Abstract
Arsenic (As) contamination of rice grains affects millions of people worldwide. In this study, we found that sulfur application (20As+120S) decreased As concentration in rice grains by 44 % compared to grains without sulfur application (20As+0S). Importantly, sulfur application decreased arsenate [As(V)] and arsenite [As(III)] concentration in rice grains significantly, while there was no significant effect on dimethylarsenate (DMA) concentration. To elucidate the molecular basis of As accumulation in rice grains, we performed Illumina sequencing to acquire the differentially expressed genes induced by arsenate and sulfur treatments. By contrast with the control, the expression of 1,000 genes was found to be changed significantly, with 46 genes up-regulated and 954 genes down-regulated in grains grown in arsenate-contaminated soil (20As+0S). Between samples of control and arsenate together with sulfur treatment (20As+120S), 1,169 genes expressed significantly differently, with 16 genes up-regulated and 1,153 genes down-regulated. Sulfur application significantly changed the expression of genes involved in As metabolism in rice grains, significantly down-regulated phosphate transporter gene OsPT23 and aquaporin gene OsTIP4;2, while ABC transporter genes (OsABCG5, OsABCI7_2 and OsABC6) and phytochelatin synthase genes (OsPCS1, OsPCS3 and OsPCS13) were up-regulated. These results provide an insight into the molecular basis of how sulfur assimilation regulates As accumulation in rice grains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inorganic arsenic (As) is a class 1 carcinogenic metalloid, and accumulation of As in crop plants can pose a serious threat to human health (Meharg and Hartley-Whitaker 2002; Zhu et al. 2008). Because rice is highly efficient in accumulating As (Su et al. 2008; Williams et al. 2007), it is urgent to reveal the mechanisms of As accumulation in rice grains.
Arsenic uptake by rice roots is performed mainly by phosphate transporters and silicon transporters (Wang et al. 2002; Ma et al. 2008). To detoxify As after uptake, plants have developed several strategies. For example, efflux of arsenite [As(III)] to the external growth medium by the roots; this strategy has been reported for many plants, such as tomato (Xu et al. 2007) and rice (Wang et al. 2008; Duan et al. 2012). Chelation of As(III) by thiol (SH)-containing compounds is another important As detoxification mechanism used, e.g., by Hydrilla verticillata and Helianthus annuus (Raab et al. 2005; Srivastava et al. 2007). The As(III)-thiolate complex can be transported into vacuoles by an ABC transporter (Raab et al. 2004; Bleeker et al. 2006). One group of principal chelators in plants is the phytochelatins (PCs), which are synthesized from reduced glutathione (GSH) by the enzyme PC synthase under As stress (Clemens et al. 1999; Heiss et al. 2003).
Sulfur (S) is a key component of thiols. Sulfur status is critical for plant tolerance to As, and also for As distribution among plant tissues (Zhang et al. 2011; Duan et al. 2013; Hernández et al. 2015). Sulfur deficiency decreased PC concentration in plant roots, and resulted in increased As translocation from root to shoot (Zhang et al. 2011; Duan et al. 2013). In rice endosperm and bran, 55.6 % and 52.9 % of As was in the form of an As(III)-thiolate complex, respectively (Lombi et al. 2009). Several studies have found that gene expression in the sulfur assimilation pathway is altered by As stress in plants (Norton et al. 2008; Chakrabarty et al. 2009; Rai et al. 2011; Yu et al. 2012; El-Zohri et al. 2015). Conversely, S deficiency caused decreased reduction of As(V) to As(III) in rice roots (Zhang et al. 2011). Application of the thiol form (thiourea) up-regulated sulfate transporters (Sultr1;1 and Sultr1;2) and increased the level of thiolic metabolites under arsenate tolerance in rice (Srivastava et al. 2014). To date, the molecular mechanism of sulfur involved in thiol synthesis and As remobilization is still unclear. Thus, we suppose that S application regulates expression of genes controlling As translocation and metabolism. By making use of the Illumina sequencing approach, we specifically investigated the effects of S on gene expression involved in As transport and metabolism in rice grains. A detailed knowledge of transcriptional changes in As transport and metabolism in rice grains provides indications that will help develop possible approaches to reduce As accumulation in rice grains.
Materials and Methods
Plant Growth and Treatments
The rice cultivar TN1 (Oryza sativa L.) was used in this study. A pot trial was carried out at the Scientific and Educational Farm of Henan Agricultural University (113°66′ E, 34°75′ N), Zhengzhou in central China. The background concentration of As in the soil was 2.78 mg kg−1, and the soil pH was 7.2. To prepare test soils with As, the soil was contaminated artificially with 20 mg kg−1 As (Na2HAsO4·12H2O, 20As+0S). Sulfur (S) treatments were applied by adding 120 mg kg−1 (Na2S2O3·5H2O) to the As-contaminated soil (20As+120S).
