Abstract
Calmodulin (CaM) and calmodulin-like (CML) proteins, a group of Ca2+ sensors, play an important role in a large number of different biological processes, including plant growth and development, as well as the biotic and abiotic stress responses. However, CaM/CML genes have not been identified in sacred lotus (Nelumbo nucifera), an important horticultural plant, and the expressional patterns of these genes are yet to be elucidated. In this study, thirty-four CaM/CML genes from Nelumbo nucifera were identified. Phylogenetic analysis showed that they could be divided into nine groups. Gene structure and conserved motif analyses demonstrated the conservation and divergence of CaMs/CMLs in Nelumbo nucifera. Cis-acting elements analysis indicated that they might be related to plant growth and development, abiotic stress, and plant hormones. In addition, expression analysis showed that NNU-CaMs/CMLs were differentially expressed in various tissues and responded to calcium treatments in roots. Moreover, weighted gene co-expression network analysis of public transcriptome data of lotus wild variety “China Antique” with different tissues presented the expression connectivity of NNU-CaMs/CMLs, which were divided into 11 modules. Gene ontology analysis of the genes in each module demonstrated that NNU-CaMs/CMLs may be involved in extensive biological processes, such as synthesis and processing of DNA and RNA, and protein post-translational modification, and each module specifically correlated with the phenotypes including the development of leaves’ petiole and lotus seed, implying the wide regulation of NNU-CaMs/CMLs in lotus. Taken together, our results will enhance the understanding and lay a foundation for further study of the functions of the NNU-CaMs/CMLs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium is an important nutrient, and its ion (Ca2+) is a versatile intracellular messenger in all eukaryotes. In plants, Ca2+ acts as a secondary messenger involved in plant growth and development including stress responses. Stimuli from the surrounding environment and developmental processes elicit calcium signals, and the free Ca2+ concentration rapidly increases in the cytosol (Sarwat et al. 2013). To maintain Ca2+ homeostasis, plant cells have developed calcium channels and pumps for compartmentalization and extrusion of the extra ion (Luan and Wang 2021). Meanwhile, Ca2+-binding proteins with EF-hand motifs that are Ca2+-binding structural motifs bind Ca2+ to reduce its levels in the cell. Calmodulin (CaMs) and calmodulin-like proteins (CMLs) (Zielinski 1998), calcium-dependent protein kinase (CDPKs) (Harmon et al. 2000), and calcineurin B-like proteins (CBLs) (Luan et al. 2002) are the three important EF-hand family proteins in plants.
CaM and CML gene family had been identified and analyzed in various plant species, including Arabidopsis (McCormack and Braam 2003), rice (Boonburapong and Buaboocha 2007), apple (Li et al. 2019), Brassica napus (He et al. 2020), Papaya (Ding et al. 2018), Chinese cabbage (Nie et al. 2017), woodland strawberry (Zhang et al. 2016), Grapevine (Vandelle et al. 2018), Lotus japonicas (Liao et al. 2017), wild tomato (Shi and Du 2020), and tomato (Munir et al. 2016). Genome-wide identification of CaM/CML family from plants showed plants encoded more CMLs than CaMs, and the number is independent of genome size. CaMs are highly conserved proteins containing four EF-hand motifs without any other functional domains. CMLs contain 1 to 6 Ca2+-binding EF-hand motifs (DeFalco et al. 2009). Furthermore, the majority of CMLs are intron-less, whereas CaMs are intron-rich (Mohanta et al. 2017). However, CaMs/CMLs have not been identified in Nelumbo nucifera.
As Ca2+ sensors, CaMs/CMLs have been reported to be involved in various developmental processes (Perochon et al. 2011; Campos et al. 2018). In pollen germination and pollen tube elongation in Arabidopsis thaliana, the level of pollen germination in loss-of-function mutant cam2 was reduced (Landoni et al. 2010); AtCML25 regulated the K+ influx, and loss-of-function mutation in the AtCML25 caused a major reduction in the rate of pollen germination and the elongation of the pollen tube (Wang et al. 2015b). Mutation of cml24 leads to an obvious reduction of root length and decreased lateral root density in Arabidopsis (Zha et al. 2016). Cotton GhCaM7 promoted the elongation of cotton fiber by regulating the production of reactive oxygen species (ROS) (Tang et al. 2014). AtCML39 promoted the light-dependent seedling establishment and loss-of-function cml39 mutants displayed growth arrest after germination in the absence of exogenous sucrose (Bender et al. 2013). In addition, AtCML39 was involved in regulating ovule and fruit development where the loss of AtCML39 resulted in reduced seed number in shorter siliques and the number of ovules (Midhat et al. 2018).
In addition, the roles of CaMs/CMLs in plant responses to both abiotic and biotic stimuli have also been reported (Ranty et al. 2016; Zeng et al. 2015). AtCML20 acts as a negative regulator of ABA and drought stress responses in Arabidopsis (Wu et al. 2017), while AtCML37 is a positive regulator of ABA accumulation induced by drought stress (Scholz et al. 2015). Besides, AtCML37 functions as an active regulator of plant resistance to lepidopteran herbivores (Scholz et al. 2014). Overexpression of AtCML8 enhances the plants’ resistance to pathogenic bacteria (Zhu et al. 2017), indicating that AtCML8 is a positive regulatory factor in plant immunity. Overexpression of the GmCaM4 gene in soybean enhanced its resistance to three plant pathogens and improved its tolerance to high salt conditions (Rao et al. 2014). Moreover, AtCML41, AtCML46, and AtCML47 in Arabidopsis and type III CaM subtype in Tobacco had been documented to participate in the biotic and abiotic stress response (Xu et al. 2017; Lu et al. 2018; Takabatake et al. 2007).
