significantly correlated with THITL only under HN conditions. FOITL was significantly negatively correlated with PHCI, SITCI, TITCI, and FOITCI under both LN and HN conditions, and FIFITL was significantly negatively correlated with PHCI, SITCI, TITCI, and FOITCI under both LN and HN conditions. In addition, FIFITL was significantly positively correlated with FIITCI under LN conditions.
Correlation Analysis Between Plant Height/Internode Component Index and Yield-Related Traits in the KJ-RIL Population
The correlation coefficients between yield-related traits and both PHCI and ICIs in the KJ-RIL population are shown in Table 4. TKW was significantly positively correlated with PHCI under LN conditions but was significantly negatively correlated with TITCI under LN conditions as well as with FOITCI under both LN and HN conditions. SNPP was significantly negatively correlated with PHCI only under HN conditions as well as with FIITCI under both LN and HN conditions. KNPS and TITCI were significantly positively correlated with each other only under HN conditions. YPP was significantly negatively correlated with PHCI, TITCI, and FOITCI simultaneously under HN conditions. The above findings indicated that PHCI and ICIs might affect yield potential to some extent, especially under HN conditions, and that common genetic factors underlying PHCI, ICIs and yield-related traits might exist.
Putative Additive QTLs for Internode and Plant Height Component Indices
Up to 57 putative additive QTLs for the four ICIs and PHCI were detected in the KJ-RIL population. Together, they covered all of the 21 wheat chromosomes with the exception of 1B (Table 5). Of these, 21, 14, and 22 QTLs were mapped to the A, B, and D subgenomes, respectively. There were 10, 17, 6, 11, and 13 QTLs detected for FIITCI, SITCI, TITCI, FOITCI, and PHCI, respectively, which individually explained 1.16–57.40% of the phenotypic variance with LOD scores ranging from 2.02 to 51.05 (Fig. 2).
Location of QTLs for internode and plant height component indices identified in four different environments based on a population of 187 KJ-RILs derived from a cross between ‘KN9204’ and ‘J411’. The chromosome number is marked at the top of each chromosome. The positions of markers are listed on the left of the bars, and the names of markers and QTLs are listed on the right of the corresponding chromosomes. The colored segments on the chromosome indicate the confidence interval of the corresponding QTL, and the segment of red, green, black, fluorescent green, and pink colors represent the traits for the first internode component index (FIITCI), the second internode component index (SITCI), the third internode component index (TITCI), the fourth internode component index (FOITCI), the plant height component index (PHCI), respectively. The environments where the corresponding QTLs detected are shown in parenthesis
A total of ten putative, additive QTLs for FIITCI were identified in the four environments and the BLUE analysis (Fig. 2; Table 5). Individually, they explained 1.16–57.40% of the phenotypic variance (Table 5). Of these, QFiitci-KJ-1D.1, QFiitci-KJ-4A.1, QFiitci-KJ-6A.1, QFiitci-KJ-6B.1, and QFiitci-KJ-7A.1 were repeatedly detected in at least two datasets including the BLUE analysis. Among them, QFiitci-KJ-6B.1 was a major, stable QTL with high LOD scores of 4.77 to 51.05 and high phenotypic variance explained (PVE) values of 6.09 to 57.40%; the allele of QFiitci-KJ-6B.1 which caused increases in FIITCI was from parent ‘J411’. In addition, both QFiitci-KJ-4D.2 and QFiitci-KJ-4D.3 were major QTLs with LOD scores ≥ 3.0 and PVEs ≥ 7.0%, although QFiitci-KJ-4D.3 was an environment-specific QTL, detected in only one environment. There were eight and two QTL alleles donated by the parents ‘J411’ and ‘KN9204’, respectively, which increased FIITCI.
For SITCI, 17 QTLs were identified across the four environments (Fig. 2), which individually explained 3.50–29.00% of the phenotypic variance (Table 5). QSitci-KJ-3A.1 could be detected in multiple environments of E1, E3, E4, and the BLUE analysis. QTLs QSitci-KJ-3A.2 and QSitci-KJ-4B.1 had high LOD scores of 5.85 and 18.20, respectively, with high PVEs of 15.06% and 29.00%, respectively, although they were both identified in only one environment. In addition, QSitci-KJ-1A.1, QSitci-KJ-1A.2, QSitci-KJ-4B.2, QSitci-KJ-4D.2, QSitci-KJ-4D.2, and QSitci-KJ-6D.1 also displayed environment-dependent expression, with LOD scores ≥ 3.0 and PVEs ≥ 7.0%, which were defined as environment-dependent, major QTLs for SITCI. Alleles of eleven and six QTLs, which caused increasing SITCI, were contributed by ‘J411’ and ‘KN9204’, respectively.
