Abstract
Background
Biological control (biocontrol) organisms are the key component of the sustainable agriculture. Although the majority of the research on biocontrol organisms focused on fungi and bacteria, recent studies revealed the importance of predatory protists in pathogen suppression through direct (by feeding on plant pathogens) and indirect (by enhancing bacterial activities related to pathogen suppression) mechanisms. However, a review of the literature related to predatory protists and plant pathogen interaction is still lacking.
Scope
In this review, we aimed to 1) provide the current body of knowledge on the roles of predatory protists in plant pathogen suppression, and 2) highlight the importance of predatory protists as potential biocontrol agents. We also provided information on isolation techniques and application methods of predatory protists that can be used as biocontrol agents in agricultural systems.
Conclusion
We highlighted that predatory protists can be an important solution for the sustainable management of plant pathogens. Since there is a huge knowledge gap in this area, further studies should focus on protist-pathogen interaction and its application for sustainable plant productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainable agriculture is essential for meeting the challenges that agricultural and food systems around the world are facing. The aim of sustainable agriculture is to improve food security and nutrition for all, alleviate poverty, conserve natural resources, mitigate climate change, and build more sustainable, resilient, and inclusive food systems. One of the main challenges in maintaining sustainable agriculture is the plant pathogens, which are mainly controlled by excessive usage of chemicals, especially pesticides, and are harmful to the environment (Kaur et al. 2024).
The major plant pathogenic microorganisms include bacteria, fungi and protists (mainly oomycetes). The plant pathogens in the rhizosphere, the soil surrounding the roots of the living plants, have serious negative effects on plant health (Dean et al. 2012; Mansfield et al. 2012). Infection by plant pathogens leads to changes in primary metabolism that affect plant growth and development, as well as changes in secondary metabolism based on the induction of plant defenses (Asadi and Millar 2024). Thus, the attack by the plant pathogens on the plant roots causes a decrease in the yields, even when the pathogen-plant interactions do not show obvious disease symptoms (Chaloner et al. 2021). Protection against plant pathogens is one of the primary necessities to maintain the agricultural production. Pesticides are the most common approach to disease control in agriculture. However, some pesticides have adverse effects on human health, the environment, and living organisms (Kaur et al. 2024), which makes researchers around the world to search for alternative strategies to pesticides. Currently, the best alternative strategy is the microbial biological control agents (biocontrol agents) that are the key components of sustainable agriculture, offering potentially eco-friendly and long-term solutions for integrated plant pathogens and disease management (Trivedi et al. 2020).
Research on biocontrol agents and their use in agriculture to reduce the infection density and disease-causing activity of pathogens has spread rapidly in recent decades. Biocontrol-mediated disease suppression is the results of interactions between plants, pathogens and microbial communities in the rhizosphere soil (Trivedi et al. 2020). Several bacterial genera such as Bacillus, Pseudomonas and Agrobacterium, and fungal genera such as Trichoderma, Talaromyces and Candida have been registered and are currently being used as biocontrol agents (Trivedi et al. 2020). The biocontrol agents belonging to bacteria and fungi employ a range of mechanisms including antimicrobial activity, resource competition with plant pathogens, interference with pathogen virulence, and induction of plant defenses to protect plants from diseases caused by the pathogens (Thambugala et al. 2020; Bonaterra et al. 2022). In recent years, it has been recognized that not only bacteria and fungi but also protists play important roles in the pathogen control (Xiong et al. 2020). However, despite countless studies that have been conducted for bacteria and fungi in the biocontrol context, less is known about protists, the vast majority of eukaryotes. To the best of our knowledge, this is the first review directly focusing on predatory protists as biocontrol agents.