Rice seeds were sown in a nursery plate and grown for 25 days. Pots without a drainage hole at the bottom were filled with 3.0 kg soil. Sulfur was mixed thoroughly with soil containing base fertilizers, and As was added in liquid form. The base fertilizers applied to each pot contained 0.40 g urea, 1.32 g monopotassium phosphate and 0.70 g potassium chloride. After a 2-week soil equilibration, the seedlings were transferred to the pots. Four replicates with one plant per pot were set for each treatment and the pots were placed randomly in the greenhouse. At 20 days after anthesis, rice grains were harvested, frozen immediately in liquid nitrogen and then stored at −80 °C.
RNA Extraction and Digital Gene Expression Library Sequencing/Analysis
Total RNA was extracted from rice grains of the three treatments: 0 mg kg−1 As+0 mg kg−1 S (0As+0S, control), 20 mg kg−1 As+0 mg kg−1 S (20As+0S, As) and 20 mg kg−1 As +120 mg kg−1 S (20As+120S, As-S) using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). A minimum of 6 μg total RNA was used for cDNA library sequencing on the Illumina sequencing platform at Beijing Genomics Institute (BGI), according to the manufacturer’s protocols as follows: after extracting total RNA, Oligo(dT) magnetic beads were used for adsorption to purify mRNA, and an oligo(dT) primer was then used to synthesize first and second-strand cDNA. The bead-bound cDNA was subsequently digested with the restriction enzyme NlaII. Fragments other than the 3′ cDNA fragments connected to oligo(dT) beads were washed away and the Illumina adaptor 1 was ligated to the sticky 5′-end of the digested bead-bound cDNA fragments. After 15 cycles of linear PCR amplification, 105 bp fragments were purified by 6 % TBE PAGE gel electrophoresis. After denaturation, the single-chain molecules were fixed onto an Illumina Sequencing Chip (flowcell, http://www.illumina.com/). Each tunnel will generate millions of raw reads with a sequencing length of 49 bp. Image analysis, sequencing quality evaluation, and a data production summary were conducted as described by Liu et al. (2012). Adaptor sequences, low quality tags (tags with unknown nucleotides N), empty reads and tags that were too long or too short, and tags with only one copy were removed to get clean tags. For annotation, all tags were mapped to the reference sequences of the Rice Database (http://rice.plantbiology.msu.edu). Gene expression levels within a given sample were normalized as transcripts per million clean tags (TPM), highlighting the number of transcript copies in every 1 million clean tags.
Quantitative RT-PCR
A real-time quantitative PCR (qRT-PCR) reaction was conducted using an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Primer sequences for each selected gene are listed in Table S1. PCR amplifications were performed according to the manufacturer’s specifications (SYBR® Green qRT-PCR Master Mix, Toyobo, Osaka, Japan). Reaction were set up by mixing 5 μL 1:20 diluted template cDNA, 10 μL 2× SYBR green reaction mix, 0.5 μL each of the forward and reverse primers, and 4 μL diethylpyrocarbonate (DEPC)-treated dH2O. The amplification program was as follows: 95 °C for 10 min; 15 s at 95 °C and 1 min at 60 °C for 40 cycles followed by 95 °C for 1 min,60 °C for 1 min. β-Actin was used as a control gene. The comparative Ct method was used for data analysis. All experiments were repeated at least three times.
Analysis of Arsenic Species in Rice Grains
Oven-dried grain materials were extracted with 7 mL 1 % nitric acid in a microwave-accelerated reaction system (CEM Microwave Technology, Matthews, NC). The extracts were centrifuged and filtered through a 0.45 μm membrane filter. As species in the extraction solution were determined by high-performance liquid chromatography inductively coupled to plasma mass spectrometry (HPLC-ICP-MS) (7500a, Agilent Technologies, Santa Clara, CA) (Zhu et al. 2008). A reagent blank and standard reference plant material (GBW080684 rice flour, from the National Research Center for Standard Materials in China) were included to verify the precision of analytical procedures. The As recovery of the standard reference material of rice flour ranged from 92.31 % to 109.10 %.
Analysis of Total As in Rice Grains
Oven-dried grain materials were digested in nitric acid, and then measured by an atomic fluorescence spectrometry (AFS-2202E, Beijing Haiguang Analytical Instrument Co., Beijing, China). A reagent blank and standard reference plant material (GBW07605, from the National Research Center for Standard Materials in China) were included to verify the precision of analytical procedures.