Six putative, additive QTLs for TITCI were identified in the four environments, which individually accounted for 4.62–31.81% of the phenotypic variance, with LOD scores from 2.65 to 26.93 (Fig. 2; Table 5). QTitci-KJ-4B.1 was a major, stable QTL with LOD scores of 6.62–26.93 and PVEs of 6.14–31.81%, which was consistently identified in three different environments as well as under BLUE analysis. In addition, QTitci-KJ-3A.1 was another stable, major QTL that was detected repeatedly in two of the four environments as well by BLUE analysis. In total, there were three and three QTL alleles increasing TITCI from parents ‘KN9204’ and ‘J411’, respectively.
Eleven putative, additive QTLs for FOITCI were detected across the four environments (Fig. 2). These QTLs individually explained 4.59–38.76% of the phenotypic variance with LOD scores ranging from 2.10 to 18.44 (Table 5). Five QTLs, namely QFoitci-KJ-2D.3, QFoitci-KJ-3A.1, QFoitci-KJ-5A.1, QFoitci-KJ-6A.2, and QFoitci-KJ-6B.1, could be identified in two environments. Of these, QFoitci-KJ-6B.1 was a major, stable QTL with LOD score of 4.08–8.43 and PVE of 11.71–17.74%; QFoitci-KJ-6B.2 was approximately 10 Mb away from QFoitci-KJ-6B.1, and it had a LOD score of 18.44 and a PVE of 38.76% in environment E2; ‘KN9204’ and ‘J411’ contributed positive alleles of QFoitci-KJ-6B.1 and QFoitci-KJ-6B.2, respectively, that increased FOITCI. Four and seven QTL alleles increasing FOITCI were derived from ‘KN9204’ and ‘J411’, respectively.
For PHCI, 13 putative, additive QTLs were identified across all the tested environments and the BLUE analysis (Fig. 2; Table 5), which individually explained 2.65–34.85% of the phenotypic variance, with LOD scores ranging from 2.02 to 26.49. Six and seven QTL alleles which increased PHCI were derived from ‘KN9204’ and ‘J411’, respectively. At least four QTLs (QPhci-KJ-3A.1, QPhci-KJ-3A.2, QPhciKJ-3A.3, and QPhci-KJ-3D.1) were reproducibly detected in two different environments. Of these, only QPhci-KJ-3D.1 was a major QTL with a PVE of 4.84 to 9.83. In addition, QPhci-KJ-5D.1, QPhci-KJ-5D.2, and QPhci-KJ-6B.1 were major QTLs that could be identified in only one environment; however, they had relatively high LOD scores of 26.49, 15.92, and 15.88, respectively, and contributed 34.85%, 18.26%, and 18.13% of the phenotypic variance, respectively.
Epistatic QTL Analysis for Internode and Plant Height Component Indices
For FIITCI, only one pair of epistatic QTLs was detected (Table 6; Fig. 3). This interactive effect referred to the chromosomal regions of 20.13 Mb on chromosome 1A and 589.67 Mb on chromosome 4D. No significant additive effects existed from these two loci. These two loci came from parent ‘KN9204’ and could reduce FIITCI, and their interaction could explain 8.87% of the FIITCI phenotypic variation.
