Abstract
Pakchoi plants were grown in 32 mM NO3− nutrient solution with or without 2.5 mM γ-aminobutyric acid (GABA) to investigate metabolite changes, gene and protein expression levels, and the activities of key enzymes related to nitrate metabolism in the leaves over a period of 0–12 days. High-nitrogen treatment enhanced plant growth and the NO3−, NO2−, NH4+, Gln, and Glu contents in the leaves; promoted the gene and protein expression of nitrate reductase (NR) and glutamate decarboxylase (GAD); and increased the activities of NR, nitrite reductase (NiR), glutamine synthetase (GS), glutamate synthase (GOGAT), and GAD. The endogenous GABA concentration in the leaves was enhanced in parallel with the increase in GAD activity. The GABA-treated leaves displayed the greatest increases in the gene and protein expression levels of NR and GAD and in the activities of NR, NiR, GS, GOGAT, and GAD. In addition, accelerated rates of nitrate reduction and assimilation were detected, and these changes occurred concurrently with the observed increases in gene or protein expression and enzyme activity. As a result, the concentrations of NH4+, Gln, Glu, and endogenous GABA were significantly elevated, and the NO3− and NO2− contents were significantly decreased, in GABA-treated leaves compared with plants exposed to nitrogen-rich conditions. Our results reveal a potential positive that GABA may act as a nitrogen source to improve the plant growth and the most prominent effect of decreasing nitrate contents by accelerating NO3− reduction and assimilation. Exogenous GABA plays an important role in reducing the NO3− content of leaves, and thereby improves the ability to harvest leafy vegetables containing higher levels of endogenous GABA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen is an essential element for accelerated shoot growth, rapid flower bud development, and effectively increasing crop productivity. Furthermore, the effective supply and management of nitrogenous fertilizer favors the formation of amino acids or protein, which is beneficial for increasing crop quality (Chen et al. 2004). However, the amount of nitrogen fertilizer used in vegetable production has far exceeded the recommendations for open fields and polypropylene-covered or solar greenhouses in China, resulting in serious problems, such as poor plant growth, soil acidification and secondary salinization, and eutrophication of nearby aquatic bodies. Green leafy vegetables are known for their capacity to accumulate nitrate, and thus for their contribution to a high total nitrate intake in people’s daily diet (Colonna et al. 2016). Although nitrate itself is relatively non-toxic, debates regarding its benefits have continued because its reaction products and metabolites (e.g., NO2−, NO, and nitrosamines) exert adverse health effects that result in suffocation, particularly in infants, children, and adults (Bryan et al. 2012). Consequently, some organizations, such as the World Health Organization (WHO) and the European Commission, have established regulatory limitations or thresholds for nitrate concentrations in some leafy vegetables and/or criteria for acceptable daily nitrate intake levels according to body weight (Cavaiuolo and Ferrante 2014).
Traditionally, inorganic nitrogen, particularly nitrate, has been among the most commonly supplied nutrients, and commercial fertilizers applied to increase output represent the major cost of plant production (Masclaux-Daubresse et al. 2010). However, many factors, especially the amount, timing, and method of nitrate fertilization used, affect the nitrate concentration in leafy vegetables (Colonna et al. 2016). External nitrate has positive regulatory effects on the nitrate uptake itself and on many genes of primary nitrogen metabolism in many plant species (Beuve et al. 2004). Furthermore, it has been widely established that organic forms of nitrogen, particularly small-molecule amino acids (e.g., arginine, glutamate (Glu), glutamine (Gln), alanine, methionine, and glycine), can also be taken up by a wide variety of plants from rhizosphere soil or nutrient solutions (Näsholm et al. 2009). When the rates of root uptake of nitrogen sources are compared, the general conclusion is that for most plant species, the absorption of amino acid is higher than that of nitrate when several N sources are present simultaneously (Näsholm et al. 2009; Persson et al. 2006; Thornton and Robinson 2005). Indeed, several informative studies have confirmed the ability to produce low-nitrate leafy vegetables (e.g., spinach, lettuce, and pakchoi) by applying organic nitrogen as a substitute or partial substitute for nitrate in nutrition solution, a process that is closely related to the regulation of nitrate uptake and the shifting of the metabolic flux of the roots (Liu et al. 2014; Wang et al. 2014b). However, this effect is influenced by many factors, including the type and amount of amino acid, the duration and method of application, and the concentration of inorganic ions in the soil or solution (Muller and Touraine 1992; Wang et al. 2007). Nonetheless, the available direct lines of evidence regarding the influence of amino acids on nitrogen metabolism, particularly the key enzymes and substances associated with nitrate reduction following the absorption of amino acids, remain insufficient.
γ-Aminobutyric acid (GABA) is a non-protein amino acid widely present in plant tissue. The synthesis pathway of GABA is regulated by glutamic acid degradation catalyzed by GAD (EC 4.1.1.15). Concurrently, the promotion of GABA production technology accelerates the production of GABA-rich vegetables, teas (Wang et al. 2006), and health-oriented plant products (Liu et al. 2016). GABA plays a role in many important physiological metabolic pathways that have been elucidated in previous studies; these functions include regulating pH, facilitating the bypass of tricarboxylic acid cycle, metabolizing nitrogen, regulating antioxidants, providing an efficient growth-supporting nitrogen source, and acting as a signaling molecule (Fait et al. 2008; Hellmann et al. 2000). Some studies have demonstrated that GABA might act as a long-distance signal to upregulate nitrate uptake and utilization in Brassica napus (Beuve et al. 2004) and Arabidopsis thaliana seedlings (Barbosa et al. 2010). Furthermore, exogenous GABA can be absorbed by plants as an alternative organic nitrogen fertilizer after it is applied to the rhizosphere or sprayed on leaves (Ma et al. 2016; Salvatierra et al. 2016). However, Arabidopsis seedlings fed significant concentrations of GABA still demonstrate low levels of GABA accumulation in tissues; additionally, GABA promoted root growth and NO3− uptake at a low NO3−level (5 mM NO3−), while GABA inhibited root elongation and NO3− uptake at high NO3− level (40 mM NO3−) (Barbosa et al. 2000). Although some regulatory models can theoretically explain the relationship between the role of GABA role in plants and NO3− levels in the rhizosphere, related experiments did not provide a clear explanation of the role of GABA in reducing nitrate in plant tissue, or the precise molecular conversions that occur after the absorption of GABA.