Results
Arsenic Speciation and Concentration in Rice Grains
Concentrations of three As species in rice grains are shown in Fig. 1. As(III), As(V) and dimethylarsenate (DMA) were detected in all samples, while no monomethylarsenate (MMA) was detected in the grains. Among different As and S treatments, there was no significant difference in DMA concentration. Arsenate and As(III) concentrations in 20As+0S-treated rice grains were significantly higher than in the control (0S+0As) and sulfur treatment (20As+120S). Regarding total As concentration, 20As+0S treatment significantly increased As concentration in rice grains, and sulfur application significantly reduced As concentration in rice grains (P < 0.05). By comparing As concentration in grains treated with 20As+0S and 20As+120S, it was observed that sulfur application decreased As concentration in rice grains by 44 %. These results demonstrated that sulfur application significantly reduced inorganic As concentration in rice grains, while not significantly affecting organic As concentration.
Analysis of Digital Gene Expression Libraries
To elucidate the molecular mechanisms of As transport and metabolism in rice grains, we performed high throughput Tag-seq analysis of rice grain libraries treated with As and/or S. After filtering low quality tags from the raw data, the total number of clean tags per library varied from 3.51 million to 3.57 million. The numbers of clean tags that could be mapped to the reference unigene were 2.67, 2.79 and 2.82 million, with the corresponding percentage in all clean tags ranging from 78.42 % to 82.79 %. Among the distinct clean tags, approximately 3 % had copy numbers higher than 100 counts, approximately 37 % of the clean tags detected had 6–100 copies, and about 60 % of the distinct clean tags had 2–5 copies. The control library had the highest number of both distinct tags and distinct clean tags; the 20As+120S library had the lowest number of both distinct tags and distinct clean tags. These data suggested that more genes were detected in the control library than in the 20As and 20As+120S libraries, and that, in all three libraries, most transcripts were expressed at low levels.
Arsenic and Sulfur Cause Changes in Global Gene Expression in Rice Grains
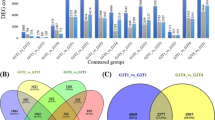
The expression of 18,832 genes was detected in all samples, and 17,533, 16,693 and 16,092 genes were detected in the control, 20As+0S and 20As+120S, respectively, indicating that the number of expressed genes was comparable among the three libraries. The number of differentially expressed genes (DEGs) in As and S treatments was analyzed (Fig. 2). Comparing control and 20As+0S samples, 1,000 genes showed altered expression, with 46 genes up-regulated and 954 genes down-regulated. Between control samples and 20As+120S, 1,169 genes showed differential expression, with 16 genes up-regulated and 1,153 genes down-regulated (Fig. 2a). Comparing with the control, 7 genes were significantly up-regulated in both 20As+0S and 20As+120S treatments, and 430 genes were significantly down-regulated at least 2-fold in both 20As+0S and 20As+120S treatments (Fig. 2b). Between samples of 20As+0S and 20As+120S, 165 genes were found to be significantly altered in expression, with 51 genes up-regulated and 113 genes down-regulated. These results indicated that 20As+0S induced a greater number of down-regulated genes in comparison to 20As+120S treatment. The expression of DEGs in each of the As and S treatments was analyzed by Pearson correlation (Figs. 3, 4, 5, 6 and 7).
a Number of differentially expressed genes (DEGs) in rice grains treated with S and/or As. b Venn diagram of the number of DEGs in rice grains treated with S and/or As. The number of genes up-regulated (bold) and down-regulated (italic) compared between treatments: control ©), 20As+0S (As) and 20As+120S (As-S), using threshold values false discovery rate (FDR) ≤0.001 and |log2ratio| ≥ 1 for controlling FDR
Expression profile of As transporter genes in rice grains treated with S and/or As. a Expression profile of ten phosphate transporters. b The expression profile of eight aquaporins. c The expression profile of five citrate transporters. The genes were selected by threshold values transcripts per million clean tags (TPM) ≥ 1 and | ≥ 1.0| fold change in at least one comparison between control ©), 20As+0S (As) and 20As+120S (As-S) treatments. The fold changes in gene expression were calculated as the log2ratio of TPM in two treatments: blue log2ratio of −2 (down-regulation), red log2ratio of 2 (up-regulation)
Expression profile of sulfate transporter genes in rice grains treated with S and/or As. The genes were selected by threshold values TPM ≥ 1 and | ≥ 1.0| fold change in at least one comparison between control ©), 20As+0S (As) and 20As+120S (As-S) treatments. The fold changes in gene expression were calculated as the log2ratio of TPM in two treatments: blue log2ratio of −2 (down-regulation), red log2ratio of 2 (up-regulation)