Epistatic QTL analysis for the first internode component index (FIITCI) and the third internode component index (TITCI). The values on the line represent the phenotypic variance explained by the two interacting QTLs, and the values in the ellipse represent the physical position (Mb) on the ‘KN9204’ genome
For TITCI, three pairs of epistatic QTLs were detected (Table 6; Fig. 3). The first interaction effects referred to the chromosomal regions of 792.17 Mb on chromosome 3B and 480.70 Mb on chromosome 4B. No significant additive effects existed in these two loci. The two loci genotypes being the same as those in ‘KN9204’ could reduce TITCI, and this interactive effects could explain 9.46% of the TITCI phenotypic variation. The second interaction referred to the chromosomal regions of 289.67 Mb on chromosome 4D and 659.67 Mb on chromosome 4D. No significant additive effects existed in these two loci. The two loci genotypes being the same as those in ‘KN9204’ could increase TITCI, and their interactive effect could explain 16.27% of the TITCI phenotypic variation. For the third pair of epistatic QTLs, the two chromosomal regions involved were 812.17 Mb on chromosome 3B and 372.88 Mb on chromosome 5D, and no significant additive effects were detected in these two loci. The two loci genotypes being the same as those in ‘KN9204’ could increase TITCI, and this interactive effect could explain 7.00% of the phenotypic variation of TITCI.
No epistatic QTLs were detected for SITCI, FOITCI, or PHCI, indicating that single, additive effects play key roles in the phenotypic variation of these traits in the 187 KJ-RILs.
Discussion
Independent Genetic Basis Underlying Plant Height, Plant Height Components, and Their Component Indices Exist in Most Cases
The introduction of Rht genes reducing PH without altering the yield components has resulted in yield increases in wheat and other cereals (Carrillo et al. 1985; Hedden 2003). Thereafter, numerous studies focused on characterizing the genetic basis of PH and identifying novel Rht genes (Law et al. 1978; Wu et al. 2010; Cui et al. 2011; Liu et al. 2011; Li et al. 2013; He et al. 2016; Zhang et al. 2017; Chai et al. 2019). Previous studies have proved that PH and PHC might share their individual genetic basis in most cases (Carrillo et al. 1985; Cui et al. 2012; Ren et al. 2014; Zhang et al. 2017; Li et al. 2020). To date, no report regarding the genetic relationships between PH, PHC, PHCI, and ICIs at the QTL level has been published.
Traits with stronger genetic correlations tend to share more common QTL regions than those with weaker genetic correlations. In the present study, PH and internode lengths showed moderate or even weak correlations with PHCI and ICIs in most cases, implying that limited co-located QTLs or QTL clusters could be identified (Table 3). We performed QTL analysis for PH, spike length, internode length, and their component indices based on a high-density physical map (data not shown). The results revealed that 20 QTLs (35.1%) for ICIs and/or PHCI were co-localized with QTLs for PH, SL, and internode length, which comprised ten QTL clusters (Fig. 2; Table S1), indicating that ICIs and PHCI had influences on PH and its components in some cases. Approximately 64.9% of the QTLs for ICIs and PHCI have no association with either PH or individual internode lengths. This finding was consistent with their moderate or even weak phenotypic correlations in Table 3, indicating that ICIs and PHCI exhibited their specific genetic basis independent of PH and internode lengths in most cases. Therefore, it is essential to perform QTL analysis for ICIs and PHCI in order to better understand the genetic basis of plant architecture and thus to improve plant architecture in molecular breeding programs designed to improve yield.
Plant Height Component Indices and Internode Component Indices Affect Yield Formation in Some Cases
In general, PH has considerable effects on both yield potential and yield stability, especially in terms of environmental adaptation (Zhang et al. 2021). Gao et al. (2020) showed that PH was strongly, negatively correlated with grain yield under irrigation conditions, whereas a significant positive correlation was detected under no-irrigation condition. Zhang et al. (2021) demonstrated that four QTLs for PH were strongly associated with yield-related traits by using seven pairs of near-isogenic wheat lines. Rht gene that had no adverse effects on yield potential was of great value in high-yielding molecular breeding programs (Tian et al. 2021). As mentioned above, ICIs and PHCI shared their individual genetic basis in most cases. No study regarding genetic association analysis among ICI, PHCI, and yield-related traits had previously been published.
In this study, moderate or even weak correlations were observed among PHCI, ICI, and yield-related traits at the phenotypic level (Table 4). This finding implied that PHCI and ICIs might affect yield potential in some cases, especially under HN conditions. It is worth mentioning that more significant negative associations were observed between yield-related traits and PHCI/ICIs, especially for TITCI and FOITCI, albeit with moderate or low correlation coefficients (Table 4). This finding implied that the plant architecture with gradually bottom-up shortened internodes tended to have high-yielding potential, especially for the uppermost internode.