Nelumbo nucifera (N. nucifera) belongs to the small family of Nelumbonaceae (Wang and Zhang 2005), and is named as sacred lotus due to its significance in the religions of Buddhism and Hinduism (Shen-Miller 2002). It is also a popular ornamental, vegetable, and medicinal plant with great economic value (Lin et al. 2019). It is critical to obtain a deeper understanding on its growth and development regulation, as well as its response to different stimuli. Since Ca2+ is one of the most important secondary messengers, it is necessary to figure out its signaling. Nevertheless, there is limited information about CaMs/CMLs and their functions in lotus. In this study, we have identified 34 CaMs/CMLs in N. nucifera based on the genome-wide analysis and have performed bioinformatics analysis that included phylogenetic analysis, gene structures, conserved motifs, and promoter cis-acting elements. The expression patterns in 15 different tissues and weighted gene co-expression network analysis were investigated. Additionally, CaMs/CMLs in response to calcium signaling under calcium treatment were studied. The findings of this study provide potential clues in illuminating the detailed function of CaMs/CMLs involved in the growth, development, and calcium signaling response of N. nucifera in the future.
Materials and Methods
Plant Materials and Treatments
The seeds of China Antique (CA, wild lotus) were placed in the tap-water and germinated in the growth chamber maintained at 22–28 °C, with a photoperiod of 12 h light/12 h dark. For calcium treatment, 2-week-old sacred lotus seedlings were transferred to 10 mM CaCl2, 10 mM EDTA, and H2O (control) until collected for further experiments. Roots were collected at 1, 2, 4, and 12 h after CaCl2, EDTA, and H2O treatments. Three biological replicates were set for each treatment. All the samples were frozen in liquid nitrogen immediately and stored at − 80 °C until used for RNA extraction.
Identification of CaM/CML Family Genes of Lotus
The protein sequences of CaM/CML in Arabidopsis (AtCaM/AtCML) (McCormack and Braam 2003) and rice (OsCaM/ OsCML) (Boonburapong and Buaboocha 2007) were downloaded from TAIR database (https://www.arabidopsis.org/) and Rice Annotation Project Database (https://rapdb.dna.affrc.go.jp/index.html), respectively. The lotus genomic sequences and protein sequences were downloaded from the lotus database (http://lotus-db.wbgcas.cn/) (Ming et al. 2013; Wang et al. 2015a). All the AtCaM/CML and OsCaM/CML protein sequences were used to search for lotus CaM and CML proteins by local BALSTP in BioEdit version 7.0.9.0 with an e-value lower than 10−5. To prove the reliability of the candidate sequences, the core domain of the sequences was analyzed by Pfam (http://pfam.xfam.org/) and SMART (http://smart.embl-heidelberg.de/smart/batch.pl). Proteins containing the EF-hand domain as well as not any other identifiable domains were considered as putative N. nucifera CaM/CML proteins (NNU-CaMs/CMLs) (Wang et al. 2015a). The theoretical molecular weight (Mw) and isoelectric point (pI) were predicted using an online pI/Mw tool (https://web.expasy.org/compute_pi/). The subcellular location was predicted in the online software WoLF PSORT (https://wolfpsort.hgc.jp/).
Gene Structure, Conserved motif, and Promoter Analysis
The intron–exon gene structure of NNU-CaMs/CMLs was analyzed by alignment of coding sequences (CDS) and its corresponding genomic DNA sequences in GSDS 2.0 (http://gsds.gao-lab.org/) (Hu et al. 2015). MEME (http://meme-suite.org/tools/meme) (Bailey et al. 2009) was used to determine the conserved motif of NNU- CaMs/CMLs, and the number of motifs was set to 10, which were further used in the InterPro database (https://www.ebi.ac.uk/interpro/search/sequence/) to annotate motif. The cis-acting elements of 2 kb upstream promoter sequences of NNU-CaMs/CMLs were analyzed by the online software PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Phylogenic Analysis
Multiple sequences of the identified CaMs/CMLs from Arabidopsis (McCormack and Braam 2003), rice (Boonburapong and Buaboocha 2007), and N. nucifera were aligned by ClustalW. The phylogenetic tree was constructed by Neighbor-Joining using MEGA-X (Kumar et al. 2018) with the Bootstrap method, and the number of Bootstrap Replications was set at 1000. Finally, beautification of the phylogenetic tree was done using the online tool ITOL (https://itol.embl.de/) (Letunic and Bork 2016).
Expression Profiling of CaM/CML Family Genes
The genome-wide transcriptome data of sacred lotus from 15 different tissues or developmental stages including anther, carpel, cotyledon, seed coat, and rhizome (Zhang et al. 2019; Li et al. 2018, 2020; Ming et al. 2013) were downloaded from National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/), which were accessed and filtered through FastQC (V0.11.3) and Trimmomatic (V0.38) (Bolger et al. 2014). The obtained clean reads were aligned to the reference genome of N.nucifera from lotus database (http://lotus-db.wbgcas.cn/) (Wang et al. 2015a) using Hisat2 (v2.0.5) (Kim et al. 2015), and the featureCount (V1.6.4) (Liao et al. 2014) was applied to quantify and standardize the read count and TPM (transcripts per kilobase per million) value of each expressed gene. The mRNA expression data of NNU-CaMs/CMLs were extracted from genome-wide expression data, and then, the heatmap was drawn via the R package “pheatmap” (www.r-project.org). The weighted gene co-expression network analysis (WGCNA) method (Langfelder and Horvath 2008) was utilized to further characterize the expression profile of CaM/CML family genes in N.nucifera. All of the genes were used for constructing an expression matrix, and genes with similar expression patterns were clustered into the same module. The relationships between the genes in the module and the different samples were calculated, and the modules containing CaM/CML family genes were identified. In each module, the co-expressed genes associated with CaM/CML family genes were applied to analyze the gene ontology (GO) using the online software AgriGO V2.O (http://systemsbiology.cau.edu.cn/agriGOv2/index.php) (Du et al. 2010).