Protists are extremely diverse and abundant and play important roles in the soil ecosystems including the rhizosphere (Geisen et al. 2018; Murase and Asiloglu 2023). Nearly half of the protist taxa are predators feeding on microbes. The decomposer protists are one of the key microorganisms in nutrient cycling through the decomposition of organic matter (Geisen et al. 2018). Protists with photosynthesis ability are crucial members of soil carbon cycling and they mainly inhabit the top layer of soil where the sunlight is adequate (Jassey et al. 2022). Several members of protists are parasites and plant pathogens that have important negative impacts on host health and plant productivity, respectively (Mahé et al. 2017). Among the functional groups, predatory protists that feed on bacteria, fungi, and other microbes are the most abundant and taxonomically diverse group of protists in several soil ecosystems (Gao et al. 2019). There are important consequences of predatory protists feeding on the prey microorganisms. Firstly, the predatory activity of protists is one of the major factors controlling community composition and population of the soil microbiome (Asiloglu et al. 2021a). Once a protist feeds on a microorganism, excess nutrients including nitrogen, phosphorus, and other microelements are released into the environment, which then become available for non-preyed microorganisms and plants (Gao et al. 2019). Thus, predators do not only decrease the targeted bacterial populations but also have a positive impact on the remained or the non-preyed microbial communities. The presence of protists in the rhizosphere soil has a positive impact on plant growth and productivity through nutrient turnover and enhanced bacterial activities (Bonkowski 2004; Gao et al. 2019; Murase and Asiloglu 2023).
In addition to the effects of predatory protists on microbial communities and plant growth, predatory protists play important roles in pathogen suppression through direct (by feeding on plant pathogens) and indirect (by enhancing bacterial activities related to pathogen suppression) mechanisms. This review focuses on the role of predatory protists in controlling plant pathogens. We argued that predatory protists can be promising biological control agents, presenting current research findings. Furthermore, we explained how to isolate and use predatory protists for agricultural applications.
Roles of predatory protists in the suppression of plant pathogens
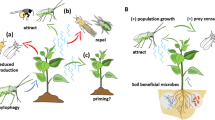
Predatory protists are involved in the suppression of plant pathogens and have a vast potential to be used as biocontrol agents. Two distinct mechanisms are involved in how predatory protists suppress the growth and disease incidence of the plant pathogens: the indirect mechanism, where protists enhance bacterial secondary metabolite production that is involved in pathogen suppression, and the direct mechanism through predation on the plant pathogens (Fig. 1).
Mechanisms of pathogen suppression by predatory protists in the rhizosphere soil. Presence of predatory protists enhance bacterial secondary metabolite production (prey defense), which is known to suppress the growth of plant pathogens, protecting plants. Organic fertilization causes an increase in the abundance of predatory protists, enriching the pathogen-suppressive bacteria. It often triggers the indirect mechanism (Left). Predatory protists directly feed and grow on plant pathogenic microorganisms and suppress their growth and disease incidences via a direct mechanism (Right)
The indirect mechanism
Predatory protists have selective feeding behavior meaning that not all bacteria can be preyed upon (Gao et al. 2019). Bacteria can sense chemical cues from protists and can survive predation through adaptations such as changes in cell size and shape, increased motility, filamentous formation, and secretion of defensive secondary metabolites (Matz and Kjelleberg 2005). Among them, the secretion of defensive secondary metabolites is also known to suppress the growth of plant pathogens (Bonaterra et al. 2022). This was first shown by Jousset et al. (2006) who studied the interactions of three different protists (amoeba, ciliate, and flagellate) with Pseudomonas fluorescens strain CHA0, a root-colonizing biocontrol agent. They showed that the exometabolite production in P. fluorescens CHA0 was contributed to the avoidance of protist predation and helped P. fluorescens CHA0 to sustain higher populations in the rhizosphere. Later, the same group also showed that predator–prey interactions were determinants of toxin production by Pseudomonas fluorescens in the rhizosphere (Jousset and Bonkowski 2010). Although the studies by Jousset et al. (2006, 2010) did not focus on plant pathogens, their findings on how predatory protists enhance bacterial secondary metabolite production including toxins became a key component of the later studies on the protist-pathogen interaction.
