Abstract
Background and aims
Priming effect (PE) plays a crucial role in driving soil organic carbon (SOC) decomposition and it is strongly affected by C addition types and nitrogen (N) addition. However, the understanding of the strength and microbial mechanisms of PE in response to specific root exudates (glucose and oxalic acid) and N addition remains inadequate.
Methods
In this study, we carried out a 60-day incubation experiment using simulated root exudates (i.e., glucose and oxalic acid) and inorganic N in coniferous and broad-leaved forest soils to estimate their effects on PE and microbial mechanisms.
Results
Oxalic acid addition resulted in positive PE through “co-metabolism” (i.e., the accelerated microbial decomposition of native SOC), which was supported by an increase in microbial biomass C (MBC), and the activities of enzyme involved in the C and N metabolism in both forest soils. In contrast, N addition significantly lowered positive PE by moderating N mining, as supported by the decreased ratios of fungi: bacteria (F: B), oxidase activities: hydrolase activities (O: H), and C: N enzyme activities, and increased CO2 derived from root exudate per MBC. These results indicated that stoichiometric decomposition increased with the partial preferential use of the root exudate. The pattern of increased ratios of F: B, O: H, and C: N enzymes with incubation time revealed the dominance of microbial N mining.
Conclusion
Collectively, our results demonstrate that shifts in driving PE from stoichiometric decomposition to microbial N mining over time predominantly depend on N availability, thereby providing insightful evidence for accurately assessing soil C dynamics for future climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is the largest carbon (C) reservoir in terrestrial ecosystems, and its C pool is higher than global vegetation and the atmosphere combined (Lehmann and Kleber 2015). The soil C pool is governed by the balance between the C input from plants and the output of C mainly derived from soil organic carbon (SOC) decomposition (Cotrufo et al. 2015; Castellano et al. 2015; Zhang et al. 2021). Exogenous fresh C input into soils can affect the microbial mineralization of native SOC, causing a priming effect (PE) that exerts a crucial influence on global soil C dynamic (Kuzyakov et al. 2000; Qiao et al. 2014; Kan et al. 2022). Some studies have estimated the effect of fresh C addition on PE in various ecosystems (Deng et al. 2021; Qiu et al. 2023; Siles et al. 2022; Ren et al. 2022), with 380% stimulation or 50% suppression (Cheng et al. 2014). These divergent results suggest that there exists uncertainty regarding the interactions between fresh C and microorganisms. Hence, it is important to further explore the potential microbial mechanisms of PE.
Root exudates are a labile C source that can promote microbial respiration (Finzi et al. 2015; Haichar et al. 2014). Sugar and organic acids are common compounds of root exudates and have various functions in mediating soil biogeochemical processes. The underlying mechanism can be either (1) root exudates directly change soil properties (Haichar et al. 2014) and microbial attributes (Coskun et al. 2017) or (2) root exudates like oxalic acid could interfere with or weaken the stability of mineral-organic interactions, releasing protected SOC that was previously bound to minerals (Keiluweit et al. 2015; Liao et al. 2022). These two mechanisms affect PE. Moreover, the direction and magnitude of PE would be altered with incubation time (Bernard et al. 2022; Fang et al. 2018; Zhang et al. 2021;), For instance, some studies found that PE decreased with time (Fang et al. 2018; Chao et al. 2019), while Zhang et al. (2021) suggested that PE first decreased to negative and then increased to positive effects. The main reason for these divergent results may be the altered substrate and nutrient supply, which could influence the microbial community. Nevertheless, it is unclear how different components of the exudate input interact with the microbial community over time, thus affecting PE dynamics.
Increased human activities have led to dramatic increase in nitrogen (N) deposition over the past few decades (Ackerman et al. 2019). Although N deposition has dramatically improved soil N availability throughout the world (Peñuelas et al. 2013), it can also largely mediate the intensity and direction of PE by changing soil properties and microbial attributes (Li et al. 2019; Hicks et al. 2019; Mehnaz et al. 2019). The effect of N addition on the PE remains controversial with positive (Chen et al. 2014), negative or no effect (Hicks et al. 2019; Parajuli et al. 2022; Nottingham et al. 2015). There exist two opposing microbial theories explaining the variation of PE generated by the C and N addition, namely “microbial N mining” and “microbial stoichiometry decomposition”. For microbial N mining, microorganisms use labile C as a source of energy to produce more enzymes to decompose recalcitrant soil organic matter for N acquisition, likely leading to a positive PE. Accordingly, N addition is expected to reduce PE intensity by alleviating microbial nutrient deficiency. While microbial stoichiometric decomposition indicates that fast-growing microorganisms may enhance SOC decomposition by improving microbial biomass and activity when the substrate stoichiometry meets microbial physiological requirements. Consequently, a positive PE is also predominated by microbial stoichiometric decomposition theory. Previous studies have suggested that these two competing microbial mechanisms are linked and can shift over time (Fang et al. 2018). Specific microbial groups with the activity of K-strategists (e.g., fungi) mainly dominated during the microbial N mining, while the activity of r-strategists (e.g., bacteria) was important in the PE mediated by the microbial stoichiometric (Chen et al. 2014; Razanamalala et al. 2018). Nevertheless, these contrasting microbial processes have not been validated considering the relationship between PE and microbial processes under N addition over time in contrasting soils.