Gene Expression Analysis by Quantitative Real-Time PCR
The TRIZOL reagent was used to extract total RNA, and the kit of HiScript II Q RT SuperMix for qPCR with gDNA wiper (Vazyme, China) was applied to synthesize cDNA according to manufacturer’s protocol. The gene-specific primers were designed through an online software Primer3Plus (https://primer3plus.com/). qRT-PCR was conducted using gene-specific primers (Table S1) in a 10μL reaction with a 2 × iTaq™ Universal SYBR Green Supermix (BioRad, USA) under the annealing temperature of 54 °C for 10 s, in which the internal reference NnActin (NNU-02553) (Deng et al. 2015) was used to normalize the expression data. Relative expression levels were calculated according to the 2−∆∆CT (cycle threshold) method (Livak and Schmittgen 2001).
Results
Identification and Phylogenetic Analysis of CaM/CML Genes in Lotus
In order to identify the NNU-CaMs/CMLs gene family, 56 CaMs/CMLs in Arabidopsis and 37 CaMs/CMLs in rice (Table S2) were blasted against the lotus protein sequences using local BLASTP in BioEdit. A total of 217 putative lotus protein sequences were identified by local BLASTP under the given criterion, which was further subjected to analysis in Pfam and SMART. Pfam and SMART predicted the presence of EF-hand motif in 82 and 34 protein sequences, respectively. A total of 34 lotus proteins (Table S3) predicted by both were finally selected as the presumed NNU-CaM/CML family proteins. Among all the NNU-CaM/CML proteins, 10, 7, and 4 had four, three, and two EF-hand motifs (Table 1). The numbers of EF-hand motifs in the remaining 13 proteins were inconsistently predicted by the two software. The length of the protein sequences ranged from 82 amino acids (NNU_17378) to 294 amino acids (NNU_22784). The theoretical molecular weights (MW) and isoelectric point (pI) ranged from 9.2 to 31.6 kDa and from 3.96 to 6.89, respectively. The genome of the sacred lotus has been assembled into nine major anchored megascaffolds and several small scaffolds (Ming et al. 2013). Among all the NNU-CaM/CML genes, 27 were distributed on 9 of the largest scaffolds (Table 1). The prediction results of subcellular localization indicated that most of the NNU-CaMs/CMLs were located on the nuclear and cytosol, but some were plastid proteins such as chloroplast proteins and mitochondria proteins. Moreover, an NNU-CaM/CML was predicted to be plasma membrane protein (Table 1).
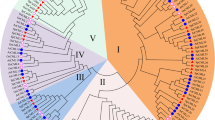
The phylogenetic tree was constructed by Neighbor-Joining and Bootstrap method to study the evolutionary relationship of CaM/CML family genes in Arabidopsis, rice, and lotus (Fig. 1). The results showed that all CaM/CML family genes were divided into 12 groups (Group 1 to Group 12), and 34 NNU-CaMs/CMLs were distributed in 9 groups including Group 1, Group 2, and Group 6 to 12. Groups 8 and 10 were the largest group that contained 7 members of the NNU-CaM/CML genes, respectively. Groups 1 and 2 only contained one NNU-CaM/CML gene.
Phylogenetic analysis of CaM/CML proteins from Arabidopsis, rice, and lotus. The numbers 1–12 indicate different groups, and different groups are represented by different colors in the outermost circle, and the color on the phylogenetic tree is the same as the group color. NNU-CaMs/CMLs are distinguished using red color. The unrooted neighbor-joining (Nj) phylogenetic tree was constructed by MEGA-X. Protein sequences were downloaded from TAIR database (https://www.arabidopsis.org/), Rice Annotation Project Database (https://rapdb.dna.affrc.go.jp/index.html), and the lotus database (http://lotus-db.wbgcas.cn/) (Wang et al. 2015a), respectively
Gene Structure, Conserved Motifs, and Analysis of the Promoters Cis-Acting Elements
Exon–intron structure analysis showed that seven NNU-CaM/CML genes contained one to seven introns and the remaining 27 NNU-CaM/CML genes had only one exon, in which NNU_10822 contained only one intron, while NNU_06974 contained seven introns (Fig. 2). A total of 10 conserved motifs were identified in 34 NNU-CaM/CML proteins using MEME. Members of NNU-CaMs/CMLs contained different number of motifs, and each NNU-CaM/CML protein had two to six conserved motifs. A total of 18 NNU-CaM/CML proteins contained four conserved motifs. Motif sequence analysis showed that motif 1–7 were EF-hand domains for Ca2+ binding and all the NNU-CaM/CML proteins had Motif 1 and motif 2 (Fig. 3). A total of 887 cis-acting elements were discovered in NNU-CaM/CML genes, including 430 light-responsive elements and 244 hormone-responsive elements (Fig. 4). In addition, numerous cis-acting elements related to plant growth and development, abiotic stress, and plant hormones were found in the promoter region of NNU-CaM/CML genes, including two development-related elements CAT-box and GCN4_motif; eight hormone-responsive elements such as ABRE, CGTCA-motif, AuxRR-core, TCA-element, and TATC-box; five abiotic stress–related elements such as MBS, ARE, and LTR; and fifteen light-responsive elements.