Because pakchoi (Brassica campestris ssp. chinensis Makino) exhibits the advantages of high yields, good adaptability, and resistance, it is one of the most widely cultivated leafy vegetables in China (Luo et al. 2006). However, the growth and, ultimately, the yield and quality of pakchoi are greatly affected by the level and status of nitrogen nutrition, particularly that of nitrate, in the rhizosphere. Increased inorganic nitrogen fertilizer inputs have improved the production of pakchoi in the past 20 years, but this procedure poses a great risk to the quality of the vegetables due to the accumulation of nitrate in the leaves (Wang et al. 2014b). Currently, amino acids’ substitution technology has become an effective method for decreasing the nitrate concentration in vegetables. Our experiments have shown that adding GABA to the nutrient solution, soaking seeds, and foliar spray reduced the nitrate content in leafy vegetables and increased the endogenous GABA contents (Li et al. 2016; Li et al. 2017). However, to the best of our knowledge, the mechanism by which GABA absorption decreases nitrate and improves plant growth, particularly the effects on gene and protein expression of key enzymes and on metabolite changes, remains ambiguous. We can propose a model in which GABA, after uptake by the roots, may act as a nitrogen source to improve plant growth, with the most prominent effect of decreasing nitrate contents by accelerating nitrate reduction and assimilation, followed by inhibiting nitrate uptake. Thus, based on the identification and molecular cloning of BcNR and BcGAD from pakchoi, the present study evaluated the effects of exogenous GABA on the gene and protein expression of BcNR and BcGAD and the metabolite contents in pakchoi leaves cultured with a nitrogen-rich nutrient solution. The results obtained regarding the effects of GABA on nitrogen metabolism might provide a new basis for elucidating the mechanism through which GABA reduces nitrate contents in pakchoi at a relatively high NO3− supply level.
Materials and Methods
Plant Materials
The pakchoi (Brassica campestris ssp. chinensis Makino) cultivar “Wuyueman,” which is known for its late bolting capability, was used as the experimental material. The seeds were sown in a seedling-raising plate with wet quartz sand after surface sterilization with water at 55 °C. The seedlings were grown under controlled greenhouse conditions with a 16/8-h photoperiod, a photosynthetic photon flux density of 350 μmol m−2 s−1, a temperature of 26 °C/16 °C, and a relative humidity of 60–75%. The seedlings were watered with half-strength Hoagland solution after cotyledon expansion.
Experimental Treatments
At the four-true-leaf stage, seedlings exhibiting consistent growth were selected and divided into three groups for continued growth in a hydroponic system. Twenty seedlings were planted in 60 × 40 × 20-cm plastic pots (length × width × height) filled with 48 L of Hoagland solution (containing 14 mM NO3−, 1 mM NH4+, EC 2.0–2.2 mS cm−1, pH 6.5 ± 0.1) with a dissolved oxygen concentration of 8.0 ± 0.2 mg L−1, which was controlled using air pumps. After 10 days of growth, when the plants exhibited seven leaves, they were subjected to the experimental treatments in renewed nutrient solution. For the treatments, the seedlings were cultured with one of three solutions:
-
(1)
Control: the nitrate concentration in the Hoagland solution was maintained at 14 mM without GABA;
-
(2)
High-nitrogen treatment (HN): 18 mM NaNO3 was added to the Hoagland solution to obtain a final nitrate concentration of 32 mM, while the NH4+ concentration was maintained at 1 mM. Although 32 mM nitrate is lower than the actual nitrogen level used for leafy vegetable production in protected environments in China, this concentration was used based on plant growth determination experiments performed with different nitrate concentrations (8, 16, 24, 32, and 40 mM) in Hoagland nutrient solution for 22 days.
-
(3)
Treatment with 2.5 mM GABA (HN + GABA): 2.5 mM GABA (Sigma Chemical Co., USA) and 18 mM NaNO3 were simultaneously added to the Hoagland solution to obtain a final nitrate concentration of 32 mM.
The concentration of GABA was determined based on our previous experiments, the results of which indicated that nitrate metabolism in pakchoi is more significantly affected in the presence of 2.5 mM GABA than in the presence of 1.25, 3.75, 5.0, 7.5, or 10 mM GABA (data not shown). The experimental design was repeated four times for every group, and each treatment included a total of 12 pots (80 plants per treatment) arranged using a completely randomized block design. Leaf samples were collected from new leaves of the control plants to clone the NR and GAD gene sequences. Furthermore, key enzymes and metabolites related to nitrate metabolism were assessed in the second-uppermost well-developed leaves collected from plants subjected to the three treatments for 0, 3, 6, 9, and 12 days, and the gene and protein expression levels were evaluated after 0, 0.5, 1, 2, 3, 4, 6, 9, and 12 days.
Measurements of Plant Weight
Twenty uniform plants were selected from each treatment (five plants per pot) to measure the fresh and dry weights of the leaves and roots after 6 and 12 days of treatment, and plants treated with different nitrate concentrations (8, 16, 24, 32, and 40 mM) were collected after 22 days. All of the leaves and roots were washed with distilled H2O and weighed individually to determine their fresh masses. All of the shoots and roots were then dried in an oven at 80 °C to obtain their dry masses.
Analysis of the Relative Transcript Levels of BcNR and BcGAD
According to our previous experiment, the structures of the NR and GAD genes of pakchoi were cloned, and their GenBank accession numbers are KP852556 and KP852557, respectively.
In this experiment, three independent new leaf samples from individual plants were collected and immediately frozen in liquid N2 to detect the relative transcript levels of BcNR and BcGAD. Gene expression was determined using the Agilent MX3000P real-time PCR system (Agilent Technologies) according to the method described by Wang et al. (2014a) with some modifications. Approximately 100 mg of tissue powder from each sample was used for total RNA extraction employing the EASY Spin Plus Plant RNA Mini kit (Aidlab). The concentration and integrity of the RNA were then routinely assessed based on the absorption ratio determined with a Thermo Scientific Nano Drop 2000/2000C ultraviolet spectrophotometer and 1% (w/v) agarose gel electrophoresis, respectively. BcNR and BcGAD gene-specific forward and reverse primers and reference gene primers were designed using Primer Design Software 5.0. The primer pairs for each gene were as follows: BcNR, forward, GGAGGGAAGAAGGTAACGAG, and reverse, AGAAACACCAGCACCAGAAC; BcGAD, forward, TTCTGCGTGTTGTCATTAGG, and reverse, CCTCTGCTCCCATCTTCCTA; and Actin, forward, GGTATGGGTCAGAAAGATGC, and reverse, CTGTGAGTAGAACTGGGTGC.