QTLs for yield-related traits have been documented in Cui et al. (2016, 2017). Among the 57 QTLs for PHCI and four ICIs, 19 QTLs (32.8%) were co-located with those for yield-related traits, which represented 12 QTL clusters (Table S2). Taking the fourth cluster of yield (CY4) as an example, QPhci-KJ-3A.1, QSitci-KJ-3A.1, and QTitci-KJ-3A.1 were co-localized with two QTLs for yield-related traits (namely YPP, KNPS) on chromosome 3A. The favorable alleles from ‘KN9204’ increased KNPS and YPP, while reducing PHCI, SITCI, and TITCI. For CY8, QPhci-KJ-4B.1, QSitci-KJ-4B.1, and QTitci-KJ-4B.1 were co-localized with QTLs for TKW, KNPS, and YPP (Table S2). The alleles from ‘KN9204’ increased PHCI, TITCI, KNPS, and YPP but reduced SITCI and TKW. Single-marker QTL analysis for TKW, KNPS, SNPP, and YPP in CY8 was performed based on phenotypic values across eight environments by Cui et al. (2016, 2017), with the aim of characterizing the genetic effects of PHC, SITCI, and TICI on yield-related traits in detail. The results confirmed that alleles from ‘KN9204’ increased PHCI, TITCI, KNPS, and YPP but reduced SITCI and TKW in multiple environments (Fig. 4). Moreover, significant signals for SNPP were identified, with alleles from ‘KN9204’ increasing SNPP (Fig. 4). These significant correlations and QTL co-localizations among PHCI, ICIs, and yield-related traits provide new genetic evidence supporting the hypothesis that PHCI and ICIs have influences on yield-related traits in wheat in some cases.
Single-marker QTL analysis for TKW, KNPS, SNPP, and YPP on chromosome 4B (KN4B:15–36 Mb) based on phenotypic values in eight environments in Cui et al. (2016, 2017). The left-hand figures show the LOD scores of the corresponding traits in eight different environments; the right-hand figures show the additive effect values of the corresponding traits in eight different environments. Of the eight scatter diagrams, a and b, c and d, e and f, and g and h indicate the QTL LOD profiles and additive effects for kernel number per spike (KNPS), thousand-kernel weight (TKW), spike number per plant (SNPP), and yield per plant (YPP), respectively
Stable QTLs for Plant Height/Internode Component Indices Are of Value to Further Explore Candidate Genes
Closely linked markers are essential tools for molecular breeding in wheat (Landjeva et al. 2007; William et al. 2007). In general, a stable QTL is less affected by the environment, and the corresponding linked markers are of great value in molecular breeding programs. In the present study, we defined a relative stable QTL that was confirmed in at least two of the four environments. A total of 21 of the 57 QTLs (36.8%) for PHCI and the four ICIs could be repeatedly identified in no less than two of the four environments as well as the BLUE analysis.
Of the 21 environment-reproducible QTLs, the effects of QSitci-KJ-3A.1, QTitci-KJ-4B.1, and QFiitci-KJ-6B.1 could be determined in three of the four environments as well as the BLUE analysis. In the region of QSitci-KJ-3A.1, there were 44 candidate genes distributed (Fig. S1). As the primary interval is still relatively large, it is difficult to predict which one is the most likely candidate gene behind QSitci-KJ-3A.1. In addition, it was approximately 0.06–4.1 Mb away from the SNP markers of AX-109607322 and AX-110446594 (Fig. 2). Moreover, AX-94476859, AX-108925203, AX-110496730, and AX-109580196 were closely linked with QSitci-KJ-3A.1. Therefore, QSitci-KJ-3A.1 could be efficiently used in molecular breeding programs. PCR-based markers need to be developed based on probes specific for the sequences of these SNP markers in the future. QTitci-KJ-4B.1 overlapped with Rht-B1 and co-located with a major, stable QTL for PH (Fig. 2; Table S1 and S2). Therefore, we predict that Rht-B1 might be the candidate gene of QTitci-KJ-4B.1, which showed pleiotropic effects on PH, PHC, PHCI, and ICIs. QFiitci-KJ-6B.1 were mapped to a more concentrated region with the peak values of LODs at 591.77, 598.77, 616.77, and 616.76 Mb in E1, E2, E4, and the BLUE analysis, respectively. In view of its stability, higher LODs, and PVEs, fine-mapping analysis of this QTL should be performed to furtherly determine its precise physical position, albeit the high-density map of this chromosomal region.