Motifs analysis of 34 N. nucifera CaMs/CMLs. Ten motifs identified by the MEME tool are represented by different colored boxes, and their sequences are shown at the bottom. The motifs of NNU-CaMs/CML proteins were grouped according to the phylogenetic classification in Fig. 1
Expression Characterization of NNU-CaM/CML Genes
To clarify the potential function of NNU-CaMs/CMLs, their expression patterns in 15 different tissues including cotyledon, petiole (initial vertical leaves’ petiole and floating leaves’ petiole), seed coat (6, 12, and 18 days after pollination), carpel (pollinated and unpollinated), anther (mature and immature), receptacle (mature and immature), petal, rhizome apical meristem, and rhizome elongation zone derived from published transcriptome data (Zhang et al. 2019; Li et al. 2018, 2020; Ming et al. 2013) were analyzed (Fig. 5). All NNU-CaM/CML genes had higher expression in the petiole, seed coat (DAP6, DAP12, DAP18), anther (mature and immature), rhizome apical meristem, and rhizome elongation zone, and most of the NNU-CaM/CML genes had lower expression in the cotyledon, carpel, receptacle, and petal. NNU_07289, NNU_02678, NNU_12734, NNU_07897, and NNU_16648 had the highest expression in initial floating leaves’ petiole floating on the water surface, whereas NNU_20720, NNU_09004, NNU_00630, NNU_26426, NNU_17318, NNU_09260, and NNU_06974 showed higher expression levels in immature-anther compared with other tissues. NNU_26510, NNU_09549, NNU_17382, NNU_26454, and NNU_11273 had low expression in most tissues.
Expression analysis of N. nucifera CaM/CML genes in different tissues. The transcriptome data were obtained from NCBI (Zhang et al. 2019; Li et al. 2018, 2020; Ming et al. 2013). Red and blue represent high and low expression intensity of NNU-CaM/CML genes in the heat map, respectively. The phylogenetic tree is shown on the left. The different tissues are noted on the bottom and on the right is NNU-CaM/CML gene ID. DAP, days after pollination
Expression Analysis of NNU-CaMs/CMLs in Response to Calcium Signaling
To understand the potential regulation of NNU-CaM/CML genes in response to calcium signaling, expression profile of NNU-CaM/CML genes under high concentration of calcium and calcium inhibitor (EDTA) treatments was analyzed using qRT-PCR (Fig. 6). Fifteen candidate NNU-CaM/CML genes were selected based on the phylogenetic tree classification (Table S1). The expression of fourteen NNU-CaMs/CMLs was altered in response to calcium and EDTA treatment, and only NNU_15888 had no specific trend after 10 mM CaCl2 and EDTA treatment. Among them, the expression levels of nine genes (NNU_03952, NNU_12734, NNU_09004, NNU_09864, NNU_17533, NNU_11273, NNU_20720, NNU_23744, NNU_22784) increased within 2 h after the calcium treatment. The highest expression levels were observed for NNU_09004 and NNU_09864, which showed 15- and 20-fold higher expression levels at 2 h in 10 mM CaCl2 treatment than control. After 10 mM EDTA treatment, the relative quantitative expression of NNU-CaMs/CMLs had no distinct change in the early stage. These results suggest that increased concentration of Ca2+ affected the NNU-CaMs/CMLs spatial expression patterns and NNU-CaMs/CMLs are involved in calcium response.
NNU-CaMs/CMLs Involved in Diverse Biological Processes
In order to elucidate the potential function of NNU-CaMs/CMLs involvement in growth and development, the weighted gene co-expression network, GO enrichment of genes co-expressed with NNU_CaMs/CMLs, and the relationships between traits and modules involved in NNU-CaMs/CMLs were analyzed (Fig. 7). A total of 25 modules were identified based on the co-expression network, and thirty-four NNU-CaM/CML genes were divided into 11 different modules including green module, black module, brown module, turquoise module, pink module, yellow module, tan module, blue module, light-cyan module, dark-green module, and green-yellow module (Fig. 7a). The number of NNU-CaMs/CMLs in each module ranged from one to eight. Eight NNU-CaM/CML genes (NNU_20720, NNU_09004, NNU_07895, NNU_00630, NNU_09260, NNU_13842, NNU_17378, and NNU_06974) were assigned to brown module, which contained the largest number of NNU-CaM/CML genes. The second was the green module, which contained 7 NNU-CaM/CML genes (NNU_26454, NNU_03952, NNU_10822, NNU_14160, NNU_02112, NNU_22775, and NNU_19002). Only one NNU-CaM/CML gene was assigned to the blue (NNU_26426), light-cyan (NNU_26510), dark-green (NNU_09864), and green-yellow (NNU_05464). The number of co-expression genes, which had similar expression patterns with NNU-CaMs/CMLs, ranged from 153 (NNU_09864) to 4776 (NNU_17533), and the average number of genes was 1560. The results showed that NNU-CaMs/CMLs co-expressed with numerous lotus genes.
Weighted gene co-expression network analysis (WGCNA) of NNU-CaMs/CMLs. a Gene module of co-expression. The number of co-expression genes is indicated by the height of the column, and different colors show different weight ranges. The module of each NNU-CaMs/CMLs is located at bottom. b GO enrichment. The different modules distinguished using red color and green shape showed the biological process involved. c Analysis of the module-trait relationships. Each row represents a module, and each column represents a sample. The numbers on the top and bottom of each cell represent the correlation and significant p-values, respectively
GO enrichment analysis of genes in each module connected by NNU-CaM/CML genes showed that 9 modules had significant Go terms except tan and green-yellow module (Fig. 7b). The genes in the turquoise module were involved in a large number of biological processes, mainly related to the synthesis and processing of DNA and RNA. The process of protein post-translational modification was simultaneously enriched in three modules, including the dark-green, green, and pink module. The genes in the light-cyan module participated in protein folding and the genes in the brown module were mainly involved in the biological process of transport. Module-trait relationships analysis displayed a significant correlation between different modules and phenotypes. The nine modules including green, black, pink, yellow, dark-green, turquoise, brown, blue, and light-cyan modules were significantly correlated with different tissues (Fig. 7c). The brown module was significantly correlated with immature anther (R = 0.99, p = 9e − 14), the dark-green module was significantly correlated with rhizome apical meristem (R = 0.99, p = 2e − 12), the development of seed coat was significantly associated with the green module (DAP6, R = 0.96, p = 1e − 08) and the black module (DAP18, R = 0.95, p = 1e − 07), and the petiole types were correlated with pink (floating leaves’ petiole, R = 0.98, p = 4e − 10), and yellow module (vertical leaves’ petiole, R = 0.78, p = 7e − 04), indicating that different NNU-CaM/CML genes may involve in different development processes.