The indirect mechanism of pathogen suppression by predatory protists often studied in field conditions where addition of organic fertilizers enhanced predatory protists' abundance, which then enhanced the bacterial activities on secondary metabolite production, resulting in suppression of the plant pathogens (Fig. 1). One of the initial studies showing the indirect mechanism of predatory protists to control plant pathogens were conducted by Xiong et al. (2020), who studied the rhizosphere microbiome of the healthy and diseased tomato plants. They showed a negative correlation between the abundance of predatory protists and the plant pathogens. The bacterial metabolism gene analysis showed that the predatory protists enhanced bacterial activities in pathogen-suppressing secondary metabolite production, which mitigated success in pathogen control. Later, Guo et al. (2022) investigated soil and root microbiomes in banana cultivation under long-term conventional and organic fertilization systems, which was suffering from the Fusarium wilt disease. In the treatments with organic fertilization, disease incidence was reduced and yield was increased. Their results showed that the decrease in the disease incidence was best explained by relatively higher abundance of predatory protists and pathogen-suppressive bacteria in the organic fertilization treatments. The confirmation experiments showed that the interaction between Cercomonas spp. and Bacillus spp. suppressed the plant pathogens and enhanced the plant health (Guo et al. 2022). Similarly, the protist-bacteria interaction suppressing the plant pathogen, Fusarium sp., in the rhizosphere of broad bean has been also shown previously (Bahroun et al. 2021).
In a more recent study, Gao et al. (2024) studied the protist communities in the soil of healthy and diseased chili peppers affected by Fusarium wilt disease, and integrated the data with the bacterial and fungal communities from previous studies. The results showed the enrichment of predatory protists in diseased chili peppers. Differences were also observed in the networks among predatory protists, bacteria, and fungi in healthy and diseased chili peppers. The relative abundance of several functional genes associated with bacterial prey defense was found to be increased in diseased chili peppers, along with the increased relative abundance of predatory protists. Their results highlighted the indirect role of protists in the rhizosphere under pathogenic stress by influencing microbial community and functionality (Gao et al. 2024). As predatory protists indirectly reducing plant diseases is a recent hot topic, the number of studies has been increasing for several plant species under different environmental conditions. Although in each condition, predatory protist species, protist-affected biocontrol bacterial species, and pathogens are changing (Guo et al. 2024), the mechanism remains the same (Fig. 1). Further efforts on enhancement of pathogen-suppressing predatory protist abundances in field conditions should allow us to create sustainable management of the plant pathogens.
The direct mechanism
Although facultative and obligate mycophagous protists that feed on plant pathogenic fungi have long been recognized (Drechsler 1936; Old and Darbyshire 1978; Chakraborty and Old 1982; Petz et al. 1985; Ekelund 1998), the majority of the researchers treated protists as solely bacterivorous; probably due to that it is relatively easier to study bacterivorous protists via the traditional cultivation methods (Geisen et al. 2016). Nevertheless, several studies have proved that protists directly feed on important plant pathogens (Drechsler 1936; Chakraborty and Old 1982). As early as 1936, two testate amoeba species attacking oospores of Pythium ultimum, an important pathogen of hundreds of plants, have been reported (Drechsler 1936). Since then, studies focused on amoeba preying on fungal species. Among those studies, a few showed that protists can directly feed on plant pathogenic fungi, leading to the development of the first idea using protists (amoeba) as biocontrol agents in 1970s (Old and Patrick 1979). Later studies isolated protists from the pathogen-infected fields. For instance, an amoeba that was isolated from a field experiencing a decline in the take-all disease was tested for its efficiency in feeding on three plant pathogenic fungi (Chakraborty and Old 1982). Although promising results for protists feeding on pathogens are obtained, to the best of our knowledge, all studies conducted in laboratory cultures and protists have not been tested as biocontrol in the presence of plants with either pot or field experiments.