Expression profile of As metabolism genes in rice grains treated with S and/or As. The columns represent the contrasts between treatments: control ©), 20As (As) and 20As+120S (As-S). Each gene in the three treatments is represented by a colored line indicating the mean relative transcript level: blue log2ratio of −2 (down-regulation), red log2ratio of 2 (up-regulation). a Schematic illustration of As metabolism pathway. b Expression profile of 31 DEGs associated with As metabolism in rice grains, including thee arsenic reduction (RS) genes, one γ-glutamylcysteine synthetase (γ–ECS) gene, three glutathione synthetase (GS) genes, seven phytochelatin synthase (PCS) genes, two ATPS (ATP sulfurylase) genes, two sulfite reductase (SiR) genes, nine O-acetylserine (thiol) lyase (OAS-TL) genes and four serine acetyltransferase (SAT) genes. c Expression profile of four PCSs. The genes were selected by threshold values TPM ≥ 1 and | ≥ 1.0| fold change in at least one comparison between control ©), 20As+0S (As) and 20As+120S (As-S) treatments
Expression profile of ABC transporter genes in rice grains treated with S and/or As. The genes were selected by threshold values TPM ≥ 1 and | ≥ 1.0| fold change in at least one comparison between control ©), 20As+0S (As) and 20As+120S (As-S) treatments. The fold changes in gene expression were calculated as the log2ratio of TPM in two treatments: blue log2ratio of −2 (down-regulation), red log2ratio of 2 (up-regulation)
Expression profile of glutathione S-transferase (GST) genes in rice grains treated with S and/or As. The genes were selected by threshold values TPM ≥ 1 and | ≥ 1.0| fold change in at least one comparison between control ©), 20As+0S (As) and 20As+120S (As-S) treatments. The fold changes in gene expression were calculated as the log2ratio of TPM in two treatments: blue log2ratio of −2 (down-regulation), red log2ratio of 2 (up-regulation)
As Transporter Genes
We analyzed the global expression profiles of genes encoding inorganic As transporters (Fig. 3), specifically phosphate transporter (PT) (Liu et al. 2011) and aquaporin. A total of ten phosphate transporters [OsPT1 (LOC_Os03g05620.1), OsPT14 (LOC_Os02g38020.1), OsPT15 (LOC_Os02g52860.1), OsPT16 (LOC_Os03g15690.2), OsPT17 (LOC_Os04g37600.1), OsPT20 (LOC_Os09g38100.1), OsPT21 (LOC_Os01g17240.1), OsPT23 (LOC_Os05g37820.1), OsPT24 (LOC_Os09g39680.1) and OsPT26 (LOC_Os12g07970.1)] were significantly regulated in As and S treatments (Fig. 3a). Among these ten phosphate transporter DEGs, three genes (OsPT1, OsPT15 and OsPT26) were down-regulated ≥1-fold (TPM ≥1) by As addition, while OsPT20 was up-regulated by about 6.46-fold and 7.20-fold in the 20As+0S and 20As+120S treatments, respectively. Expression of these transporters in the 20As+0S and 20As+120S treatments was compared to investigate the effects of S on phosphate transporters. OsPT1 has been found to be expressed abundantly and constitutively in rice tissue, which regulates inorganic phosphate uptake and translocation under phosphate starvation (Sun et al. 2012). Our results showed that most other P transporters were down-regulated, except OsPT1, which was up-regulated by about 6.46-fold by S application, which probably increased the demand for P in response to the excessive S application to rice.
A total of eight aquaporin genes were changed significantly in As and S treatments. In comparison to the control, six aquaporin genes ]OsNIP1;1 (LOC_Os02g13870.1), OsNIP3;1 (LOC_Os10g36924.1), OsPIP2;2 (LOC_Os02g41860.2), OsPIP2;6 (LOC_Os04g16450.1), OsPIP2;7 (LOC_Os09g36930.1) and OsTIP1;1 (LOC_Os03g05290.1)] were observed to be down-regulated in the 20As+0S treatment, and six genes [OsNIP1;1, OsNIP2;2/OsLsi6 (LOC_Os06g12310.1), OsNIP3;1, OsPIP2;6, OsPIP2;7 and OsTIP4;2 (LOC_Os01g13130.1)] were down-regulated in the 20As+120S treatment (Fig. 3b). However, two genes, OsPIP2;2 and OsPIP2;7, were significantly up-regulated in the 20As+120S treatment, compared with the 20As+0S treatment.
A homolog of plant citrate transporters, OsLsi2 is reported to be involved in arsenite translocation (Yu et al. 2012; Ma et al. 2008). Five DEGs encoding citrate transporter (LOC_Os02g57620.1, LOC_Os09g02214.3, LOC_Os09g29430.1, LOC_Os09g31130.1 and LOC_Os10g31040.3) were regulated by As and S treatments. In contrast to control samples, LOC_Os10g31040.3 was up-regulated in the 20As+0S and 20As+120S treatments, and LOC_Os02g57620.1, LOC_Os09g29430.1 and LOC_Os09g31130.1 were down-regulated in the 20As+0S treatment. Compared with 20As+0S treatment, LOC_Os10g31040.3 was down-regulated in the 20As+120S treatment. However, no significant expression of OsLsi2 (LOC_Os03g01700) were detected in our grain samples.