Here, an incubation experiment was performed to determine the influence of the simultaneous input of different root exudates and N on PE over time, and test whether PE was regulated by the shift in microbial community composition and activities in two different forest soil (i.e., coniferous forest and broad-leaved forest with Pinus yunnanensis and Quercus pannosa as the dominant species, respectively; Table S1). The soil properties were different in the two forests (e.g., pH, SOC, and C: N ratio). We hypothesized that (I) root exudate addition causes positive PE and the PE intensity under oxalic acid addition could be stronger due to liberating the mineral-associated C into soil for microbial decomposition and utilization (Keiluweit et al. 2015); (II) N addition decreases the intensity of PE in root exudate addition due to alleviating the microbial N mining, (III) “microbial stoichiometry decomposition” dominates during the early incubation due to limited N availability (Razanamalala et al. 2018), and microbial N mining is a dominant mechanism with time after exhaustion of available N in soil (Fanin et al. 2020).
Materials and methods
Study site and experimental design
The study site was located in Yulong Snow Mountain (27°10′–27°40′N, 100°10′–100°20′E; 3200 m a.s.l) in Yunnan province in China, which is regarded as a hotspot for t biodiversity research (Niu et al. 2020). Pinus yunnanensis (P. yunnanensis) is the dominant species in e coniferous forest and Quercus pannosa (Q. pannosa) is the dominant species in broad-leaved forest. These two forest types are widely distributed in the Yunnan Province. This area has a subtropical South Asian monsoon climate, with a mean annual temperature of about 18.2 ℃, and annual precipitation of 964.7 mm. The climate was characterized by a strong dry season and wet season. Soil types were classified as Luvisols in both forests, according to the US Soil Taxonomy Series.
In November 2020, soil samples were collected in coniferous and broad-leaved forests. The two forests was 300 m apart. In each forest type, three replicates of remixed soil samples were collected from five random locations in three random plots (0–10 cm). The litter floor above the mineral soil was removed, and the soil samples were collected and mixed into one composite sample. Soil samples were immediately transferred to the laboratory and sieved (2 mm), and the visible fine root fragments were manually picked out. Finally, subsamples of soils were air-dried to measure SOC, total N, and pH. The fresh subsamples were stored in a refrigerator (< 4 °C) for further analyses such as analyzing soil available C and N (see Methods in Supporting Information). The primary soil properties are exhibited in Table S1.
Experimental design and laboratory incubation
The experimental units consisted of a 250 mL triangular flask with 30 g (dry weight equivalent) of fresh and sieved soils. All soil samples were pre-incubated at 20 °C for 14 days to reduce the influence of physical disturbance after the sampling and sieving. Before incubation, the soil moisture achieved 60% of the water-holding capacity by adding distilled water.
In order to simulate root exudate C input, six treatments were established with nine replicates using 13C-labelled glucose and oxalic acid solution (representing two groups of sugars and organic acids in root exudate, respectively) after the pre-incubation: (1) soil without root exudate and N input (Control), (2) soil with N input (N), (3) soil with glucose input (G), (4) soil with inputs of glucose and N (G + N), (5) soil with oxalic acid input (O) and (6) soil with inputs of oxalic acid and N (O + N). Then, 1614.29 μg C g−1 and 1337.61 μg C g−1 of stimulated root exudate were added into coniferous soil and broad-leaved soil, respectively, which was equal to total soil microbial biomass C (MBC). This amount is similar to previous priming experiments (Bastida et al. 2013; Li et al. 2017; Chen et al. 2019). Further, 161.43 μg N g−1 and 133.76 μg N g−1 of N were added to coniferous soil and broad-leaved soil, respectively, corresponding to the C: N = 10 of the added substrate with NH4Cl. Soil CO2 efflux rates and the δ13C were sampled on 1, 3, 5, 7, 10, 15, 20, 30 and 60 days following the beginning of treatments. To study the effect of microbial properties on PE, three soil sample replicates were destructively sampled on the 7th, 30th, and 60th days, respectively to analyze soil MBC using chloroform fumigation-extraction method (methods see supporting information) and microbial attributes (phospholipid fatty acids (PLFAs) and enzymes).
Analysis of phospholipid fatty acids (PLFAs)
PLFAs were extracted using the method depicted by Wilkinson et al. (2002). In brief, lipids were extracted twice using freeze-dried soil samples (8 g) into a buffering solvent containing a chloroform, methanol and citric acid (1:2:0.8, v/ v/ v), and using mild alkaline methanolysis to transesterify to the extracting phospholipid to FAMEs (fatty acid methyl esters). The transesterified FAMEs were determined using a gas chromatograph equipped with the MIDI Sherlock Microbial Identification System (MIDI, Inc., Newark, DE, USA) and by co-elution with standards. The PLFAs i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0was used to represent gram-positive bacteria (GP) and 16:1ω7c, 16:1ω9c, 18:1ω7c, cy17:0 and cy19:0 was used to represent gram-negative bacteria (GN) (Zhang et al. 2021). Total bacterial counts were the sum of GP and GN. The 18:1ω9c and 18:2ω6c represent the fungi biomarkers, and 10Me18:0 was used to indicate actinomycetes (ACT) (Joergensen 2022; Lekberg et al. 2022). PLFAs were separated into four groups: GP, GN, F, and ACT.