After 1980s, protist-plant pathogen interactions have been mostly neglected until the study conducted by Geisen et al. (2016), in which the mycophagous protists in the soil food web were revisited. Thanks to the recent development in molecular biology, they were able to show that mycophagous protists had a greater diversity than previously assumed and feed on a variety of fungi, including plant pathogens (Fusarium sp.). Their study was conducted to investigate eight protist isolates (Acanthamoeba sp., Acanthamoeba castellanii, two Cercomonas sp., Cryptodifflugia operculate, Leptomyxa sp., two Mayorella sp., and Thecamoeba sp.) for their feeding habits on diverse fungi (Saccharomyces cerevisiae, Cryptococcus laurentii, and Fusarium culmorum). Two flagellates of the genus Cercomonas, the testate amoeba Cryptodifflugia operculata, and four genera of naked amoebae (Acanthamoeba sp., Leptomyxa sp., two Mayorella sp. and Thecamoeba sp.) that was previously assumed to be solely bacterivorous feed on fungi. Four genera (Cercomonas sp., Leptomyxa sp., Mayorella sp., and Thecamoeba sp.) grew on spores of the plant pathogenic fungi, F. culmorum. In addition, Guo et al. (2024) investigated microbial mechanisms of suppression of Rastonia solanacearum. From the rhizosphere soil of tomato, they isolated the predatory protist, Colpoda sp., that was thought to suppress R. solanacearum. The greenhouse experiments using a sterilized soil showed that Colpoda sp. directly consumed R. solanacearum. This indicates that Colpoda sp. had a direct impact on the incidence of disease. Ren et al. (2023) studied the soil microbiome of bulk and rhizosphere soil of sorghum with inorganic and organic fertilizers in the long term. Although their research does not directly prove that protists feed on pathogens, they showed that the decrease in the relative abundance of fungal plant pathogens was significantly correlated with the enhanced relative abundance of the predatory protists and they conclude that rather than bacterial taxa, predatory protist were the predictors of the decrease in the abundance of fungal pathogens. Similarly, a study in the paddy fields showed a negative correlation between the relative abundance of predatory protists and Pythium sp. (Asiloglu et al. 2021c). In conclusion, predatory protists have the potential to directly control plant pathogens, which is likely to be a better solution than the indirect mechanism. Although recently, studies focusing on the direct effect of protists on plant pathogens have been increasing (Sacharow et al. 2023; Guo et al. 2024), more studies are needed to evaluate the efficiency of protists as biocontrol agents. Perhaps, isolating potential biocontrol protist species and testing the efficiency with pot and field experiments would allow the development of the first protist biocontrol agent.
Contradictory section: association of protists with the pathogens
Although the majority of the studies on predatory protist–plant pathogen interactions reported the positive impact of predatory protists, potentially protists can carry, protect, and enhance the survival of pathogenic microorganisms, especially bacteria (Gourabathini et al. 2008). Indeed, the association of foodborne pathogenic bacteria with predatory protists is considered as an important factor for the maintenance of these pathogens in the environment (Vaerewijck et al. 2014). Protists are common in food-related environments and even on foods (Vaerewijck et al. 2014). Since several bacterial species cannot be fully digested by protists, some bacteria can survive and grow inside the food vacuole of predatory protists (Gourabathini et al. 2008) and even escape into the environment (Santos and Enninga 2016). Studies showed that predatory protist species such as Acanthamoeba sp. and Tetrahymena sp. act as a host to foodborne pathogenic bacteria and protect internalized bacteria from desiccation and exposure to disinfectants (Snelling et al. 2006) and low concentrations of calcium hypochlorite (Brandl et al. 2005). Although, so far, no similar case has been reported in the soil ecosystem and almost all of the related studies focused on the foodborne pathogens, similar interactions may occur between predatory protists and soil-borne pathogenic bacteria or fungal spores. Therefore, the possibility of protists being carriers or protectors of plant pathogens should be carefully investigated before they can be used as biocontrol agents. In addition, although so far, no risks of using predatory protists as biocontrol agents have been reported in the soil ecosystem, potentially, predatory protists can feed on beneficial bacteria and enhance the growth of plant pathogens. The risks of using predatory protists as biocontrol agents should be confirmed strictly in further studies.