Approximately 4 μg of total RNA was used to synthesize the first strand of cDNA with the PrimeScript™ II 1st-Strand cDNA Synthesis Kit (TaKaRa) using an oligo d(T) primer, according to the manufacturer’s recommended protocol. The real-time PCR system consisted of 25 μL of the SYBR® Premix Ex Taq™ II (TaKaRa) mixture, which contained 2 μL of cDNA template (after dilution), 12.5 μL of Premix Ex Taq, 1 μL of the forward primer (10 mM), 1 μL of the reverse primer (10 mM), and 8.5 μL of RNase-free ddH2O. The cycling temperature parameters consisted of 95 °C for 1.5 min followed by 40 cycles of 95 °C for 10 s, 55 °C for 15 s, and 72 °C for 30 s and a final step of 72 °C for 90 s. The samples were maintained at 95 °C for 15 s and 60 °C for 15 s, subjected to fluorescence detection for 20 min, and then maintained at 95 °C for 15 s. Melting curve analysis was performed at 95 °C for 1 min, 55 °C for 30 s, and 95 °C for 30 s. The relative transcript levels of BcNR and BcGAD were calculated with reference gene correction and transformed into a linear relationship using the 2−△△ct method.
BcNR and BcGAD Protein Expression Assays
The BcNR and BcGAD protein expression levels were measured using the enzyme-linked immunosorbent assay (ELISA) method and commercially available plant NR and plant GAD kits according to the manufacturer’s instructions (TSZ). New leaf samples (0.5 g of each sample) were homogenized in 10 mM PBS (pH 7.4) buffer containing 1 mM EGTA and 1 mM PMSF. The homogenate was maintained in a water bath at 90–95 °C for 3 min and then immediately cooled to 10 °C. After allowing the homogenate to settle for 5 min, it was centrifuged at 3000 rpm and 4 °C for 20 min. Fifty microliters of the supernatant was pipetted into coated microtiter wells and incubated for 45 min at 37 °C. After washing four times with buffer, biotinylated anti-IgG was added. After incubated for 30 min at 37 °C, streptavidin-HRP was added, and the plates were incubated for 15 min at 37 °C. The absorbance at 450 nm was measured with a microtiter plate reader, and the NR and GAD concentrations were calculated using the standard curve.
Analysis of Nitrate Reductase (NR), Nitrite Reductase (NiR), and GAD Enzyme Activity
Approximately 0.5 g of each leaf sample was used to measure the activities of NR (EC1.6.6.1) and NiR (EC1.7.7.1) according to the methods described by Gao et al. (2011) and Takahashi et al. (2001), respectively. NR and NiR activities were defined as the production or reduction of NO2− per hour in micromole (μmol NO2−·h−1 g−1FW). GAD activity was assessed using the increase in the amount of GABA according to Wang et al. (2014a) and is presented as μmol GABA h−1 g−1FW to allow convenient comparison of the different enzymes.
Nitrate (NO3 −), Nitrite (NO2 −), and Ammonium (NH4 +) Content Assays
The contents of NO3− and NO2− were measured according to the method described by Petersen and Stoltze (1999) with minor modifications. Fresh samples of pakchoi leaves (5 g of each sample) were rinsed and weighed in a volumetric flask with a rubber plug. Approximately 2.5 mL of 10% NaOH and 40 mL of deionized distilled water were added to the flask to maintain the pH at 10. The flask was then placed in a water bath at 50 °C, and 10 mL of 0.7 M ZnSO4 was added to the solution. After filtering, the nitrite level was measured at 540 nm, and the amount of nitrate was calculated.
To determine the amount of NH4+, 0.5 g of the leaf samples was ground with pentafluorobenzoyl chloride containing 5% NaHCO3, extracted with ethyl acetate, and washed with 6% H3PO4, as described by Oliveira et al. (2013).
Determination of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) Activities
GS (EC 6.3.1.2) and NADH-GOGAT (EC1.4.7.14) were extracted from the leaf samples (0.5 g each) and assayed according to the method described by Lin and Kao (1996). GS activity was initiated after addition of the enzyme extract to a 1-mL reaction mixture containing 80 μM Tris-HCl buffer(pH 8.0), 8 μM ATP, 40 μM L-Glu, 24 μM MgSO4·7H2O, 16 μM hydroxylamine, and 0.15 mL of the supernatant followed by incubation at 30 °C for 30 min. The reaction was then stopped by the addition of 2 mL of a solution of 2.5% FeCl3·6H2O (w/v) and 5% trichloroacetic acid (w/v) in 1.5 M HCl. After centrifugation at 3000g, GS activity was determined at 540 nm and presented as the formation of γ-glutamyl hydroxamate (GHA) (μmol GHA h−1 g−1FW). For the determination of NADH-GOGAT activity, the reaction mixture contained 1.75 mL of 25 mM Tris-HCl buffer (pH 7.6), 0.4 mL of 20 mM L-Gln, 0.05 mL of 0.1 M 2-oxoglutarate, 0.1 mL of 10 mM KCl, 0.2 mL of 3 mM NADH, and 0.5 mL of the enzyme extract. The reaction was immediately initiated by adding L-Gln, and GOGAT activity was defined as the decrease in NADH at 340 nm (μmol NADH h−1 g−1FW). The extraction and determination of Fd-GOGAT (EC 1.4.7.1) from leaves were performed according to the method described by Gibon et al. (2004), and activity was defined as the increase in Glu and presented as nmol Glu h−1 g−1FW.
Analysis of Gln, Glu, and GABA Contents
Leaf samples of approximately 1.0 g were ground in 5 mL of 2% sulfosalicylic amino acid at 4 °C. The pH of the homogenate was controlled at 2.0 by the addition of 0.02 M HCl, and the samples were then centrifuged at 10,000×g and 4 °C for 15 min. The supernatant was used for determination of the Gln, Glu, and GABA contents with a Hitachi 835-50 amino acid analyzer (Gao et al. 2011).