Conclusion
Up to 57 putative additive QTLs were detected for PHCI and the four ICIs. Three major and stable QTLs of QSitci-KJ-3A.1, QTitci-KJ-4B.1, and QFiitci-KJ-6B.1 are of value to further explore the candidate genes behind. PH, PHC, PHCI, and ICI appear to have their own individual genetic basis at the QTL level in most cases. Moreover, we first specify the genetic correlations among ICIs, PHCI, and yield-related traits and found that PHCI and ICIs might have an influence on yield formation.
References
Carrillo JM, Monteagudo A, Sanchez-Monge E (1985) Inheritance of internode and culm length in hexaploid Triticale. Theor Appl Genet 69:285–291
Chai LL, Chen ZY, Bian RL, Zhai HJ, Cheng XJ, Peng HR, Yao YY, Hu ZR, Xin MM, Guo WL, Sun QX, Zhao AJ, Ni ZF (2019) Dissection of two quantitative trait loci with pleiotropic effects on plant height and spike length linked in coupling phase on the short arm of chromosome 2D of common wheat (Triticum aestivum L.). Theor Appl Genet 132:1815–1831
Cui F, Li J, Ding AM, Zhao CH, Wang L, Wang XQ, Li SS, Bao YG, Li XF, Feng DS, Kong LR, Wang HG (2011) Conditional QTL mapping for plant height with respect to the length of the spike and internode in two mapping populations of wheat. Theor Appl Genet 122:1517–1536
Cui F, Li J, Ding AM, Zhao CH, Li XF, Feng DS, Wang XQ, Wang L, Wang HG (2012) QTL detection of internode length and its component index in wheat using two related RIL Population. Cereal Res Commun 40:373–384
Cui F, Fan XL, Zhao CH, Zhang W, Chen M, Ji J, Li JM (2014) A novel genetic map of wheat: utility for mapping QTL for yield under different nitrogen treatments. BMC Genet 15:57
Cui F, Fan XL, Chen M, Zhang N, Zhao CH, Zhang W, Han J, Ji J, Zhao XQ, Yang LJ, Zhao ZW, Tong YP, Wang T, Li JM (2016) QTL detection for wheat kernel size and quality and the responses of these traits to low nitrogen stress. Theor Appl Genet 129:469–484
Cui F, Na Z, Fan XL, Zhang W, Zhao CH, Yang LJ, Pan RQ, Chen M, Han J, Zhao XQ, Ji J, Tong YP, Zhang HX, Jia JZ, Zhao GY, Li JM (2017) Utilization of a Wheat660K SNP array-derived high-density genetic map for high-resolution mapping of a major QTL for kernel number. Sci Rep 7:3788
Evans LT (1998) Crop evolution, adaptation and yield. Photosynthetica 34:56–60
Fan XL, Cui F, Ji J, Zhang W, Zhao XQ, Liu JJ, Meng DY, Tong YP, Wang T, Li JM (2019) Dissection of pleiotropic QTL regions controlling wheat spike characteristics under different nitrogen treatments using traditional and conditional QTL mapping. Front Plant Sci 10:187
Gao ZX, Wang YX, Tian GY, Zhao YK, Li CH, Cao Q, Han R, Shi ZL, He MQ (2020) Plant height and its relationship with yield in wheat under different irrigation regime. Irrig Sci 38:365–371
Hedden P (2003) The genes of the Green Revolution. Trends Genet 19:5–9
He XY, Singh PK, Dreisigacker S, Singh S, Lilemo M, Duveiller E (2016) Dwarfing genes Rht-B1b and Rht-D1b are associated with both type I FHB susceptibility and low anther extrusion in two bread wheat populations. PLoS ONE 11:e0162499
Kato K, Miura H, Sawada S (1999) QTL mapping of genes controlling ear emergence time and plant height on chromosome 5A of wheat. Theor Appl Genet 98:472–477
Landjeva S, Korzun V, Börner A (2007) Molecular markers: actual and potential contributions to wheat genome characterization and breeding. Euphytica 156:271–296
Li AX, Yang WL, Lou XY, Liu DC, Sun JZ, Guo XL, Wang J, Li YW, Zhan KH, Ling HQ, Zhang AM (2013) Novel natural allelic variations at the Rht-1 loci in wheat. J Integr Plant Biol 55:1026–1037
Law CN, Snape JW, Worland AJ (1978) The genetical relationship between height and yield in wheat. Heredity 40:133–151