Discussion
Calcium, a universal second messenger, plays a key role in the signal transduction process during plant growth, development, and stress response (McAinsh and Pittman 2009). CaMs are conserved Ca2+ sensors in all eukaryotic cells. In addition to the CaMs, there are plant-specific calmodulins-like proteins (CMLs) that exist in plants (DeFalco et al. 2009). The previously reported genome sequence of the sacred lotus (Wang et al. 2015a; Ming et al. 2013) facilitates the study of genome-wide identification and expression analysis of NNU-CaMs/CMLs. In this study, we identified 34 NNU-CaMs/CMLs which showed extensive variations in gene length, theoretical molecular weight (Mw), and isoelectric point (pI) (Table 1), indicating the diversity of the NNU-CaMs/CMLs. The number of NNU-CaMs/CMLs was more than those in Lotus japonicas (7 LjCaMs and 19 LjCMLs), but less than those in Arabidopsis (6 AtCaMs and 50 AtCMLs), rice (3 OsCaMs and 32 OsCMLs), apple (4 MdCaMs and 58 MdCMLs), and grapevine (3 VviCaMs and 62 VviCMLs) (Liao et al. 2017; McCormack and Braam 2003; Boonburapong and Buaboocha 2007; Li et al. 2019; Vandelle et al. 2018). It may be that the incomplete genome sequencing and rigorous screening criteria lead to a small number of NNU-CaMs/CMLs. The predicted number of EF-hand in NNU-CaMs/CMLs ranged from one to four, which is consistent with those in rice (Boonburapong and Buaboocha 2007).
CaM proteins do not contain any palmitoylation or myristoylation signaling sequence (Mohanta et al. 2017, 2019), and the majority of their targets are cytosolic or nuclear proteins (Perochon et al. 2011). Therefore, CaM proteins are often found in the nucleus and cytoplasm (Wu et al. 2012; Zeb et al. 2020). The majority of CMLs also do not have localization signatures, and nuclear-cytoplasmic localization has been observed (Perochon et al. 2010; Trande et al. 2019). Meanwhile, CMLs were reported in various locations, such as AtCML30 localized in mitochondria (Chigri et al. 2012); AtCML36 (Astegno et al. 2017) and OsCML3 (Chinpongpanich et al. 2015) were localized in the plasma membrane. In this study, the NNU-CaMs/CMLs were predicted to mainly localize in the nucleus and cytoplasmic, and they were also predicted to be plastid proteins and plasma membrane proteins (Table 1), in consonance with the previous reports. The results of subcellular localization imply that NNU-CaMs/CMLs have diverse functions in the process of calcium signing transduction.
Based on the phylogenetic tree, CaMs/CMLs from Arabidopsis, rice, and lotus clustered within the same group and were classified into 12 subgroups (Fig. 1). NNU-CaMs/CMLs tightly clustered with AtCaMs/CMLs than with OsCaMs/CMLs, which was similar to those of woodland strawberry (Fragaria vesca) (Zhang et al. 2016), suggesting that CaMs/CMLs family is conserved in eudicots and monocots. Interestingly, NNU-CaMs/CMLs presented in Group 1, Group 2, and Group 8 contained several introns, while the other members only contained one exon (Fig. 2). In agreement with AtCaMs/AtCMLs and OsCaMs/OsCMLs (McCormack and Braam 2003; Boonburapong and Buaboocha 2007), most of NNU-CaMs/CMLs are intronless. In the evolutionary process of Ca2+ signing molecules, some calcium signaling genes were lost in the monocot lineage (Mohanta et al. 2019). In a previous study, FvCMLs were more clustered with AtCMLs than with OsCMLs (Zhang et al. 2016). The present study revealed Groups 7 and 8 only contained AtCMLs and NNU-CMLs, but no OsCMLs, probably because this cluster only represented CMLs in eudicots. These results indicated that the structure of NNU-CaMs/CMLs was diverse and conserved in eudicots, which may result in functional diversity.
The specific expression of genes is determined by the cis-acting elements in the promoter region. Previous studies have shown that certain CML genes with special cis-acting elements in the promoter region participated in hormonal or abiotic stress responses, such as AtCML9 (Magnan et al. 2008) and MtCML40 (Wang et al. 2019). In our study, NNU-CaMs/CMLs had various cis-acting elements related to plant growth and development, abiotic stress, plant hormones (Fig. 4). More than half of the NNU-CaM/CML genes contained cis-acting elements related to ABA (ABRE) and drought (MBS) stresses, indicating that NNU-CaM/CML genes may extensively participate in ABA and drought regulation. Half of the NNU-CaM/CML genes had anaerobic-responsive elements (ARE), which might be related to the adaptability to the aquatic environment.
The expressional patterns of NNU-CaM/CML genes in 15 different sacred lotus tissues had powerful specificity, which demonstrated that these genes are mainly involved in the regulation of initial floating leaves’ petiole, immature-anther, and seed coat development processes (Fig. 5). The results clarify that NNU-CaMs/CMLs involved in the regulation of development. The variety of petiole rigidity produces two petioles types of lotus, initial floating leaves’ petiole, and initial vertical leaves’ petiole. A previous study showed the process of cell wall biosynthesis and lignin biosynthesis affects lotus petioles rigidity formation (Li et al. 2020). In this study, five NNU-CaMs/CMLs (NNU_07289, NNU_02678, NNU_12734, NNU_07897, NNU_16648) were highly expressed in initial floating leaves’ petiole, implying that they may be involved in the regulation of cell wall biosynthesis, which was further supported by the observation that PdIQD10 interaction with two calmodulin proteins and involved in secondary cell wall biosynthesis in Populus (Badmi et al. 2018). Seven NNU-CaMs/CMLs showed high expression levels in the immature-anther, indicating that they may be associated with early anther development in lotus. Furthermore, WGCNA had shown that NNU-CaMs/CMLs were divided into 11 modules and GO enrichment analysis of co-expressed genes in each module also demonstrated that these genes are involved in a wide range of biological processes such as regulation, post-translational protein modification, and the response to stress, of which each module was significantly correlated with development traits in lotus (Fig. 7). These results collectively indicated that NNU-CaM/CML genes might have undergone functional differentiation, participating in multiple growth and development processes and interacting with multiple genes to regulate physiological responses. It is especially special that the results of the module-trait relationships showed the leaves’ petiole preferential expressed gene NNU_12734 correlated with two petioles types in sacred lotus, which contain ARE cis-acting regulatory element essential for the anaerobic induction. Meanwhile, GO enrichment analysis indicated that NNU_12734 was involved in the cellular polysaccharide metabolic process. There is consistent data that NNU_12734 might participate in the pathway of petiole rigidity formation in lotus.