Soil enzyme activities
The activities of eight enzymes, including six hydrolases, α-glucosidase (AG), β-glucosidase (BG), N-acetylglucosaminidase (NAG), xylanase (XYL), cellobiohydrolase (CBH), and leucine aminopeptidase (LAP), and two oxidases, polyphenol oxidase (PHO) and peroxidase (PEO), were measured using fluorometric techniques. Eight replicate wells per sample per assay were used following a modified method from Saiya-Cork et al. (2002). Soil slurries were prepared by suspending 1 g of soil in 100 ml of 50 mM sodium acetate buffer (pH5.5). In brief, the activities of AG, BG, NAG, CBH, XYL, and LAP were analyzed fluorometrically by adding 50 μL of 200 μmol L−1 4-methylumbelliferyl-α-D-glucopyranoside, 4-methylumbelliferyl-β-D-glucopyranoside, 4-methylumbelliferyl-N-acetyl-β-D-glucosaminidedehydrate, 4-methylumbelliferyl-β-Dcellobioside, 4-methylumbelliferyl-β-Dxylopyranoside and L-leucine-7-amino-4-methylcoumarin plus 200 μL of soil suspension to 96-well microtiter plates, respectively. The microplates were incubated in the dark at 20 °C for 4 h (Liao et al. 2020). Hydrolytic activity was measured fluorometrically using a microplate reader (M200 PRO; Tecan, Austria) with excitation at 365 nm and emission at 450 nm. For the oxidative enzyme, 50 μl of supernatant was added to the 200 μL of soil suspension and incubated in the dark at 20 °C for 20 h. Absorbance was measured at 460 nm using a microplate reader (M200 PRO, Tecan, Austria). The enzyme functions are listed in Table S2.
Calculations
The soil CO2 release rate from the native SOC and root exudate during incubation was calculated as follows:
where RSOC is the soil CO2 release rate from native SOC and Rroot is the soil CO2 release rate derived from root exudate. Rtreatment is the total CO2 release rate with root exudate addition, δ13 Rtreatment and δ13 Rcontrol is the isotope value of the total released CO2 with root exudate addition and control, respectively. δ13 Croot is the isotope value of root exudate added to the soil samples. This equation assumed that the isotope value of root exudate and SOC are homogeneous.
During incubation, the PE caused by root exudates was calculated by comparing SOC mineralization in exudate-amended samples with control samples.
where Rcontrol is the CO2 release rate in control without exudate addition.
Statistical analysis
A three-way repeated measures one-way analysis of variance (ANOVA) was used to evaluate the effects of root exudate, N addition, and forest types and their associated interactions on the total CO2 rate, native cumulative CO2 flux rate, exudate-derived CO2 flux rate, relative PE, and soil microbial variables. One-way ANOVA was used to test the effect of different treatments (N and root exudate treatments) on soil enzyme activities and the main soil microbial groups at each sampling point for every forest soil sample and explore the differences between sampling times. ANOVA was performed using SPSS version 21.0. Random Forest analysis was conducted to clarify the relative contribution of enzyme activity and the main microbial groups over time in each forest soil type. Random forest analysis was performed using the R “randomForest” package in the R Statistical Environment (Version 4.1.0, R Core Team). Variable importance was indicated by the percentage increase in the mean error (% IncMSE) if a specific variable was removed, which was calculated by shuffling the values of the out-of-bag samples.
Results
SOC mineralization and PE under simulated root exudate and N addition
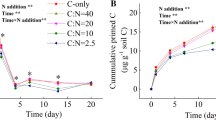
Native SOC mineralization and PE were significantly regulated by forest type, substrate type, N addition, and their interactions (p < 0.05, Table S3). Root exudate input increased native SOC decomposition, resulting in positive PE. The PE intensity was gradually decreased with time, whereas N addition significantly decreased PE in both forest soils (Figs. 1, S1, S2 and S3). The PE induced by oxalic acid was higher than that induced by glucose over the entire incubations (Fig. 1). Furthermore, Glucose addition led to a significantly higher PE in coniferous forest soil compared with broad-leaved forest soil, but the oxalic acid input did not cause significant difference in PE between the two forest types (Figs. 1 and S4).