Isolation and culture
Traditional methods are still used to isolate predatory protists. Briefly, an appropriate dilution of soil can be cultured in 96-well plates including Amoeba Saline nutrient solution (Page 1988) supported with Escherichia coli as a food source, which is one of the most often used methods. Although the dilution rates can be changed depending on soil type and the aim, 50 µL of × 1000 dilution of soil solution and 100 µL Amoeba Saline nutrient solution with E. coli cells (final volume of 107 cells mL) often provide good results. However, the isolation of protists that directly feed on plant pathogens requires some modifications. Two steps are crucially important, the food microorganism and the separation method of different protist species. A small modification in the food source can provide an efficient solution to isolate predatory protists that feed on pathogenic organisms. Using the plant pathogen species, even its spores, as a sole food source would allow almost exclusive growth of predatory protists that can feed on the pathogens (symbiotic protists should be considered). Then serial dilutions till a few or a single cell is left can be efficient enough. Other methods such as the migration method (Neff 1958), centrifugation, and pipetting method (works for big-size protists such as ciliates) can be used for separating different types of protists. Since the separation of the plant pathogens and protists might be tricky, we recommend using heat- or autoclave-killed pathogens in the first step. Once the protist isolation is done, the efficiency of protists to feed on living pathogens can be further checked in the same way. Please note that most likely bacterial cells will accompany the isolated protists and some protist cysts can carry pathogenic bacteria in them which is an issue for potential pathogens (Vaerewijck et al. 2014). Therefore, potential pathogens should be checked in the cultures of the isolated protists. To obtain axenic protist isolates, different methods such as antibiotic treatment can be used (Jones et al. 1973). Isolation of protists that can directly feed on plant pathogens is a key point in conducting experiments. For instance, once several protists are isolated, screening a variety of protist species may help discover which protistan groups suppress which type of plant pathogens. It should be noted that methodology and technology of large-scale cultivation of bacteria and fungi is more advanced than protists and there are still challenges in the large-scale cultivation of protists at the application level compared to bacteria and fungi.
Preparation and application of predatory protists
One of the major problems of the application of microbial biocontrol agents is the variability in efficacy, especially the survival rate; therefore, the effectivity of bacterial and fungal biocontrol agents can be very low (Xiao and Tang 2008). In addition, the application of biocontrol agents in agricultural systems may have negative impacts. For instance, bacterial biocontrol agents producing chitinase or toxins may harm non-target fungi, nematodes, and earthworms (Jangir et al. 2019). On the other hand, predatory protists are known to stimulate microbial activity and enhance soil fertility and plant productivity, while no negative side effects of predatory protists on soil microbial life and soil fertility have been reported so far (Gao et al. 2019). Additionally, the survival rate of predatory protists is much higher in soil compared to the introduced bacterial species. Almost all predatory protists build cysts allowing them to survive under extreme conditions such as lack of food, water, high and low temperatures etc. The cyst stage can make dry formulation possible, which can significantly increase the shelf life of the biocontrol agents. Additionally, the stock solutions can be stored at room temperature making it easy to handle the product and transportation. Once successful results are obtained through in vitro and pot experiments, protist biocontrol agents can be applied to fields with pathogen problems. Taken together, the usage of predatory protists as biocontrol agents potentially has beneficial impacts.
Several application methods can be used similar to bacterial and fungal biocontrol agents. Although we did not test the application methods, potentially, seed coating, introducing protists to irrigation water or drip irrigation systems can be effective. Predatory protists also play a role as catalyzers of organic matter breakdown (Geisen et al. 2021). Indeed, we previously showed that the presence of protists enhanced the positive effect of biochar on plant growth (Asiloglu et al. 2021b). Therefore, potentially, protists can also be applied to fields within organic fertilizers such as biochar and compost. Additionally, protists are known to have synergistic interaction with PGPR and biocontrol agents. For instance, protists enhance the survival of plant growth-promoting bacterial species, therefore, they can also be applied in combination with PGPR species (Asiloglu et al. 2020). In addition, protists are known to enhance the secondary metabolite production of bacterial species, which then suppress pathogens (Jousset et al. 2006, 2010). Although most of the results introduced above are obtained from soil protists, protists are an important component of leaf microbiota as predators (Flues et al. 2017). Therefore, protists can also be used for the potential suppression of leaf pathogens through foliar application.