In light of calcium signal playing an important role in plant stress response, the expression patterns of NNU-CaMs/CMLs in response to calcium treatment may be useful in exploring its functional responsibility in calcium signaling. We analyzed the expression of 15 NNU-CaMs/CMLs in response to CaCl2 and the chelating compounds EDTA by qRT-PCR. Approximate 60% NNU-CaMs/CMLs were up-regulated after 10 mM CaCl2 treatment (Fig. 6), indicating that the high concentration of exogenous calcium ion stimulates cells and activates Ca2+-permeable channels, increasing intracellular Ca2+ concentration to generate calcium signaling, and the expression of calcium-binding receptors NNU-CaMs/CMLs is elevated afterward. The NNU-CaMs/CMLs has a typical Ca2+ signaling process (Tian et al. 2020) after calcium treatment. The expression of NNU-CaMs/CMLs had no significant change after the early stage of 10 mM EDTA treatment, which chelate Ca2+, Mg2+ by diamine, and four carboxylate groups (Clapham 2007), demonstrating that the low intracellular Ca2+ concentration would not induce NNU-CaMs/CMLs expression. These results indicated that the concentration of calcium ions will affect the NNU-CaMs/CMLs to perceive the calcium signaling in the lotus. Previous studies have suggested exogenous calcium reduced harmful effects caused by salt stress in Phyllanthus amarus (Jaleel et al. 2008), sunflower (Helianthus annuus L.) (Sohan et al. 1999), and rice (Oryza sativa L.) (Rahman et al. 2016; Roy et al. 2019). Therefore, NNU-CaMs/CMLs response to the change in exogenous calcium concentration can provide new avenues for further studies on NNU-CaMs/CMLs role in abiotic stress including excess ions.
The gene structure and conserved domain in specific proteins determine the molecular function involved in growth, development, and stress response, and genes in a designated family have a similar and differential function because of the variation in the promoter sequence and coding sequence. Thus, investigating the characterization of members in a gene family will improve our understanding of their potential function in regulating biological processes. In the present study, NNU-CaMs/CMLs gene family was analyzed based on genome sequencing, and the gene structure, protein domain, expression profiling, and the response to Ca2+ treatment were expounded. The results presented here could provide a theoretical basis on the molecular mechanism of CaM/CML genes family in lotus (NNU-CaMs/CMLs) in response to calcium signaling. Novel information obtained will contribute to further research on the effect of exogenous Ca2+ on stress tolerance, as well as to select potential candidate genes in N. nucifera for functional studies. In addition, this study will pave the way for developing cultivars with improved tolerance against high calcium environments in lotus.
References
Astegno A, Bonza MC, Vallone R, La Verde V, D’Onofrio M, Luoni L, Molesini B, Dominici P (2017) Arabidopsis calmodulin-like protein CML36 is a calcium (Ca2+) sensor that interacts with the plasma membrane Ca2+-ATPase isoform ACA8 and stimulates its activity. J Biol Chem 292(36):15049–15061
Badmi R, Payyavula RS, Bali G, Guo HB, Jawdy SS, Gunter LE, Yang X, Winkeler KA, Collins C, Rottmann WH, Yee K, Rodriguez MJr, Sykes RW, Decker SR, Davis MF, Ragauskas AJ, Tuskan GA, Kalluri UC (2018) A new calmodulin-binding protein expresses in the context of secondary cell wall biosynthesis and impacts biomass properties in Populus. Frontiers in plant science 9: 1669
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208
Bender KW, Rosenbaum DM, Vanderbeld B, Ubaid M, Snedden WA (2013) The Arabidopsis calmodulin-like protein, CML39, functions during early seedling establishment. Plant J 76(4):634–647
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: aflexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Boonburapong B, Buaboocha T (2007) Genome-wide identification and analyses of the rice calmodulin and related potential calcium sensor proteins. BMC Plant Biol 7:4
Campos WF, Dressano K, Ceciliato P, Guerrero-Abad JC, Silva AL, Fiori CS, Morato DCA, Bergonci T, Claus L, Silva-Filho MC, Moura DS (2018) Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein 38. J Biol Chem 293(6):2159–2171
Clapham DE (2007) Calcium Signaling. Cell 131(6):1047–1058
Chigri F, Flosdorff S, Pilz S, Kölle E, Dolze E, Gietl C, Vothknecht UC (2012) The Arabidopsis calmodulin-like proteins AtCML30 and AtCML3 are targeted to mitochondria and peroxisomes, respectively. Plant Mol Biol 78(3):211–222
Chinpongpanich A, Phean-O-Pas S, Thongchuang M, Qu LJ, Buaboocha T (2015) C-terminal extension of calmodulin-like 3 protein from Oryza sativa L.: interaction with a high mobility group target protein. Acta Biochim Biophys Sin (Shanghai) 47(11):880–9
DeFalco TA, Bender KW, Snedden WA (2009) Breaking the code: Ca2+ sensors in plant signalling. Biochem J 425(1):27–40
Deng J, Fu Z, Chen S, Damaris RN, Wang K, Li T, Yang P (2015) Proteomic and epigenetic analyses of lotus (Nelumbo nucifera) petals between red and white cultivars. Plant Cell Physiol 56(8):1546–1555