Priming effect (PE) of root exudate and N addition on cumulative native soil CO2 flux and relative PE in the coniferous forest (a and c) and broad-leaved forest (b and d) soils during the 60-day incubation. Values are means with standard error (n = 3). Six treatments included that (1) soil without root exudate and N additions (Control), (2) soil with N addition (N), (3) soil with glucose addition (G), (4) soil with combined additions of glucose and N (G + N), (5) soil with oxalic acid addition (O) and (6) soil with combined additions of oxalic acid and N (O + N)
Enzyme activities and main microbial groups under simulated root exudate and N addition
Hydrolase and oxidase activities were significantly affected by forest type, substrate type, N addition and their interactive effects (Table S4). All enzyme activities generally decreased with time, except for higher NAG activity, which was observed during mid-incubation in the coniferous forest soil (Fig. S5c). In the coniferous forest soil, glucose and oxalic acid addition significantly increased the activities of PHO and PEO across the three sampling times (Fig. S5g, h). N addition significantly decreased the activities of LAP and NAG during the first two sampling periods (Fig. S5). In the broad-leaved forest soil, root exudate addition significantly increased the activities of BG, NAG, and LAP during the first sampling period (Fig. S6b-d, f) in coniferous or broad-leaved forest. The addition of oxalic acid increased the PHO and PEO activities across the three sampling times (Fig. S6).
The ratio of C: N enzymes [(AG + BG + CBH + XYL): (NAG + LAP)] was the highest under glucose and N addition and then generally decreased with time (Fig. 2a, b). The oxidase: hydrolase ratio (O: H enzymes) [(PHO + PEO): (AG + BG + CBH + XYL + NAG + LAP)] was significantly higher with oxalic acid input in coniferous forest soil (Fig. 2c), whereas it was significantly higher with glucose addition in broad-leaved forest soil (Fig. 2b, d). The oxidase-to-hydrolase ratio gradually increased over time in both forest soil types (Fig. 2d).
Effect of root exudate and N addition on soil C:N enzyme activity ratio [(AG + BG + CBH + XYL): (NAG + LAP)]and oxidase: hydrolase activity ratio [(PHO + PO):(AG + BG + CBH + XYL + NAG + LAP)] during three sampling times in the coniferous (a and c) and broad-leaved (b and d) forest soils. AG (α-Glucosidase); BG (β-Glucosidase); NAG (N-acetyl-β-glucoasminidase); CBH (Cellobiohydrolase); XYL (Xylosidase); LAP (Leucine amino peptidase); PHO (Polyphenol oxidase) and PEO (Peroxidase). Different capital letters displayed at the top of periods represent significant difference among periods under treatments. Different lowercase letters above bars represent significant differences among treatments within periods. Significance levels are set at p < 0.05. See Fig. 2 for abbreviations
The microbial community composition groups were also affected by substrate type, N addition, and sampling time (Table S4). The GP: GN and F: B ratios increased with time, and N addition significantly decreased the F: B ratio in both the glucose and oxalic acid treatments across the three sampling times in both forest soils (Figs. 3 and 4).
Effect of root exudate and N addition on main soil microbial groups during three sampling times in the coniferous forest soil including (a) gram-positive bacteria (GP); b gram-negative bacteria (GN); c Fungi (F); d Actinomyces (ACT); e the ratio of GP and GN; f the ratio of fungi and bacteria. Values are means with standard error (n = 3). Different capital letters at the top of periods represent significant difference among periods under treatments. Different lowercase letters above bars represent significant differences among treatments within periods. Significance levels are set at p < 0.05
Effect of root exudate and N addition on main soil microbial groups during three sampling times in the broad-leaved forest soil including (a) gram-positive bacteria (GP); b gram-negative bacteria (GN); c Fungi; d Actinomyces; e the ratio of GP and GN; f the ratio of fungi and bacteria. Values are means with standard error (n = 3). Different capital letters displayed at the top of periods represent significant difference among periods under treatments. Different lowercase letters above bars represent significant differences among treatments within periods. Significance levels are set at p < 0.05. See Fig. 5 for abbreviations
Drivers of PE with incubation time
Random forest analysis indicated that the importance of oxidase: hydrolase, C: N enzyme activities, PEO activity, and N availability to PE increased with the extending of incubation time, whereas the importance of AG, ACT, and NAG activities to PE decreased with incubation time in coniferous forest soil (Fig. S7a). For broad-leaved forest soil, the importance of the oxidase: hydrolase ratio, PHO, C: N enzyme activities, and N availability to PE increased with the extending of incubation time, whereas the importance of PEO, AG, and BG activities and the GP: GN ratio on PE decreased with incubation time (Fig. S7b).
Discussion
Our results showed that root exudate input had a positive priming effect by increasing the SOC decomposition rate in both forest soils (Fig. 1 and S1-S3). The findings were similar with previous studies reporting a positive PE in response to labile C input from different forest types (Chao et al. 2019; Lyu et al. 2018; Sullivan and Hart 2013; Wild et al. 2014). The observed positive priming effect under root exudate input could be attributed to microbial co-metabolism (Fontaine et al. 2003; Perveen et al. 2019), namely, the accelerated microbial decomposition of native SOC using the added root exudate as energy compound (Fontaine et al. 2003), as supported by the increase in MBC under root exudate addition compared with the control (Figs. S5, S6, and S7). The positive relationship between PE and enzyme activity also supports this observation (Fig. S9).