Li C, Tang HP, Luo W, Zhang XM, Mu Y, Deng M, Liu YX, Jiang QT, Chen GY, Wang JR, Qi PF, Pu ZE, Jiang YF, Wei YM, Zheng YL, Lan XJ, Ma J (2020) A novel, validated, and plant height-independent QTL for spike extension length is associated with yield-related traits in wheat. Theor Appl Genet 133:3381–3393
Liu G, Xn SB, Ni ZF, Xie CJ, Qin DD, Li J, Lu LH, Zhang JP, Peng HR, Sun QX (2011) Molecular dissection of plant height QTL using recombinant inbred lines from hybrids between common wheat (Triticum aestivum L.) and spelt wheat (Triticum spelta L.). Chinese Sci Bull 56:1897–1903
Ma JJ, Pei WF, Ma QF, Geng YH, Liu GY, Liu J, Cui YP, Zhang X, Wu M, Li XL, Li D, Zang XS, Song JK, Tang SR, Zhang JF, Yu SX, Yu JW (2019) QTL analysis and candidate gene identification for plant height in cotton based on an interspecific backcross inbred line population of Gossypium hirsutum × Gossypium barbadense. Theor Appl Genet 132:2663–2676
Maria B, Hermann B (2016) The semi-dwarfing alleles Rht-D1b and Rht-B1b show marked differences in their associations with anther-retention in wheat heads and with fusarium head blight susceptibility. Phytopathology 106:1544–1552
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green Revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261
Pinthus MJ, Levy AA (1983) The relationship between the Rht 1 and Rht 2 dwarfing genes and grain weight in Triticum aestivum L. spring wheat. Theor Appl Genet 66:153–157
Qiao B, Wang Y, Zhu X, Hong D (2007) QTL analysis of the uppermost internode length in rice under different growing environments. Hereditas 29:1001–1007
Ren XF, Sun DF, Dong WB, Sun GL, Li CD (2014) Molecular detection of QTL controlling plant height components in a doubled haploid barley population. Genet Mol Res 13:3089–3099
Singh A, Knox RE, DePauw RW, Singh AK, Cuthbert RD, Kumar S, Campbell HL (2016) Genetic mapping of common bunt resistance and plant height QTL in wheat. Theor Appl Genet 129:243–256
Sourdille P, Snape JW, Cadalen T, Charmet G, Nakata N, Bernard S, Bernard M (2000) Detection of QTLs for heading time and photoperiod response in wheat using a doubled-haploid population. Genome 43:487–494
Tian XL, Wen W, Xie L, Fu LP, Xu DG, Fu C, Wang DS, Chen XM, Xia XC, Chen QJ, He ZH, Cao SH (2017) Molecular mapping of reduced plant height gene Rht24 in bread wheat. Front Plant Sci 8:1379
Tian XL, Xia XC, Xu DA, Liu YQ, Xie L, Hassan MA, Song J, Li FJ, Wang D, Zhang Y, Hao YF, Li GY, Chu CC, He ZH, Cao SH (2022) Rht24b, an ancient variation of TaGA2ox-A9, reduces plant height without yield penalty in wheat. New Phytol 233:738–750
Wu XS, Wang ZH, Chang XP, Jing RL (2010) Genetic dissection of the developmental behaviours of plant height in wheat under diverse water regimes. J Exp Bot 61:2923–2937
William HM, Trethowan R, Crosby-Galvan EM (2007) Wheat breeding assisted by markers: CIMMYT’s experience. Euphytica 157:307–319
Worland AJ, Korzun V, Röder MS, Ganal MW, Law CN (1998) Genetic analysis of the dwarfing gene Rht8 in wheat. Part II. The distribution and adaptive significance of allelic variants at the Rht8 locus of wheat as revealed by microsatellite screening. Theor Appl Genet 96:1110–1120
Würschum T, Langer SM, Longin CF, Tucker MR, Leiser WL (2017) A modern green revolution gene for reduced height in wheat. Plant J 2:892–903
Würschum T, Liu W, Busemeyer L, Tucker MR, Reif JC, Weissmann EA, Hahn V, Ruckelshausen A, Maurer HP (2014) Mapping dynamic QTL for plant height in triticale. BMC Genet 15:59