Ding XC, Zhang LP, Hao YW, Xiao SL, Wu ZX, Chen WX, Li XP, Zhu XY (2018) Genome-wide identification and expression analyses of the calmodulin and calmodulin-like proteins reveal their involvement in stress response and fruit ripening in papaya. Postharvest Biol Tec 143:13–27
Du Z, Zhou X, Ling X, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38:W64–W70
Harmon AC, Gribskov M, Harper JF (2000) CDPKs — a kinase for every Ca2+ signal? Trends Plant Sci 5(4):154–159
He X, Liu W, Li W, Liu Y, Wang W, Xie P, Kang Y, Liao L, Qian L, Liu Z, Guan C, Guan M, Hua W (2020) Genome-wide identification and expression analysis of CaM/CML genes in Brassica napus under abiotic stress. J Plant Physiol 255:153251
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31(8):1296–1297
Jaleel CA, Kishorekumar A, Manivannan P, Saankar B, Gomathinayagam M, Panneerselvam R (2008) Salt stress mitigation by calcium chloride in Phyllanthus amarus. Acta Bot Croat 67:53–62
Kim D, Langmead B, Salzberg SL (2015) HISAT: A fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Landoni M, De Francesco A, Galbiati M, Tonelli C (2010) A loss-of-function mutation in Calmodulin2 gene affects pollen germination in Arabidopsis thaliana. Plant Mol Biol 74(3):235–247
Langfelder P, Horvath S (2008) WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 9:559
Letunic I, Bork P (2016) Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44(W1):W242-245
Li C, Meng D, Zhang J, Cheng L (2019) Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in apple (Malus x domestica). Plant Physiol Biochem 139:600–612
Li J, Shi T, Huang L, He D, Nyong’A T, Yang P (2018) Systematic transcriptomic analysis provides insights into lotus (Nelumbo nucifera) seed development. Plant Growth Regul 86(3):339–350
Li M, Hameed I, Cao D, He D, Yang P (2020) Integrated Omics analyses identify key pathways involved in petiole rigidity formation in sacred lotus. Int J Mol Sci 21(14):5087
Liao J, Deng J, Qin Z, Tang J, Shu M, Ding C, Liu J, Hu C, Yuan M, Huang Y, Yang R, Zhou Y (2017) Genome-wide identification and analyses of calmodulins and calmodulin-like proteins in lotus japonicas. Front Plant Sci 8:482
Liao Y, Smyth GK, Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30(7):923–930
Lin Z, Zhang C, Cao D, Damaris RN, Yang P (2019) The latest studies on lotus (Nelumbo nucifera)—an emerging horticultural model plant. Int J Mol Sci 20(15):3680
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Luan S, Wang C (2021) Calcium signaling mechanisms across kingdoms. Annu Rev Cell Dev Biol 37:18.1–18.29
Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W (2002) Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14:S389-400
Lu Y, Truman W, Liu X, Bethke G, Zhou M, Myers CL, Katagiri F, Glazebrook J (2018) Different modes of negative regulation of plant immunity by calmodulin-related genes. Plant Physiol 176(4):3046–3061
Magnan F, Ranty B, Charpenteau M, Sotta B, Galaud JP, Aldon D (2008) Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J 56(4):575–589
McAinsh MR, Pittman JK (2009) Shaping the calcium signature. New Phytol 181(2):275–294
McCormack E, Braam J (2003) Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol 159(3):585–598
Midhat U, Ting M, Teresinski HJ, Snedden WA (2018) The calmodulin-like protein, CML39, is involved in regulating seed development, germination, and fruit development in Arabidopsis. Plant Mol Biol 96(4–5):375–392
Ming R, VanBuren R, Liu Y, Yang M, Han Y, Li LT, Zhang Q, Kim MJ, Schatz MC, Campbell M et al (2013) Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn.). Genome Biol 14(5):R41
Mohanta TK, Kumar P, Bae H (2017) Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol 17(1):38
Mohanta TK, Yadav D, Khan AL, Hashem A, Abd_Allah EF, Al-Harrasi A (2019) Molecular players of EF-hand containing calcium signaling event in plants. Int J Mol Sci 20(6):1476
Munir S, Khan MR, Song J, Munir S, Zhang Y, Ye Z, Wang T (2016) Genome-wide identification, characterization and expression analysis of calmodulin-like (CML) proteins in tomato (Solanum lycopersicum). Plant Physiol Biochem 102:167–179
Nie S, Zhang M, Zhang L (2017) Genome-wide identification and expression analysis of calmodulin-like (CML) genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Genomics 18(1):842
Perochon A, Aldon D, Galaud JP, Ranty B (2011) Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 93(12):2048–2053
Perochon A, Dieterle S, Pouzet C, Aldon D, Galaud JP, Ranty B (2010) Interaction of a plant pseudo-response regulator with a calmodulin-like protein. Biochem Biophys Res Commun 398(4):747–751
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci 12(7):609
Ranty B, Aldon D, Cotelle V, Galaud JP, Thuleau P, Mazars C (2016) Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front Plant Sci 7:327
Rao SS, El-Habbak MH, Havens WM, Singh A, Zheng D, Vaughn L, Haudenshield JS, Hartman GL, Korban SS, Ghabrial SA (2014) Overexpression of GmCaM4 in soybean enhances resistance to pathogens and tolerance to salt stress. Mol Plant Pathol 15(2):145–160
Roy PR, Tahjib-Ul-Arif M, Polash MAS, Hossen MZ, Hossain MA (2019) Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol Mol Biol Plants 25(3):611–624
Sarwat M, Ahmad P, Nabi G, Hu X (2013) Ca2+ signals: the versatile decoders of environmental cues. Crit Rev Biotechnol 33(1):97–109