We found that oxalic acid input led to a larger PE than did glucose input in both forest soils (Fig. 1), suggesting that the type of root exudate was one of the major drivers of the priming effect. These results are consistent with our first hypothesis. Several possible reasons may be responsible for this result. First, oxalic acid can disrupt physically protected mineral-organic associations (Wang et al. 2021), and increase available C pools for soil microorganisms and their enzyme activities (Clarholm et al. 2015). This process strongly promotes microbial SOM decomposition, ultimately leading to a stronger priming effect. Partially consistent with this hypothesis, we observed higher C availability with the input of oxalic acid at the beginning of the incubation in broad-leaved forest (Fig. S10). Second, microorganisms prefer to utilize glucose with a lower degree of reduction of C for incorporation into microbial biomass relative to oxalic acid (Manzoni et al. 2012; Mehnaz et al. 2019), implying that microorganisms have higher C-use efficiency under glucose addition than under oxalic acid addition, possibly lowering the priming effect under glucose addition. The higher substrate-derived MBC confirmed a lower priming effect owing to higher C utilization with glucose addition (Fig. S8). Third, oxalic acid addition stimulated fungal growth (Figs. 3 and 4), and fungi efficiently degraded recalcitrant SOC with higher oxidase activity (Fig. S9; Fontaine et al. 2003, 2011). Consequently, higher fungal abundance with oxalic acid addition could enhance the priming effect. These results contradict those of a previous study showing that oxalic acid addition caused negative or no PE owing to the structural rearrangement of recalcitrant materials (Hamer and Marschner 2005). Collectively, our results indicate that oxalic acid addition promotes SOC mineralization jointly by abiotic and biotic processes, emphasizing that the two exudate compounds affect soil C decomposition through various regulation (Wang et al. 2021).
Consistent with our second hypothesis, we also observed that N addition lowered the positive priming effect regardless of the root exudate components (Fig. 1), which could be attributed to a reduction in the “microbial N-mining” theory (Dimassi et al. 2014; Fontaine et al. 2011; Liao et al. 2020). This was also observed by Perveen et al. (2019) and Feng et al. (2021). This may explain the decreased LAP and NAG activities under N addition, especially during the first two incubation periods (Figs. S5c, f and S6c, f).
N addition reduced microbial N mining and lower PE mediated by microbial stoichiometric decomposition (Fig. 5). The abundance of fungi and oxidase activity decreased, while bacterial abundance increased under N addition in both forest soils (Figs. 2, 3, 4, and S5-6). The microbial stoichiometric theory has been used to explain the enhanced PE in response to N addition (Meyer et al. 2018; Zhang et al. 2021), but the PE decreased in both forest soils when both root exudations were supplemented with N (Figs. 1, S1, and S2). The decrease in PE was likely due to the preferential use of root exudates, as indicated by the higher CO2 derived from root exudates per MBC under N addition in both forest soils (Fig. S11). The results emphasized the negative effect of PE to N addition due to the stoichiometric decomposition with preferential use of substrate.
The difference in the priming effect was higher under root exudates with N addition than with root exudates alone (Figs. 1 and S2), likely due to the dominance of the microbial N mining mechanism without N addition, which increased with incubation time because of the rapid depletion of N (Razanamalala et al. 2018), leading to a higher C: N imbalance for microorganisms (Chen et al. 2018). Under N-depleted conditions, microorganisms may stimulate SOM mineralization by secret oxidase to decompose recalcitrant organic matter and obtain N source s (i.e., microbial N mining) (Fanin et al. 2020). This may explain the increase in the oxidative hydrolase enzyme activity ratio and decrease in the C: N enzyme activity ratio with incubation time. Moreover, the increase in fungal abundance over time irrespective of N addition in both forest soils (Figs. 3 and 4) may further support the role of K-strategists in microbial N mining theory over time. Thus, our results indicated a shift in microbial mechanisms with enhanced microbial N mining over time, regardless of N addition in both forest soils, corresponding to our third hypothesis.
Additionally, we found that the priming effect induced by glucose was greater in the coniferous forest soil than in the broad-leaved forest soil, whereas there was no significant difference under oxalic acid addition over the entire incubation period between the two forest soils (Figs. 1 and S3). These divergent results are likely due to the different SOC recalcitrance between the two forest soils, with higher aromatic compounds in fresh leaves and higher soil DOC in the coniferous forest soil (Fig. S12), leading to higher microbial C limitation and a larger proportion of microbial dormancy (Barré et al. 2016). Consequently, the percentage of microorganisms activated by root exudate input is higher in coniferous forest soil than in broad-leaved soil, stimulating greater SOC decomposition (Blagodatskaya and Kuzyakov 2013). When oxalic acid is added, the released C induced by oxalic acid may also be recalcitrant to microbial utilization because of the higher aromatic compound content, which can balance the higher priming effect induced by stronger C limitation in coniferous forest soil (Bastida et al. 2019; Guo et al. 2016; Keiluweit et al. 2015).