Statistical Analysis
All experiments were conducted using at least three independent replicates of the samples. The growth under each treatment was analyzed using 20 independent plants in four pots. The means of the replicates were subjected to one-way ANOVA and Duncan’s multiple range tests with a significance level of 0.05 using SAS 8.1 software (SAS Institute, Cary, NC, USA).
Results
Growth of Pakchoi Plants
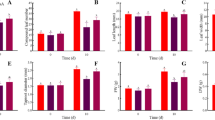
Nitrate is the most important nitrogenous fertilizer, but the administration of an appropriate amount is necessary for improving plant growth. We measured the growth of plants cultured in 8–40 mM nitrate solutions to find the optimum amount of nitrate for pakchoi. The effect of 32 mM nitrate was found to be significantly greater than the effects of the 16, 24, and 40 mM treatments, with the lowest plant growth observed in the presence of 8 mM nitrate (Fig. 1). Therefore, 32 mM nitrate was considered the closest suitable nitrogen concentration for improving pakchoi growth, and the effect of 32 mM nitrate application in nutrient solution was assessed in the subsequent HN and HN + GABA treatments experiment.
Growth of pakchoi treated with different nitrate concentrations (8, 16, 24, 32, and 40 mM) in Hoagland nutrient solution for 22 days. The individual data points represent the mean values for 20 plants from four independent replications, and the vertical bars indicate the SEs (n = 20) from 20 different seedlings. Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
To verify whether GABA acts as a nitrogen source under HN conditions, the fresh and dry weights of the leaves and roots at 6 and 12 days were measured. Compared with the control, both the HN and HN + GABA treatments significantly increased the fresh and dry weights of the pakchoi plants. Furthermore, the increase observed under the HN + GABA condition was greater than that observed under HN, and the increase at 12 days was greater than that at 6 days with HN and HN + GABA treatments (Fig. 2). After 12 days, the leaf fresh weight, leaf dry weight, root fresh weight, and root dry weight of the HN + GABA-treated plants were significantly increased by 13.65, 11.78, 10.49, and 11.11%, respectively, compared with the HN treatment. The result suggests that GABA has an effect on the growth of pakchoi as an organic nitrogen source, but the increased was marginal, only approximately 10%, indicating that GABA may play other major roles at 32 mM NO3−. Therefore, it was necessary to further study the effect of GABA on nitrate metabolism.
Fresh and dry weights of pakchoi plants exposed to high-nitrogen conditions (32 mM nitrate) and exogenous GABA (2.5 mM) for 6 and 12 days. The individual data points represent the mean values for 20 plants from four independent replications, and the vertical bars indicate the SEs (n = 20) from 20 different seedlings. Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
NO3 −and NO2 − Contents
Pakchoi is a leafy vegetable that can easily accumulate nitrates, but the content of NO3− in leaves is the combined effect result of uptake and metabolism, especially when several N sources are present simultaneously. However, at a relatively high concentration of nitrate (32 mM NO3−), it is unclear effect of GABA on nitrate content is mainly through inhibition of absorption or promotion of nitrate reduction. The results showed that the contents of NO3− and NO2− in leaves treated with control did not vary significantly during the 12 days of examined growth, where as an increasing tendency was observed under HN conditions from 3 to 12 days compared with the control (Fig. 3). However, the leaves subjected to the HN + GABA treatment consistently exhibited significant GABA-induced decreases in their NO3− and NO2− contents throughout the experimental period, indicating that the effect of GABA on promoting the reduction of nitrate was greater than its effect on inhibiting NO3− absorption. Therefore, it is important to study the role of the NR enzyme in catalyzing the conversion of NO3−, to NO2−.
Dynamic changes in endogenous nitrate and nitrite contents in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution for 0, 3, 6, 9, and 12 days. The seedlings were subjected to one of the following three treatments: control (white columns), HN (gray columns), and HN + GABA (black columns)
BcNR Gene Transcription and Protein Expression and NR Activity
The BcNR transcript and protein expression levels serve as an important indicator of NR activity. Therefore, research on the NR enzyme involves evaluations of BcNR gene and protein expression and NR activity in leaves and was conducted under sufficient nitrate supplementation with or without GABA (Fig. 4). Compared with the control, HN treatment caused significant increases in BcNR gene transcription at 0.5–6 days and BcNR protein expression at 1–6 days, and GABA application further augmented this change (Fig. 4a, b). As a result, the BcNR gene transcript and BcNR protein expression levels were significantly higher in the HN + GABA-treated leaves than those in the HN-treated leaves throughout the experimental period, and reached the peak time earlier. Meanwhile, the increase was more apparent and rapid for BcNR gene transcription than for BcNR protein expression under both HN and HN + GABA conditions; BcNR gene transcription and BcNR protein expression reached maxima at 1 and 2 days, respectively, under the HN condition and at 0.5 and 1 day, respectively under the HN + GANA condition. NR activity profiles in leaves showed similar trend with BcNR gene transcription and BcNR protein expression, that is, HN caused a large increase in NR activity, and this increase was more apparent with the addition of GABA throughout the course of the experiment (Fig. 4c). The changes in gene and protein expression and NR activity provide data support for the GABA-induced reduction of NO3− contents in pakchoi mainly through the induction of nitrate reduction.
Results of qRT-PCR analysis and ELISA of NR gene transcription and protein expression, respectively, as well as the determination of NR activity in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution over a period of 0~12 days. The seedlings were subjected to one of the following three treatments: (1) control (open squares), normal Hoagland nutrient solution containing 14 mM NO3−; (2) high-nitrogen treatment (HN, filled squares), Hoagland solution supplemented with 18 mMNO3− to obtain an NO3− concentration of 32 mM; and (3) exogenous GABA treatment (HN + GABA, filled triangles), Hoagland solution supplemented with 18 mMNO3− and 2.5 mM GABA. The individual data points represent the mean values of four independent replications, and the vertical bars indicate the SEs (n = 4) from four seedlings. Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
NiR Activity and NH4 + Contents
The NO2− in pakchoi leaves is continually reduced by NiR, and the metabolite is NH4+. The following experiments demonstrated the changes in NiR enzyme activity and NH4+ content due to HN and GABA treatment. HN treatment caused a significant increase in NiR activity at 6–12 days, and the application of GABA induced a strong increase in NiR activity from 3 to 12 days of treatment. After 6 days, NiR activity was increased by approximately 2.77-fold in HN + GABA-treated plants and by 67.17% in HN-treated plants compared with the control (Fig. 5). We speculate that the change of NiR activity was mainly affected by the increase of metabolic substrate NO2− contents under the HN condition, while higher NiR activity was the main reason for the decrease of NO2− contents under HN + GABA treatment.