Xia C, Zhang LC, Zou C, Gu YQ, Duan JL, Zhao GY, Wu JJ, Liu Y, Fang XH, Gao LF, Jiao YN, Sun JQ, Pan YH, Liu X, Jia JZ, Kong XY (2017) A TRIM insertion in the promoter of Ms2 causes male sterility in wheat. Nat Commun 8:15407
Xue H, Tian XC, Zhang KX, Li WB, Qi ZY, Fang YL, Li XY, Wang Y, Song J, Li WX, Ning HL (2019) Mapping developmental QTL for plant height in soybean [Glycine max (L.) Merr.] using a four-way recombinant inbred line population. PLoS One 14:e0224897
Zhang N, Fan XL, Cui F, Zhao CH, Zhang W, Zhao XQ, Yang LJ, Pan RQ, Chen M, Han J, Ji J, Liu DC, Zhao ZW, Tong YP, Zhang AM, Wang T, Li JM (2017) Characterization of the temporal and spatial expression of wheat (Triticum aestivum L.) plant height at the QTL level and their influence on yield-related traits. Theor Appl Genet 130:1235–1252
Zhang Y, Liu H, Yan G (2021) Characterization of near-isogenic lines confirmed QTL and revealed candidate genes for plant height and yield-related traits in common wheat. Mol Breeding 41:4
Zhang JP, Long Y, Wang LM, Dang Z, Zhang TB, Song XX, Dang ZH, Pei XW (2018a) Consensus genetic linkage map construction and QTL mapping for plant height-related traits in linseed flax (Linum usitatissimum L.). BMC Plant Biol 18:160
Zhang WQ, Li Z, Fang H, Zhang MC, Duan LS (2018b) Analysis of the genetic basis of plant height-related traits in response to ethylene by QTL mapping in maize (Zea mays L.). PLoS One 13:e0193072
Zhao CH, Zhang N, Wu YZ, Sun H, Liu C, Fan XL, Yan XM, Xu HX, Jun Ji, Cui F (2019a) QTL for spike-layer uniformity and their influence on yield-related traits in wheat. BMC Genet 20:23
Zhao CH, Sun H, Liu C, Yang GM, Liu XJ, Wang YP, Lv FX, Wu CY, Xu JW, Wu YZ (2019b) Detection of quantitative trait loci for wheat (Triticum aestivum L.) heading and flowering date. J Agr Sci-Cambridge 157:20–30
Funding
This research was supported by the Agricultural Variety Improvement Project of Shandong Province, China (2019LZGC016), the Major Basic Research Project of Natural Science Foundation of Shandong Province, China (ZR2019ZD16), the Agricultural Variety Improvement Project of Shandong Province, China (2021LZGC009), the National Natural Science Foundation of China (31871612, 32072051, 32101726), the Youth Innovation Technology Support Planning Project for Institution of Higher Education of Shandong Province, China (2019KJF002), and Yantai New and Old Kinetic Energy Conversion Research Institute and Yantai Science and Technology Achievement Transfer Demonstration Base Funded Project (2019XJDN007).
Author information
Authors and Affiliations
Contributions
Cui F, Ji J, and Qin R designed the research; Ji J and Cui F developed the KJ-RIL population; Ma TH, Cao MS, Liu XJ, Zhou XH, Hu GM, Zhong W, and Sun XH conducted phenotyping of the KJ-RIL population; Cui F and Ji J conducted genotyping of the KJ-RIL population; Qin R, Ma TH, Xiao JG, Dong JJ, Kong WC, Zhao CH, Wu YZ, and Sun H analyzed the data; Qin R, Ma TH, and Cui F wrote and revised the paper; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
QTLs for each internode component index (ICI) and plant height component index (PHCI) were identified using a recombinant inbred line (RIL) mapping population. The QTL clusters for PHCI/ICI and plant height and plant height components together with yield-related traits were simultaneously analyzed in the present study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, R., Ma, T., Cao, M. et al. QTL Detection for Internode Component Index in Wheat Using a RIL Mapping Population. Plant Mol Biol Rep 41, 195–208 (2023). https://doi.org/10.1007/s11105-022-01359-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-022-01359-1