Conclusions
In agreement with the suggested hypothesis, our results suggested that two root exudates caused positive priming effect through “co-metabolism” with a higher priming effect under oxalic acid than that under glucose. Furthermore, N addition significantly decreased the magnitude of the positive priming effect, likely because microbial N mining was reduced and microbial stoichiometry decomposition was enhanced with partial preferential use of root exudate, which was supported by the decrease in fungi to bacteria (F: B), C to N enzyme activities (C: N enzymes), and oxidase: hydrolase ratios, as well as higher root exudate-derived CO2 flux per microbial biomass C under N addition. The increased ratios of C: N enzymes, F: B, and oxidase: hydrolase with time suggest that both stoichiometric decomposition and microbial N mining could influence the priming effect, with an increasing contribution of the microbial N mining mechanism across treatments with time (Fig. 5).
Our results emphasize the shift in microbial mechanisms from stoichiometric decomposition to microbial N mining with time likely due to the declined C and N availability, thereby providing insightful evidence for accurately evaluating the soil C dynamics for future climate change. The PE is a complex process in which microbial N mining or stoichiometric decomposition theory cannot solely explain the variation of PE. Thus, combined the two theories could provide a critical clue to further explore the PE mechanisms (Feng et al. 2021). In the future, more effort is necessary to quantify the broad patterns of microbial mechanisms underlying the priming effect and reveal its response to N deposition. Nevertheless, more precise measurement of microbial communities is also urgent to further elucidate the microbial C utilization processes for a better knowledge gain of soil-C dynamics under global change.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ackerman D, Millet DB, Chen X (2019) Global estimates of inorganic nitrogen deposition across four decades. Glob Biogeochem Cycle 33:100–107. https://doi.org/10.1029/2018GB005990
Barré P, Plante AF, Cécillon L, Lutfalla S, Baudin F, Bernard S, Christensen BT, Eglin T, Fernandez JM, Chenu C (2016) The energetic and chemical signatures of persistent soil organic matter. Biogeochemistry 130:1–12. https://doi.org/10.1007/s10533-016-0246-0
Bastida F, Garcia C, Fierer N, Eldridge DJ, Bowker MA, Abades S, Delgado-Baquerizo M (2019) Global ecological predictors of the soil priming effect. Nat Commun 10:3481. https://doi.org/10.1038/s41467-019-11472-7
Bastida F, Torres IF, Hernández T, Bombach P, Richnow HH, García C (2013) Can the labile carbon contribute to carbon immobilization in semiarid soils? Priming effects and microbial community dynamics. Soil Biol Biochem 57:892–902. https://doi.org/10.1016/j.soilbio.2012.10.037
Bernard L, Basile-Doelsch I, Derrien D, Fanin N, Fontaine S, Guenet B, Karimi B, Marsden C, Maron PA (2022) Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct Ecol 36:1355–1377. https://doi.org/10.1111/1365-2435.14038
Blagodatskaya E, Kuzyakov Y (2013) Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67:192–211. https://doi.org/10.1016/j.soilbio.2013.08.024
Castellano MJ, Mueller KE, Olk DC, Sawyer JE, Six J (2015) Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob Change Biol 21:3200–3209. https://doi.org/10.1111/gcb.12982
Chao L, Liu Y, Freschet GT, Zhang W, Yu X, Zheng W, Guan X, Yang Q, Chen L, Dijkstra FA, Wang S (2019) Litter carbon and nutrient chemistry control the magnitude of soil priming effect. Funct Ecol 33:876–888. https://doi.org/10.1111/1365-2435.13278
Chen L, Liu L, Mao C, Qin S, Wang J, Liu F, Blagodatsky S, Yang G, Zhang Q, Zhang D, Yu J, Yang Y (2018) Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat Commun 9:3951. https://doi.org/10.1038/s41467-018-06232-y
Chen L, Liu L, Qin S et al (2019) Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat Commun 10:5112. https://doi.org/10.1038/s41467-019-13119-z
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Change Biol 20:2356–2367. https://doi.org/10.1111/gcb.12475
Cheng W, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Jastrow JD (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201(1):31–44. https://doi.org/10.1111/nph.12440
Clarholm M, Skyllberg U, Rosling A (2015) Organic acid induced release of nutrients from metal-stabilized soil organic matter – the unbutton model. Soil Biol Biochem 84:168–176. https://doi.org/10.1016/j.soilbio.2015.02.019
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017) How plant root exudates shape the nitrogen cycle. Trends Plant Sci 22:661–673. https://doi.org/10.1016/j.tplants.2017.05.004
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. https://doi.org/10.1038/NGEO2520
Deng S, Zheng X, Chen X, Zheng S, He X, Ge T, Hu Y (2021) Divergent mineralization of hydrophilic and hydrophobic organic substrates and their priming effect in soils depending on their preferential utilization by bacteria and fungi. Biol Fertil Soils 57:65–76. https://doi.org/10.1007/s00374-020-01503-7