As the product of nitrite reduction, the NH4+ content in control leaves was relatively low, whereas an increasing tendency was observed under HN conditions, especially under GABA treatment (Fig. 6). As a consequence, the highest NH4+ contents were found in the leaves treated with HN + GABA, followed by the leaves treated with HN, consistent with the nitrate reductive metabolism process. However, the NH4+contents in plants treated with HN + GABA peaked at 6 days and then decreased at 9–12 days, consistent with a GABA-induced change in NiR activity. The involvement of Gln and Glu in this cycle also may be the reason for the observed decrease NH4+ in the late experimental period.
Dynamic changes in endogenous ammonium contents in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution for 0, 3, 6, 9, and 12 days. The seedlings were subjected to one of the following three treatments: control (white columns), HN (gray columns), and HN + GABA (black columns)
Activities of GS and GOGAT
More than 95% of NH4+ in plants is assimilated through the GS/GOGAT cycle, which is the key pathway of inorganic nitrogen transformation. The activities of GS, NADH-GOGAT, and Fd-GOGAT in the HN-treated leaves were significantly higher than those in the control leaves, although significant changes were observed in the control-treated leaves during the 12-day experiment (Fig. 7). The most rapid increase was detected with a peak at 6 days in the presence HN + GABA conditions, and the level obtained with this treatment was significantly higher than that found in the HN and control groups throughout the experimental period. At 6 days, GS, NADH-GOGAT, and Fd-GOGAT activities in the plants treated with HN + GABA had increased by 52.00, 30.83, and 24.95%, respectively, compared with those detected in the plants treated with HN. These results indicate that GS and GOGAT play important roles in NH4+ assimilation and utilization in inorganic reduction under HN and GABA conditions.
Dynamic changes in the activities of GS, NADH-GOGAT and Fd-GOGAT in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution for 0, 3, 6, 9, and 12 days. The seedlings were subjected to one of the following three treatments: control (open squares), HN (filled squares), and HN + GABA (filled triangles)
Gln and Glu Contents
As products of the GS/GOGAT cycle, Gln is capable of assimilation and immobilization NH4+, and Glu provides a substrate for GS and ammonia for other amino acid synthesis. The Gln and Glu contents in the control leaves remained relatively low, although increases were observed during the experimental period (Fig. 8). However, the Gln and Glu contents were significantly increased in HN-treated leaves, with a peak at 6 days. HN + GABA treatment resulted in the highest Gln and Glu contents, and the resulting level was significantly higher than that obtained with HN treatment alone. However, the Gln contents in leaves were significantly lower than the Glu contents under three different conditions, which may be consistent with the rapid conversion of Gln in plants in vivo. These results for Gln and Glu contents were consistent with those of GS and GOGAT activity, which provide a support for GABA inducing the reduction and assimilation of nitrate. As the substrate of GABA synthesis, it is important to elucidate whether Glu content enhancement affects on GAD and endogenous GABA contents.
Dynamic changes in Gln and Glu contents in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution for 0, 3, 6, 9, and 12 days. The seedlings were subjected to one of the following three treatments: control (white columns), HN (gray columns), and HN + GABA (black columns)
BcGAD Gene Transcription and Protein Expression and GAD Activity
GAD is the most important enzyme that directly links the nitrate metabolism to GABA. Thus, GAD enzyme analyses involve evaluation of gene transcription, and BcNR protein expression and activity assessments in leaves to elucidate the involvement of GABA in nitrate reduction and assimilation. The control treatment did not cause obvious changes in BcGAD gene transcription, BcGAD protein expression, or GAD activity from 0.5 to 12 days, despite presenting small fluctuations (Fig. 9). However, HN treatment resulted in significant increases in BcGAD gene transcription, BcGAD protein expression, and GAD activity throughout the 12-day experiment. In response to HN, simultaneous GABA application further significantly augmented this change, which presented with a peak at 0.5, 2, and 6 days for BcGAD gene transcription, BcGAD protein expression, and GAD activity, respectively. As a result, the BcGAD gene transcription and BcGAD protein expression levels and GAD activity were all significantly higher in the HN + GABA-treated leaves than those in the HN-treated leaves throughout the experimental period. Meanwhile, the increases were more apparent and rapid for BcGAD gene transcription than those for BcGAD protein expression under both the HN and HN + GABA conditions. GAD activity is the ultimate result of gene transcription and protein expression and presents slow changes: GAD activity in the HN + GABA group was enhanced by 7.57, 12.31, and 9.08%, respectively, compared with the HN group after 3, 6, and 9 days. These results suggest that GAD plays an important role in the GABA treatment process, promoting nitrate assimilation by accelerating the conversion of Glu to GABA. Therefore, endogenous GABA content in leaves must be measured to determine whether it is closely related to GAD.
Results of qRT-PCR analysis and ELISA of GAD gene transcription and protein expression, respectively, as well as the determination of GAD activity in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution over a period of 0~12 days. The seedlings were subjected to one of the following three treatments: control (open squares), HN (filled squares), and HN + GABA (filled triangles)
Endogenous GABA Contents
Exogenous GABA can be taken up and utilized by plant roots; thus, the endogenous GABA content in leaves is regulated by GAD enzyme, but is also affected by root absorption. The endogenous GABA contents in the control leaves did not exhibit any conspicuous changes from days 3–12 (Fig. 10). HN treatment increased the endogenous GABA content, resulting in significantly higher levels compared with those observed in the control leaves over 3- to 12-day period. Furthermore, exogenous GABA treatment caused a marked increase in the endogenous GABA contents of the leaves, reaching a plateau after 6 days. Compared with the contents of the HN leaves, the endogenous GABA contents of the leaves subjected to the HN + GABA treatment for 3, 6, and 9 days were increased by 27.58, 45.58, and 24.69%, respectively; levels were significantly higher than the GAD changes induced by exogenous GABA. The result indicated that the endogenous GABA in leaves derived mainly from root absorption, followed by the metabolic synthesis catalyzed by GAD.