Scholz SS, Vadassery J, Heyer M, Reichelt M, Bender KW, Snedden WA, Boland W, Mithöfer A (2014) Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol Plant 7(12):1712–1726
Scholz SS, Reichelt M, Vadassery J, Mithofer A (2015) Calmodulin-like protein CML37 is a positive regulator of ABA during drought stress in Arabidopsis. Plant Signal Behav 10(6):e1011951
Shen-Miller J (2002) Sacred lotus, the long-living fruits of China Antique. Seed Sci Res 12(3):131–143
Sohan D, Jasoni R, Zajicek J (1999) Plant–water relations of NaCl and calcium-treated sunflower plants. Environ Exp Bot 42:105–111
Zhu X, Robe E, Jomat L, Aldon D, Mazars C, Galaud JP (2017) CML8, an Arabidopsis calmodulin-like protein, plays a role in Pseudomonas syringae plant immunity. Plant Cell Physiol 58(2):307–319
Shi J, Du X (2020) Identification, characterization and expression analysis of calmodulin and calmodulin-like proteins in Solanum pennellii. Sci Rep 10(1):7474
Takabatake R, Karita E, Seo S, Mitsuhara I, Kuchitsu K, Ohashi Y (2007) Pathogen-induced calmodulin isoforms in basal resistance against bacterial and fungal pathogens in tobacco. Plant Cell Physiol 48:414–423
Tang W, Tu L, Yang X, Tan J, Deng F, Hao J, Guo K, Lindsey K, Zhang X (2014) The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating reactive oxygen species (ROS) production. New Phytol 202(2):509–520
Tian W, Wang C, Gao Q, Li L, Luan S (2020) Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants 6(7):750–759
Trande M, Pedretti M, Bonza MC, Di Matteo A, D’Onofrio M, Dominici P, Astegno A (2019) Cation and peptide binding properties of CML7, a calmodulin-like protein from Arabidopsis thaliana. J Inorg Biochem 199:110796
Vandelle E, Vannozzi A, Wong D, Danzi D, Digby AM, Dal Santo S, Astegno A (2018) Identification, characterization, and expression analysis of calmodulin and calmodulin-like genes in grapevine (Vitis vinifera) reveal likely roles in stress responses. Plant Physiol Biochem 129:221–237
Wang K, Deng J, Damaris RN, Yang M, Xu L, Yang P (2015a) LOTUS-DB: an integrative and interactive database for Nelumbo nucifera study. Database (Oxford) 2015:bav023
Wang Q, Zhang X (2005) Colored Illustration of Lotus Cultivars in China. China Forestry Publishing House, Beijing
Wang SS, Diao WZ, Yang X, Qiao Z, Wang M, Acharya BR, Zhang W (2015b) Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ 38(11):2372–2386
Wang T, Liu M, Sun W, Zhang X, Zhang W (2019) Calmodulin-like gene MtCML40 is involved in salt tolerance by regulating MtHKTs transporters in Medicago truncatula. Environ Exp Bot 157:79–90
Wu HC, Luo DL, Vignols F, Jinn TL (2012) Heat shock-induced biphasic Ca2+ signature and OsCaM1–1 nuclear localization mediate downstream signalling in acquisition of thermotolerance in rice (Oryza sativa L.). Plant Cell Environ 35(9):1543–57
Wu X, Qiao Z, Liu H, Acharya BR, Li C, Zhang W (2017) CML20, an Arabidopsis calmodulin-like protein, negatively regulates guard cell ABA signaling and drought stress tolerance. Front Plant Sci 8:824
Xu B, Cheval C, Laohavisit A, Hocking B, Chiasson D, Olsson T, Shirasu K, Faulkner C, Gilliham M (2017) A calmodulin-like protein regulates plasmodesmal closure during bacterial immune responses. New Phytol 215(1):77–84
Zeb Q, Wang X, Hou C, Zhang X, Dong M, Zhang S, Zhang Q, Ren Z, Tian W, Zhu H, Li L, Liu L (2020) The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J Integr Plant Biol 62(7):887–896
Zeng H, Xu L, Singh A, Wang H, Du L, Poovaiah BW (2015) Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front Plant Sci 6:600
Zha G, Wang B, Liu J, Yan J, Zhu L, Yang X (2016) Mechanical touch responses of Arabidopsis TCH1-3 mutant roots on inclined hard-agar surface. Int Agrophys 30:105–111
Zhang K, Yue D, Wei W, Hu Y, Feng J, Zou Z (2016) Characterization and functional analysis of calmodulin and calmodulin-like genes in Fragaria vesca. Front Plant Sci 7:1820
Zhang Y, Nyong’A TM, Shi T, Yang P (2019) The complexity of alternative splicing and landscape of tissue-specific expression in lotus (Nelumbo nucifera) unveiled by Illumina- and single-molecule real-time-based RNA-sequencing. DNA Res 26(4):301–311
Zielinski RE (1998) Calmodulin and calmodulin-binding proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 49:697–725
Author information
Authors and Affiliations
Contributions
P. Y. designed the experiment. L.G. performed the experiments. L.G. and F. Y. analyzed the results and prepared the original manuscript. F. Y., D.N.R., and P. Y. revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
∙ A total of 34 CaM/CML genes from lotus (Nelumbo nucifera) were identified.
∙ NNU-CaMs/CMLs were differentially expressed in various tissues.
∙ NNU-CaMs/CMLs responded to calcium signaling under calcium treatments in roots.
∙ NNU-CaMs/CMLs are involved in extensive biological processes.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, L., Damaris, R.N., Yu, F. et al. Genome-wide Identification and Expression Analysis of CaM/CML Gene Family in Sacred Lotus (Nelumbo nucifera). Plant Mol Biol Rep 40, 418–432 (2022). https://doi.org/10.1007/s11105-021-01330-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-021-01330-6