Sulfate Transporter Genes
The hierarchical clustering showed that a total of four sulfate transporter genes [OsSultr1;3 (LOC_Os08g31410.7), OsSultr2;1 (LOC_Os03g09940.1), OsSultr3;2 (LOC_Os03g06520.2), and OsSultr5;1 (LOC_Os01g45830.1)] were down-regulated. Comparing control and 20As+0S treatments, one sulfate transporter gene [OsSultr3;5 (LOC_Os01g41050.1)] was observed to be significantly up-regulated (Fig. 4). Compared with the microarray data for arsenate stress (Kumar et al. 2011), the expression of OsSultr3;5, OsSultr2;1 and OsSultr3;2 in rice seedlings was different in the present study, suggesting that expression of these three sulfate transporters with respect to As stress is regulated differentially in different rice tissues. In contrast to the control samples, two genes encoding sulfate transporters (OsSultr3;5 and OsSultr5;1) were up-regulated and three genes (OsSultr1;3, OsSultr2;1 and OsSultr3;2) were down-regulated in the 20As+120S treatment. Compared with the 20As+0S treatment, OsSultr5;1 was elevated 7-fold in the 20As+120S treatment.
Genes Involved in Arsenic and Sulfur Metabolism
The global expression profiles of genes related to As and sulfate metabolism are shown in Fig. 5. None of three arsenic reduction (RS) genes (LOC_Os10g39860.1, LOC_Os03g01770.1 and LOC_Os02g49680.1), two ATPS (ATP sulfurylase) genes (LOC_Os03g53230.1 and LOC_Os04g02050.2) and one γ-glutamylcysteine synthetase (γ–ECS) gene (LOC_Os05g03820.1) were differently expressed. One (LOC_Os11g42350.1) of three glutathione synthetase (GS) genes (LOC_Os12g34380.1, LOC_Os12g16200.1 and LOC_Os11g42350.1) were both down-regulated in As and As-S compared to the control. Two sulfite reductase (SiR) genes (LOC_Os01g25484.1 and LOC_Os05g42350.1) were down-regulated in the As-S treatment compared to the control. Five of nine O-acetylserine (thiol) lyase (OAS-TL) genes (LOC_Os01g59920.1, LOC_Os01g74650.3, LOC_Os02g12900.1, LOC_Os03g11660.1, LOC_Os03g53650.3, LOC_Os04g08350.2, LOC_Os06g05700.1, LOC_Os06g36840.1 and LOC_Os12g42980.1) were down-regulated in the As treatment compared to control, and LOC_Os03g11660.1 was up-regulated in the As-S treatment compared to the As treatment. There were no differences in expression among four serine acetyltransferase (SAT) genes (LOC_Os01g52260.1, LOC_Os03g04140.2, LOC_Os03g10050.1 and LOC_Os05g45710.1) between the treatments.
Four of seven phytochelatin synthase (PCS) genes [OsPCS1 (LOC_Os05g34290.1), OsPCS2 (LOC_Os10g35460.1), OsPCS3(LOC_Os03g30250.1), OsPCS5 (LOC_Os06g01260.1), OsPCS7 (LOC_Os05g32110.4), OsPCS8 (LOC_Os07g41310.1) and OsPCS13 (LOC_Os03g18910.1)] were significantly regulated by As and S treatments. In comparison to controls, three of four PCS DEGs (OsPCS1, OsPCS3 and OsPCS13) were down-regulated in the 20As+0S treatment, and two (OsPCS3 and OsPCS7) were down-regulated in the 20As+120S treatment. Compared with 20As+0S treatment, three PCS DEGs (OsPCS1, OsPCS3 and OsPCS13) were up-regulated in the 20As+120S treatment (Fig. 5c).
ABC Transporter
Two ABCC-type transporters (AtABCC1 and AtABCC2) in Arabidopsis have been found to regulate arsenic tolerance (Song et al. 2010). Compared to the control, four ABC transporter genes were both down-regulated in the 20As+0S and 20As+120S treatments, respectively (Fig. 6). Five genes annotated as ABC transporter [OsABCB6 (LOC_Os01g50160.1), OsABCI7_2 (LOC_Os11g29850.2), OsABCG5 (LOC_Os03g17350.1), OsABCG42_2 (LOC_Os06g36090.1), and OsABCB22 (LOC_Os08g45030.1)] were up-regulated in the 20As+120S treatment compared to the 20As+0S treatment. OsABCG43 (LOC_Os07g33780.1), which is involved in cadmium accumulation (Verbruggen et al. 2009) was down-regulated in the 20As+120S treatments compared to the control. OsABCB6 and OsABCB22 were also observed to be significantly down-regulated in the 20As+120S treatment compared to the control.