Dynamic changes in endogenous GABA contents in pakchoi leaves treated with 32 mM NO3− and exogenous 2.5 mM GABA in nutrient solution for 0, 3, 6, 9, and 12 days. The seedlings were subjected to one of the following three treatments: control (white columns), HN (gray columns), and HN + GABA (black columns)
Discussion
Growth Changes Resulting from NO3 − and GABA
Terrestrial plants acquire NO3− at appropriate amounts during growth, but the NO3− uptake rate depends on the plant species and the external concentration of NO3− (Nazoa et al. 2003). Our results indicated that pakchoi cultivars grew well in the presence of 32 mM NO3− solution, and this solution resulted in increased growth compared with that observed with the 8–24 mM and 40 mM NO3−-N treatments (Fig. 1), indicating that 32 mM NO3−-N was closer to the suitable nitrogen concentration for pakchoi growth. This conclusion is supported by the results reported by Chen et al. (2004), who showed that the yields of rape and Chinese cabbage were optimized using a nitrogen supply of 0.3 g kg−1 soil, whereas an obvious decrease was observed under a nitrogen supply greater than 0.45 g kg−1 soil.
Some investigations have indicated that nitrate uptake by plant root is accomplished through a set of low- and high-affinity membrane transport systems (Williams and Miller 2001). Therefore, the uptake rates of amino acids are faster than those of NO3− for most plant species when several N sources are present simultaneously (Näsholm et al. 2009). GABA might be used as the nitrogen source to improve the growth of bacteria, fungi, and Arabidopsis seedlings (Fait et al. 2008). However, GABA stimulates root growth of Arabidopsis thaliana in the presence of an NO3− concentration below 40 mM in the medium, but exerts an inhibitory effect at concentrations above 40 mM NO3− (Barbosa et al. 2010). Our results demonstrated that the growth of pakchoi cultivars was significantly improved by GABA application under a relatively high N supply (32 mM NO3−) (Fig.1). Moreover, the addition of exogenous 2.5 mM GABA to the solution stimulated the growth of pakchoi roots and shoots, demonstrating that GABA could be absorbed from the nutrient solution via the roots. This finding is consistent with the previously reported results obtained in melon cultured with GABA under hypoxic conditions (Fan et al. 2015). These results suggested that GABA might be acting as a nitrogen source to improve plant growth after root absorbing.
Nitrate Reduction Related to NO3 − and GABA
Only small amounts of the nitrate absorbed by roots are assimilated, while the remainder is transported to the leaves; thus, the leaves are the location of nitrate and nitrite reduction and assimilation (Xu et al. 2012). In mesophyll cells, nitrate is reduced to nitrite and ammonium through reactions catalyzed by NR and NiR, respectively, and then used for amino acid synthesis to improve plant growth over time (Bryan et al. 2012). During this reductive metabolic process, NR is a substrate-inducible enzyme; hence, the nitrate content and NR activity in leaves are induced by a threshold nitrate concentration in the soil and medium (Chen et al. 2004; Xu et al. 2012). In the presence of nitrate, the increase in NR gene transcription becomes more effective, and many related metabolic reactions occur before and after the catalytic process to impact the accumulation of nitrate (Sun et al. 2008). However, transformant plants with very low (1–3% of wiled-type levels) NR activity accumulate high levels of nitrate (Scheible et al. 1997). In the experiments described herein, the NO3−level in pakchoi leaves continued to increase over time, and this increase was accompanied by the accumulation of higher concentrations of NO2− and NH4+, although the activities of NR and NiR were increased significantly in the leaves treated with a higher concentration of nitrate in solution (Figs. 3, 4, 5, and 6). Additionally, similar obviously increasing trends were found for the BcNR transcript levels, BcNR protein expression, and NR activity in HN-treated leaves. However, the significant change in BcNR transcription levels was more apparent and rapid than that of protein expression, and increased NR activity was significantly prolonged in comparison, specifically the time required to reach the maximum level (Fig. 4). These findings indicate that the pivotal roles of the nitrate supply in regulating BcNR expression at the transcriptional and protein levels and in NR activity cannot be denied. These significant effects of nitrate on BcNR gene and protein expression and enzyme activity might be mainly attributed to a substrate-inducing effect, although this effect was not sufficient to completely consume the excess nitrate in pakchoi leaves treated with HN solution. We conclude that NO3− accumulation in leaves mainly occurs when nitrate uptake exceeds nitrate reduction.
GABA can be used as organic nitrogen or as a putative long-distance inter-organ signal molecule in plants (Beuve et al. 2004). An interaction between GABA and nitrate in the growth solution is supported by the observed changes in the NO3− contents of tissues treated with exogenous GABA and the hypothetical role of GABA in the regulation of nitrate assimilation (Barbosa et al. 2010). The application of amino acids can inhibit the influx and uptake of NO3−, which are spatially and developmentally controlled at the transcriptional level (Nazoa et al. 2003). However, 1 mM GABA treatment induced a significant increase of Nrt2 mRNA expression of Brassica napus L., but had less effect on nitrate influx (Beuve et al. 2004). In the present study, pakchoi leaves exhibited the highest BcNR gene and protein expression levels and NR and NiR activities under HN + GABA conditions. These results further demonstrate that GABA decreases nitrate and nitrite accumulation by simultaneously positively regulating BcNR gene and protein expression and the activities of NR and NiR, providing additional evidence that GABA is closely related to nitrate reduction metabolism. Furthermore, the nitrate and nitrite contents were decreased to levels lower than those detected in the control by the application of exogenous GABA in HN solution. This result might be explained by the simultaneous effect of GABA application on the induction of nitrate reduction and nitrate uptake, but the influence of GABA on promoting nitrate reduction was far greater than its influence on inhibiting NO3− absorption due to the existence of competitive sites for GABA and NO3− in pakchoi cells in the presence of higher nitrate nutrient levels. Nitrate assimilation related to NO3− and GABA.