Dimassi B, Mary B, Fontaine S, Perveen N, Revaillot S, Cohan JP (2014) Effect of nutrients availability and long-term tillage on priming effect and soil C mineralization. Soil Biol Biochem 78:332–339. https://doi.org/10.1016/j.soilbio.2014.07.016
Fang Y, Nazaries L, Singh BK, Singh BP (2018) Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob Change Biol 24:2775–2790. https://doi.org/10.1111/gcb.14154
Fanin N, Alavoine G, Bertrand I (2020) Temporal dynamics of litter quality, soil properties and microbial strategies as main drivers of the priming effect. Geoderma 377:114576. https://doi.org/10.1016/j.geoderma.2020.114576
Feng JG, Tang M, Zhu B (2021) Soil priming effect and its responses to nutrient addition along a tropical forest elevation gradient. Glob Change Biol 27:2793–2806. https://doi.org/10.1111/gcb.15587
Finzi AC, Abramoff RZ, Spiller SK, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Change Biol 21:2082–2094. https://doi.org/10.1111/gcb.12816
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. https://doi.org/10.1016/j.soilbio.2010.09.017
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. https://doi.org/10.1016/S0038-0717(03)00123-8
Guo J, Yang Z, Lin C, Liu X, Chen G, Yang Y (2016) Conversion of a natural evergreen broadleaved forest into coniferous plantations in a subtropical area: effects on composition of soil microbial communities and soil respiration. Biol Fertil Soils 52:799–809. https://doi.org/10.1007/s00374-016-1120-x
Haichar FEZ, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80. https://doi.org/10.1016/j.soilbio.2014.06.017
Hamer U, Marschner B (2005) Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol Biochem 37(3):445–454. https://doi.org/10.1016/j.soilbio.2004.07.037
Hicks LC, Meir P, Nottingham AT, Reay DS, Stott AW, Salinas N, Whitaker J (2019) Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol Biochem 129:212–222. https://doi.org/10.1016/j.soilbio.2018.10.015
Joergensen RG (2022) Phospholipid fatty acids in soil—drawbacks and future prospects. Biol Fertil Soils 58(1):1–6. https://doi.org/10.1007/s00374-021-01613-w
Kan ZR, Chen Z, Wei YX, Virk AL, Bohoussou YND, Lal R, Zhang HL (2022) Contribution of wheat and maize to soil organic carbon in a wheat-maize cropping system: a field and laboratory study. J Appl Ecol 59(11):2716–2729. https://doi.org/10.1111/1365-2664.14265
Keiluweit M, Bougoure JJ, Nico PS, Pett-Ridge J, Weber PK, Kleber M (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Chang 5:588–595. https://doi.org/10.1038/nclimate2580
Kuzyakov Y, Friedel J, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498. https://doi.org/10.1016/S0038-0717(00)00084-5
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68. https://doi.org/10.1038/nature16069
Lekberg Y, Bååth E, Frostegård Å, Hammer E, Hedlund K, Jansa J, Olsson PA (2022) Fatty acid 16: 1ω5 as a proxy for arbuscular mycorrhizal fungal biomass: current challenges and ways forward. Biol Fertil Soils 58(8):835–842. https://doi.org/10.1007/s00374-022-01670-9
Li Q, Tian Y, Zhang X, Xu X, Wang H, Kuzyakov Y (2017) Labile carbon and nitrogen additions affect soil organic matter decomposition more strongly than temperature. Appl Soil Ecol 114:152–160. https://doi.org/10.1016/j.apsoil.2017.01.009
Li Q, Song X, Chang SX, Peng C, Xiao W, Zhang J, Wang W (2019) Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric for Meteorol 268:48–54. https://doi.org/10.1016/j.agrformet.2019.01.012
Liao C, Tian Q, Liu F (2020) Nitrogen availability regulates deep soil priming effect by changing microbial metabolic efficiency in a subtropical forest. J for Res 32:713–723. https://doi.org/10.1007/s11676-020-01148-0
Liao C, Men XX, Wang C, Chen R, Cheng XL (2022) Nitrogen availability and mineral particles contributed fungal necromass to the newly formed stable carbon pool in the alpine areas of Southwest China. Soil Biol Biochem 173:108788. https://doi.org/10.1016/j.soilbio.2022.108788
Lyu M, Xie J, Vadeboncoeur MA, Wang M, Qiu X, Ren Y, Jiang M, Yang Y, Kuzyakov Y (2018) Simulated leaf litter addition causes opposite priming effects on natural forest and plantation soils. Biol Fertil Soils 54:925–934. https://doi.org/10.1007/s00374-018-1314-5
Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196(1):79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Mehnaz KR, Corneo PE, Keitel C, Dijkstra FA (2019) Carbon and phosphorus addition effects on microbial carbon use efficiency, soil organic matter priming, gross nitrogen mineralization and nitrous oxide emission from soil. Soil Biol Biochem 134:175–186. https://doi.org/10.1016/j.soilbio.2019.04.003
Meyer N, Welp G, Rodionov A, Borchard N, Martius C, Amelung W (2018) Nitrogen and phosphorus supply controls soil organic carbon mineralization in tropical topsoil and subsoil. Soil Biol Biochem 119:152–161. https://doi.org/10.1016/j.soilbio.2018.01.024