Glutathione S-transferases
Arsenic treatments up-regulated one GST gene (LOC_Os03g04220.1), and down-regulated eight other GST genes (LOC_Os01g70770.1, LOC_Os03g04260.1, LOC_Os03g17470.1, LOC_Os10g34020.1, LOC_Os10g38580.1, LOC_Os10g38700.1, LOC_Os10g38730.1, LOC_Os12g02960.1) (Fig. 7). The down-regulated gene LOC_Os03g17470 is annotated as IN2-1 protein. This gene was up-regulated in rice roots treated with As(V) (Norton et al. 2008). Compared with 20As+0S treatment, one Phi class GST gene (LOC_Os03g04220.1), and three Tau class GSTs (LOC_Os07g07320.1, LOC_Os10g38700.1 and LOC_Os12g02960.1) were up-regulated in the 20As+120S treatment.
Confirmation of DGE Results by qRT-PCR
We selected five up-regulated genes (LOC_Os01g41050.1, LOC_Os08g20020.2, LOC_Os04g52100.1, LOC_Os11g23890.1, LOC_Os12g41830.1) and five down-regulated genes (LOC_Os02g07628.1, LOC_Os03g18150.1, LOC_Os06g36590.1, LOC_Os07g05040.1, LOC_Os08g15840.2) to perform qRT-PCR to confirm the results from DGE. The results of qRT-PCR showed that the expression profiles of these ten genes were consistent with Tag-seq data (Fig. 8).
Quantitative RT-PCR validation of genes from digital gene expression (DGE) of rice grains. qRT-PCR analysis of gene expression normalized to the β-actin gene and relative to the control. a Up-regulated genes in rice grains treated with S and/or As compared to control of DGE and qRT-PCR. b Down-regulated genes in rice grains treated with S and/or As compared to control of DGE and qRT-PCR
Discussion
Our previous studies have demonstrated that S nutrition affects As translocation from root to shoot in rice seedlings, which is significantly enhanced by S deficiency (Zhang et al. 2011). Sulfur or sulfate application reduced As translocation from roots to shoots by binding As(III) to the -SH group (Hu et al. 2007; Fan et al. 2013). Excessive S application (60 mg kg−1 and 120 mg kg−1) decreased As concentration in brown rice (Fan et al. 2013). These latter studies suggest that enhancement of S application restricted As translocation to rice grains. The results from the present study confirmed that excessive S application could significantly decrease the As concentration in brown rice (Fan et al. 2013). Furthermore, we found that S application mainly reduced the concentration of inorganic As, but not the DMA concentration in rice grains. It has been reported that DMA cannot be complexed by thiol compounds in plants (Chen et al. 2015). Therefore, it is reasonable that translocation of DMA from shoot to grain was not influenced by sulfur supply. In addition, S application significantly affected the transcription of genes involved in As transport and metabolism in rice grains: phosphate transporter OsPT23 and aquaporin OsTIP4;2 were down-regulated, while ABC transporter genes (OsABCG5, OsABCI7_2 and OsABC6) and phytochelatin synthase genes (OsPCS1, OsPCS3 and OsPCS13) were up-regulated. To our knowledge, this is the first report showing the effect of S application on As species and As-related genes expression in rice grains.
Previous studies have shown that arsenate and phosphate share the same transport pathway in higher plants (Meharg and Macnair 1992). Phosphate transporters such as Pht1;1 and Pht1;4 are involved in arsenate uptake and accumulation in Arabidopsis (Shin et al. 2004). The down-regulated phosphate transporters, OsPT21 and OsPT23 in S-treated rice grains might be responsible for decreasing the grain As(V) concentration (Fig. 3). Ma et al. (2006, 2008) and Zhao et al. (2010) found that a number of nodulin 26-like intrinsic proteins (NIPs), which belong to the aquaporin family, mediate silicon and arsenite accumulation in rice. We did not detect the expression of Lsi1 (LOC_Os02g51110) and Lsi2 (LOC_Os03g01700) in this study. This could be ascribed to the specific expression of these genes in rice roots but not in grains (Yu et al. 2012). We found that the expression of eight aquaporin genes changed significantly in response to As and S treatment, with most being down-regulated. For example, expression of OsNIP1;1 and OsNIP3;1 in S treatment was reduced about 7-fold. Such down-regulation could contribute to the decrease in As(III) transport from shoot to grain in rice. OsLsi6 (LOC_Os06g12310.1) and OsTIP4;2 were also down-regulated in the 20As+120S treatment compared to As treatment. In plants, As(III) binds with non-protein thiols such as phytochelatins or GSH (Zhao et al. 2010; Zhang et al. 2011). Neither γ-glutamylcysteine synthetase (γ-ECS) nor ATP sulfurylase (ATPS) genes were differentially regulated by 20As+0S in rice grains, despite these two enzymes being involved in phytochelatin synthesis. It is noteworthy that the expression of two phytochelatin synthases (PCS) and four glutathione S-transferase genes were up-regulated in the 20As+120S treatment compared to 20As+0S treatment (Fig. 5). These results indicate that the amount of PC in rice grains may be increased by sulfur supply, similar to the case in rice seedlings (Zhang et al. 2011; Dixit et al. 2015). Interestingly, three genes encoding putative methyltransferase, LOC_Os02g51030, LOC_Os03g12110 and LOC_Os10g28630 were up-regulated by As treatment in rice seedlings (Norton et al. 2008), but were not up-regulated in rice grains in this study (Table S2). The results further indicate that As cannot be methylated in rice grains.