Excessive accumulation of ammonium is harmful to plant growth but can be reduced or avoided by the rapid conversion of ammonium to amino acids, which is the most important step in nitrogen assimilation (Britto and Kronzucker 2002). Ammonium assimilation to produce Glu in plants is performed by the Gln/Glu synthesis pathway, which is catalyzed by GS/GOGAT (Lea and Miflin 2003). GS has a high affinity for ammonium and direct effect on the absorption and utilization of nitrogen (Kusano, et al. 2011). GOGAT also presents great potential for improving nitrogen use efficiency including Fd-GOGAT and NADH-GOGAT (Lu et al. 2011). The activities of GS and GOGAT increase gradually as the NH4+ concentration increases in rice roots (Ma et al. 2016). Concomitantly, the activities of GS and GOGAT show similar variation in relation to NO3−, demonstrating that low NO3− concentrations (16–112 mM) promote activity, whereas high NO3− concentrations (160 mM) inhibit activity (Han et al. 2015). The present study showed that pakchoi leaves exhibited significant increases in the activities of GS and GOGAT under HN conditions during the 12-day experiment (Fig. 7), and this effect was due, in large part, to a higher level of ammonium, promoting nitrogen assimilation. However, there was no significant difference in the NH4+ levels between the control and HN plants after 3 days of treatment (Fig. 6), whereas different GS and GOGAT activities were obtained, an effect that might be explained by the obvious rapid changes in Gln and Glu, as the reaction substrate, beginning 3 days into the experiment (Fig. 8). Furthermore, due to the dual effects of higher levels of NH4+, as well as Glu and Gln, the activities of GS and GOGAT in pakchoi leaves were significantly improved even further by GABA application, which might provide support for roles for GS and GOGAT in GABA inducing the reduction and assimilation of nitrate under HN conditions.
Endogenous GABA Resulting from NO3 − and GABA Treatment
The synthesis of GABA is the first step in the GABA shunt, which is catalyzed by GAD and utilizes Glu as the substrate. The endogenous GABA was closely related to the GABA and nitrate application in soil or solution. Previous studies have indicated that GABA application exerts an increasing effect on the endogenous GABA concentration, as well as GAD transcription and activity (Wang et al. 2014a). In addition, the influence of GABA on GAD expression is relevant, as demonstrated by the high correlation between GABA levels and GAD mRNA abundance detected in rice, soybean, and tomato plants (Hyun et al. 2013; Takayama et al. 2015). The observations on Prunus rootstock refer to GAD2 and GAD4 in particular because these genes exhibit transcriptional levels that are modulated by exogenous GABA (Salvatierra et al. 2016). Furthermore, the GABA contents and GAD activity exhibit a positive relationship under high NO3− conditions (Hu et al. 2015). The results of our study showed markedly higher levels of endogenous GABA and BcGAD gene transcription and protein expression and GAD activity in the 12-day HN-treated pakchoi leaves. These findings indicated that the addition of NO3− to the solution induced changes in BcGAD at both the transcriptional and post-translational levels, though the change in GAD activity was prolonged compared with the change in BcGAD gene transcription and protein expression (Fig. 9), representing the main reason for the increase in the endogenous GABA concentration in HN-treated leaves (Fig. 10). Furthermore, exogenous GABA significantly enhanced BcGAD gene transcription and protein expression and enzyme activity, but the increase of endogenous GABA in leaves was significantly greater than the GAD changes under HN + GABA conditions. These results suggest that endogenous GABA in leaves mainly comes from root absorption, with only a small proportion resulting from the metabolic synthesis catalyzed by GAD. In combination with the decreased NO3− concentrations in HN-GABA-treated leaves, we propose that exogenous GABA can be taken up by pakchoi roots and can be used as a nitrogen source to improve plant growth. In addition, the role of GABA in regulating nitrate levels was mainly attributed to its acceleration of nitrate reduction and assimilation rather than the inhibition of nitrate absorption.
Conclusions
In this study, exogenously applied GABA (2.5 mM) can be taken up by pakchoi roots under 32 mM nitrate solution condition, and GABA can be used as a nitrogen source to improve the plant growth. Furthermore, this treatment decreased the NO3− contents in pakchoi leaves by promoting nitrogen reduction and assimilation, as shown through assessments of molecular and metabolic changes. During the metabolic process, the gene and protein expression levels of BcNR and BcGAD are regulated under HN with or without GABA treatment. We propose a model in which a potential positive effect of promoting nitrate metabolism in leaves might be activated by the effects of high levels of NO3−combined with exogenous GABA application. Our results reveal that exogenous GABA plays an important role in reducing nitrate in leaves, resulting in the harvest of leafy vegetables containing higher endogenous GABA contents. Considering the beneficial influence of GABA application on roots exposed to high NO3− conditions, it would be interesting to evaluate the effects of the application of GABA in nutrient solution on improving the quality of leafy vegetables (Fig. 11).