Niu H, Kang S, Gao W, Sarangi C, Tripathee L, Rupakheti D, Yan X (2020) Investigation of the spatio-temporal heterogeneity and optical property of water-soluble organic carbon in atmospheric aerosol and snow over the Yulong Snow Mountain, southeastern Tibetan Plateau. Environ Int 144:106045. https://doi.org/10.1016/j.envint.2020.106045
Nottingham AT, Turner BL, Stott AW, Tanner EVJ (2015) Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biol Biochem 80:26–33. https://doi.org/10.1016/j.soilbio.2014.09.012
Parajuli B, Ye R, Szogi A (2022) Mineral N suppressed priming effect while increasing microbial C use efficiency and N2O production in sandy soils under long-term conservation management. Biol Fertil Soils 58:903–915. https://doi.org/10.1007/s00374-022-01665-6
Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Janssens IA (2013) Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4:2934. https://doi.org/10.1038/ncomms3934
Perveen N, Barot S, Maire V, Cotrufo MF, Shahzad T, Blagodatskaya E, Stewart CE, Ding W, Siddiq MR, Fontaine S (2019) Universality of priming effect: an analysis using thirty five soils with contrasted properties sampled from five continents. Soil Biol Biochem 134:162–171. https://doi.org/10.1016/j.soilbio.2019.03.027
Qiao N, Schaefer D, Blagodatskaya E, Zou X, Xu X, Kuzyakov Y (2014) Labile carbon retention compensates for CO2 released by priming in forest soils. Glob Change Biol 20:1943–1954. https://doi.org/10.1111/gcb.12458
Qiu Q, Li M, Mgelwa AS, Hu YL (2023) Divergent mineralization of exogenous organic substrates and their priming effects depending on soil types. Biol Fertil Soils 59(1):87–101. https://doi.org/10.1007/s00374-022-01682-5
Razanamalala K, Razafimbelo T, Maron PA, Ranjard L, Chemidlin N, Lelievre M, Dequiedt S, Ramaroson VH, Marsden C, Bernard L (2018) Soil microbial diversity drives the priming effect along climate gradients: a case study in Madagascar. ISME J 12:451–462. https://doi.org/10.1038/ismej.2017.178
Ren C, Wang J, Bastida F, Delgado-Baquerizo M, Yang Y, Wang J, Zhong Z, Zhou Z, Zhang S, Zhao F (2022) Microbial traits determine soil C emission in response to fresh carbon inputs in forests across biomes. Glob Change Biol 28:1516–1528. https://doi.org/10.1111/gcb.16004
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Siles JA, Diaz-Lopez M, Vera A, Eisenhauer N, Guerra CA, Smith LC, Buscot F, Reitz T, Breitkreuz C, Bastida F (2022) Priming effects in soils across Europe. Glob Change Biol 28:2146–2157. https://doi.org/10.1111/gcb.16062
Sullivan BW, Hart SC (2013) Evaluation of mechanisms controlling the priming of soil carbon along a substrate age gradient. Soil Biol Biochem 58:293–301. https://doi.org/10.1016/j.soilbio.2012.12.007
Wang Q, Yuan Y, Zhang Z, Liu D, Xiao J, Yin H (2021) Exudate components mediate soil C dynamic through different priming mechanisms in forest soils. Appl Soil Ecol 160. https://doi.org/10.1016/j.apsoil.2020.103855
Wild B, Schnecker J, Alves RJE, Barsukov P, Bárta J, Čapek P, Gentsch N, Gittel A, Guggenberger G, Richter A (2014) Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol Biochem 75:143–151. https://doi.org/10.1016/j.soilbio.2014.04.014
Wilkinson SC, Anderson JM, Scardelis SP, Tisiafouli M, Taylor A, Wolters V (2002) PLFA profiles of microbial communities in decomposing conifer litters subject to moisture stress. Soil Biol Biochem 34:189–200. https://doi.org/10.1016/S0038-0717(01)00168-7
Zhang Q, Cheng L, Feng J, Mei K, Zeng Q, Zhu B, Chen Y (2021) Nitrogen addition stimulates priming effect in a subtropical forest soil. Soil Biol Biochem 160:108339. https://doi.org/10.1016/j.soilbio.2021.108339
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (32130069), the Key Research and Development Program of Yunnan Province (No. 202303AC100009), the Project for Talent and Platform of Science and Technology in Yunnan Province Science and Technology Department (202205AM070005), and the Scientific Research Fund of Education Department in Yunnan Province (2024J0001). We are grateful to Lijiang Forest Biodiversity National Observation and Research Station and research station and Baima Snow Mountain complex ecosystem vertical transect field observation and research station for help with field work.
Author information
Authors and Affiliations
Contributions
X.L.C and C.L conceived the ideas and designed methodology; C.L and Y.B collected the data; C.L analyzed the data; X.L.C and C.L led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing of interest.
Additional information
Responsible Editor: Hans Lambers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, C., Bao, Y. & Cheng, X. Shifts in microbial mechanism regulate carbon priming effect under two simulated root exudate and nitrogen addition in coniferous and broad-leaved forest soils. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06887-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06887-1