Complexes of arsenite and PCs can be sequestered in vacuoles via ABC transporters in plants (Song et al. 2010; Zhao et al. 2010). OsABCC9 have been reported response to Cr(IV) stress (Dubey et al. 2010). Many ABC transporter genes are up-regulated in roots and shoots under As stress in rice seedlings (Yu et al. 2012). In this study, we found that 16 ABC transporters genes were significantly down-regulated and 5 ABC transporter genes were up-regulated in the comparison between the 20As+0S treatment and the control. These up-regulated ABC transporters may be linked to the decrease in inorganic As accumulation in the rice grain.
Results obtained in this study show that S application down-regulated phosphate transporter (OsPT23) and aquaporin (OsTIP4;2) genes, and up-regulated sulfate transporter (OsSultr5;1) and PCS (OsPCS1, OsPCS3 and OsPCS13) genes, which might result in a decrease in inorganic As concentration in rice grains. Further detailed investigation of these potential genes would benefit our understanding of the transcriptional regulation of arsenic-responsive genes in rice grains. The original data set has been deposited with the NCBI GEO database (access no. GSE48799).
Abbreviations
- As:
-
Arsenic
- As(III):
-
Arsenite
- As(V):
-
Arsenate
- ABC superfamily:
-
ATP-binding cassette superfamily
- ABCA:
-
ABC subfamily A
- ABCB:
-
ABC subfamily B
- ABCC:
-
ABC subfamily C
- ABCD:
-
ABC subfamily D
- ABCF:
-
ABC subfamily F
- ABCG:
-
ABC subfamily G
- ABCI:
-
ABC subfamily I
- ATPS:
-
ATP sulfurylase
- AR:
-
Arsenate reductase
- DGE:
-
Digital gene expression
- DEGs:
-
Differentially expressed genes
- DMA:
-
Dimethylarsenate
- γ–ECS:
-
γ-Glutamylcysteinesynthetase
- GSH:
-
Glutathione
- GS:
-
Glutathione synthetase
- GST:
-
Glutathione S-transferase
- MMA:
-
Monomethylarsenate
- OAS-TL:
-
O-Acetylserine (thiol) lyase
- PCs:
-
Phytochelatins
- PCS:
-
Phytochelatin synthases
- PT:
-
Phosphate transporter
- PIPs:
-
Plasma membrane intrinsic proteins
- TIPs:
-
Tonoplast intrinsic proteins
- NIPs:
-
Nodulin 26-like intrinsic membrane proteins
- SIPs:
-
Small and basic intrinsic proteins
- qRT-PCR:
-
Quantitative RT-PCR
- S:
-
Sulfur
- SAT:
-
Serine acetyltransferase
- SiR:
-
Sulfite reductase
- TMAs:
-
Trimethylarsine
References
Bleeker PM, Hakvoort HWJ, Bliek M, Souer E, Schat H (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerance Holcus lanatus. Plant J 45:917–929
Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, Tripathi RD, Tuli R (2009) Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 74:688–702
Chen Y, Moore KL, Miller AJ, McGrath SP, Ma JF, Zhao FJ (2015) The role of nodes in arsenic storage and distribution in rice. J Exp Bot 66(13):3717–3724
Clemens S, Kim E, Neumann D, Schroeder J (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18:3325–3333
Dixit G, Singh AP, Kumar A, Singh PK, Kumar S, Dwived S, Trivedi PK, Pandey V, Norton GJ, Dhankher OP, Tripathi RD (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Duan GL, Kamiya T, Ishikawa S, Arao T, Fujiwara T (2012) Expressing ScACR3 in rice enhanced arsenite efflux and reduced arsenic accumulation in rice grains. Plant Cell Physiol 53:154–163
Duan GL, Liu WJ, Chen XP, Hu Y, Zhu YG (2013) Association of arsenic with nutrient elements in rice plants. Metallomics 5:784–792
Dubey S, Misra P, Dwivedi S, Chatterjee S, Bag S, Mantri S, Asif MH, Rai A, Kumar S, Shri M, Tripathi P, Tripathi RD, Trivedi PK, Chakrabarty D, Tuli R (2010) Transcriptomic and metabolomic shifts in rice roots in response to Cr (VI) stress. BMC Genomics 11:648–667
El-Zohri M, Odjegba V, Ma L, Rathinasabapathi B (2015) Sulfate influx transporters in Arabidopsis thaliana are not involved in arsenate uptake but critical for tissue nutrient status and arsenate tolerance. Planta 241(5):1109–1118
Fan J, Xia X, Hu Z, Ziadi N, Liu C (2013) Excessive sulfur supply reduces arsenic accumulation in brown rice. Plant Soil Environ 59:169–174
Heiss S, Wachter A, Bogs J, Cobbett C, Rausch T (2003) Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J Exp Bot 54:1833–1839