References
Barbosa JM, Locy RD, Barger TW, Singh NK, Cherry JH (2000) GABA increases the rate of nitrate uptake and utilization in Arabidopsis roots. Nato Science 83:53–63
Barbosa JM, Singh NK, Cherry JH, Locy RD (2010) Nitrate uptake and utilization is modulated by exogenous γ-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol Biochem 48:443–450
Beuve N, Rispail N, Laine P, Cliquet JB, Ourry A, Le deunff E (2004) Putative role of gamma-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ 27:1035–1046
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Bryan NS, Alexander DD, Coughlin JR, Milkowski AL, Boffetta P (2012) Ingested nitrate and nitrite and stomach cancer risk: an updated review. Food Chem Toxicol l50:3646–3665
Cavaiuolo M, Ferrante A (2014) Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 6:1519–1538
Chen B, Wang Z, Li S, Wang G, Song H, Wang X (2004) Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Sci 167:635–643
Colonna E, Rouphael Y, Barbieri G, De Pascale S (2016) Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem 199:702–710
Fait A, Fromm H, Walter D, Galili G, Fernie AR (2008) Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci 13:14–19
Fan L, Wu X, Tian Z, Jia K, Pan Y, Li J, Gao H (2015) Comparative proteomic analysis of gamma-aminobutyric acid responses in hypoxia-treated and untreated melon roots. Phytochemistry 116:28–37
Gao H, Jia Y, Guo S, Lv G, Wang T, Juan L (2011) Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J Plant Physiol 168:1217–1225
Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JH, Palacios N, Cross J, Selbig J, Stitt M (2004) Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16:3304–3325
Han YR, Wang XF, Yang FJ, Wei M, Shi QH, Li QM, Cui XM (2015) Effects of NO3 − stress on photosynthetic characteristics and nitrogen metabolism of strawberry seedlings. Chin J Appl Ecol 26:2314–2320
Hellmann H, Funck D, Rentsch D, Frommer WB (2000) Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol 123:779–789
Hu X, Xu Z, Xu W, Li J, Zhao N, Zhou Y (2015) Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol Biochem 92:1–10
Hyun TK, Eom SH, Jeun YC, Han SH, Kim J (2013) Identification of glutamate decarboxylases as a γ-aminobutyric acid (GABA) biosynthetic enzyme in soybean. Ind Crop Prod 49:864–870
Kusano M, Tabuchi M, Fukushima A, Funayama K, Diaz C, Kobayashi M, Hayashi N, Tsuchiya YN, Takahashi H, Kamata A, Yamaya T, Saito K (2011) Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1; 1 in coordinating metabolic balance in rice. Plant J 66:456–466
Lea PJ, Miflin BJ (2003) Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol Biochem 41:555–564
Li JR, Tian Z, Wu XL, Zhang Y, Gong BB, Gao HB (2017) Cloning of GAD gene in Pakchoi(Brassica campestris ssp. chinensis) and induced expression analysis treated with exogenous GABA under higer nitrogen level. J Agric Biotechnol 25:1217–1227
Li JR, Tian Z, Wu XL, Gong BB, Gao HB (2016) Regulation of γ-aminobutyric acid on growth and nitrate metabolism of Pak-choi treated with high nitrogen application. Acta Horticulturae Sinica 43:2182–2192
Lin CC, Kao CH (1996) Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul 18:233–238
Liu TT, Tseng YW, Yang TS (2016) Functionalities of conjugated compounds of γ-aminobutyric acid with salicylaldehyde or cinnamaldehyde. Food Chem 190:1102–1108
Liu X, Wang L, Li Z, Huang D (2014) Nitrate/Gly ratios in nutrition influenced the growth and amino acid composition in spinach (Spinaciaoleracea L.). J Plant Nutr 37:765–776
Lu Y, Luo F, Yang M, Li X, Lian X (2011) Suppression of glutamate synthase genes significantly affects carbon and nitrogen metabolism in rice (Oryza sativa L.). Sci China Life Sci 54:651–663
Luo JK, Sun SB, Jia LJ, Chen W, Shen QR (2006) The mechanism of nitrate accumulation in Pakchoi [Brassica campestris L. ssp. Chinensis (L.)]. Plant Soil 282:291–300
Ma X, Zhu C, Yang N, Gan L, Xia K (2016) γ-Aminobutyric acid addition alleviates ammonium toxicity by limiting ammonium accumulation in rice (Oryza sativa) seedlings. Physiol Plant 158:389–401
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157
Muller B, Touraine B (1992) Inhibition of NO3 − uptake by various phloem-translocated amino acids in soybean seedlings. J Exp Bot 43:617–623
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, Tillard P, Zhuo D, Glass AD, Touraine B (2003) Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: responses to nitrate, amino acids and developmental stage. Plant Mol Biol 52:689–703
Oliveira HC, Freschi L, Sodek L (2013) Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiol Biochem 66:141–149
Persson J, Gardeström P, Näsholm T (2006) Uptake, metabolism and distribution of organic and inorganic nitrogen sources by Pinus sylvestris. J Exp Bot 57:2651–2659
Petersen A, Stoltze S (1999) Nitrate and nitrite in vegetables on the Danish market: content and intake. Food Addit Contam 16:291–299
Salvatierra A, Pimentel P, Almada R, Hinrichsen P (2016) Exogenous GABA application transiently improves the tolerance to root hypoxia on a sensitive genotype of Prunus rootstock. Environ Exp Bot 125:52–66
Scheible WR, Lauerer M, Schulze ED, Caboche M, Stitt M (1997) Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J 11:671–691
Sun F, Hou X, Li Y, Yang X (2008) Molecular cloning and characterization of nitrate reductase gene from non-heading Chinese cabbage. Sci Hortic 119:1–10
Takahashi M, Sasaki Y, Ida S, Morikawa H (2001) Nitrite reductase gene enrichment improves assimilation of NO2 in Arabidopsis. Plant Physiol 126:731–741
Takayama M, Koike S, Kusano M, Matsukura C, Saito K, Ariizumi T, Ezura H (2015) Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating γ-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol 56:1533–1545
Thornton B, Robinson D (2005) Uptake and assimilation of nitrogen from solutions containing multiple N sources. Plant Cell Environ 28:813–821
Wang C, Fan L, Gao H, Wu X, Li J, Lv G, Gong B (2014a) Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol Biochem 82:17–26
Wang H, Wu L, Wang M, Zhu Y, Tao Q, Zhang F (2007) Effects of amino acids replacing nitrate on growth, nitrate accumulation, and macroelement concentrations in Pakchoi (Brassica chinensis L.). Pedosphere 17:595–600
Wang HF, Tsai YS, Lin ML, Ou AS (2006) Comparison of bioactive components in GABA tea and green tea produced in Taiwan. Food Chem 96:648–653
Wang X, Yu W, Zhou Q, Han R, Huang D (2014b) Metabolic response of pakchoi leaves to amino acid nitrogen. J Integr Agric 13:778–788
Williams L, Miller A (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol 52:659–688
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Ann Rev Plant Biol 63:153–182
Acknowledgements
This research was performed by the Collaborative Innovation Center of vegetable Industry in Hebei, which is financially supported by the Nature Science Foundation of Hebei (No. C2014204074), the Key Program of Science and Technology of the Education of Department of Hebei (No. ZH2012048), and the Scientific Research Foundation for Returned Overseas Scholars of Hebei (No. 130601224).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Tian, Z., Wu, X. et al. Gamma-Aminobutyric Acid (GABA) Modulates Nitrate Concentrations and Metabolism in the Leaves of Pakchoi (Brassica campestris ssp. chinensis Makino) Treated with a Nitrogen-Rich Solution. Plant Mol Biol Rep 36, 530–542 (2018). https://doi.org/10.1007/s11105-018-1092-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-018-1092-0