Hernández LE, Sobrino-Plata J, Montero-Palmero MB, Carrasco-Gil S, Flores-Cáceres ML, Ortega-Villasante C, Escobar C (2015) Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J Exp Bot. doi:10.1093/jxb/erv063
Hu ZY, Zhu YG, Li M, Zhang LG, Cao ZH, Smith FA (2007) Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ Pollut 147:387–393
Kumar S, Asif MH, Chakrabarty D, Tripathi RD, Trivedi PK (2011) Differential expression and alternative splicing of rice sulphate transporter family members regulate sulphur status during plant growth, development and stress conditions. Funct Integr Genomics 11:259–273
Liu F1, Chang XJ, Ye Y, Xie WB, Wu P, Lian XM (2011) Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice. Plant Physiol 152:2211–2221
Liu Q, Guo Y, Li J, Long J, Zhang B, Shyr Y (2012) Steps to ensure accuracy in genotype and SNP calling from Illumina sequencing data. BMC Genomics13 Suppl 8:S8. doi: 10.1186/1471-2164-13-S8-S8
Lombi E, Schechel KG, Pallon AM, Pallon J, Carey YG, Zhu YG, Meharg AA (2009) Speciation and distribution of arsenic and location of nutrients in rice grains. New Phytol 184:193–201
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Meharg AA, Macnair MR (1992) Suppression of the high-affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Norton GJ, Lou-Hing DE, Meharg AA, Price AH (2008) Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59:2267–2276
Raab A, Feldmann J, Meharg AA (2004) The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134:1113–1122
Raab A, Schat H, Meharg AA, Feldmann J (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic–phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168:551–558
Rai MK, Kalia RK, Singh R, Gangola MP, Dhawan AK (2011) Developing stress tolerant plants through in vitro selection—an overview of the recent progress. Environ Expr Bot 71:89–98
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642
Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee YS, Martinoia E (2010) Arsenic tolerance in Arabidopsisis mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107:21187–21192
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f) Royle. Environ Sci Technol 41:2930–2936
Srivastava AK, Srivastava S, Mishra S, Suprasanna P, D’Souza SF (2014) Identification of redox-regulated components of arsenate (AsV) tolerance through thiourea supplementation in rice. Metallomics 6:1718–1730
Su YH, McGrath SP, Zhu YG, Zhao FJ (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 108:434–441
Sun S, Gu M, Cao Y, Huang X, Zhang X, Ai P, Zhao J, Fan X, Xu G (2012) A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol 159:1571–1581
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Wang J, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata: uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
Wang LH, Duan GL, Williams PN, Zhu YG (2008) Influences of phosphorus starvation on OsACR2.1 expression and arsenic metabolism in rice seedlings. Plant Soil 313:129–139
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Xu XY, McGrath SP, Zhao FJ (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176:590–599
Yu LJ, Luo YF, Liao B, Xie LJ, Chen L, Xiao S, Li JT, Hu SN, Shu WS (2012) Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol 195:97–112
Zhang J, Zhao QZ, Duan GL, Huang YC (2011) Influence of sulphur on arsenic accumulation and metabolism in rice seedlings. Environ Exp Bot 72:34–40
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, Meharg AA, Williams PN (2008) High percentage inorganic arsenic content of mining impacted and non-impacted Chinese rice. Environ Sci Technol 42:5008–5013
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41103062 and 41371458) and the Innovation Scientists and Technicians Troop Construction Projects of Henan Province (94200510003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, J., Zhao, CY., Liu, J. et al. Influence of Sulfur on Transcription of Genes Involved in Arsenic Accumulation in Rice Grains. Plant Mol Biol Rep 34, 556–565 (2016). https://doi.org/10.1007/s11105-015-0937-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-015-0937-z