Conclusion
Despite the vast potential of protists to be used as effective biocontrol agents, our knowledge of microbial biocontrol agents is almost exclusively derived from bacterial and fungal studies. Here we highlighted the potential mechanisms of plant pathogen suppression by predatory protists. Two main mechanisms can be classified as indirect and direct. In the indirect mechanism, predatory protists enhance the activities and populations of biocontrol bacterial species, which then suppress plant pathogens. The direct mechanism involves predatory protists’ predation power directly on plant pathogens. Although the indirect mechanism has been studied relatively better than the direct mechanism, trophic interaction between bacteria and protists is involved in the indirect mechanism, which can be a limitation in obtaining consistent results under different soil types as different soils may have different bacterial communities. The direct mechanism, on the other hand, does not rely on the presence of other microorganisms, however, finding predatory protist species that feed on various plant pathogens can be challenging as prey-predator interaction between protists and plant pathogens can be species-specific. Nevertheless, we believe that predatory protists can be an important solution for the sustainable management of plant pathogens. Since there is a huge knowledge gap in this area, further studies should focus on protist-pathogen interaction and its implications for sustainable plant productivity.
Data availability
A literature review based on the related articles available on the Web of Science, Science Direct, and Google Scholar databases was utilized for the investigation.
References
Asadi M, Millar AA (2024) Review: plant microRNAs in pathogen defense: a panacea or a piece of the puzzle? Plant Sci 341:111993. https://doi.org/10.1016/j.plantsci.2024.111993
Asiloglu R, Kenya K, Samuel SO et al (2021a) Top-down effects of protists are greater than bottom-up effects of fertilisers on the formation of bacterial communities in a paddy field soil. Soil Biol Biochem 156:108186. https://doi.org/10.1016/j.soilbio.2021.108186
Asiloglu R, Sevilir B, Samuel SO et al (2021b) Effect of protists on rhizobacterial community composition and rice plant growth in a biochar amended soil. Biol Fertil Soils 57:293–304. https://doi.org/10.1007/s00374-020-01525-1
Asiloglu R, Shiroishi K, Suzuki K et al (2021c) Soil properties have more significant effects on the community composition of protists than the rhizosphere effect of rice plants in alkaline paddy field soils. Soil Biol Biochem 161:108397. https://doi.org/10.1016/j.soilbio.2021.108397
Asiloglu R, Shiroishi K, Suzuki K et al (2020) Protist-enhanced survival of a plant growth promoting rhizobacteria, Azospirillum sp. B510, and the growth of rice (Oryza sativa L.) plants. Appl Soil Ecol 154:103599. https://doi.org/10.1016/j.apsoil.2020.103599
Bahroun A, Jousset A, Mrabet M et al (2021) Protists modulate Fusarium root rot suppression by beneficial bacteria. Appl Soil Ecol 168:104158. https://doi.org/10.1016/j.apsoil.2021.104158
Bonaterra A, Badosa E, Daranas N et al (2022) Bacteria as biological control agents of plant diseases. Microorganisms 10:1759. https://doi.org/10.3390/microorganisms10091759
Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162:617–631. https://doi.org/10.1111/j.1469-8137.2004.01066.x
Brandl MT, Rosenthal BM, Haxo AF, Berk SG (2005) Enhanced survival of Salmonella enterica in vesicles released by a soilborne Tetrahymena species. Appl Environ Microbiol. https://doi.org/10.1128/AEM.71.3.1562-1569.2005
Chakraborty S, Old KM (1982) Mycophagous soil amoeba: Interactions with three plant pathogenic fungi. Soil Biol Biochem 14:247–255. https://doi.org/10.1016/0038-0717(82)90034-7
Chaloner TM, Gurr SJ, Bebber DP (2021) Plant pathogen infection risk tracks global crop yields under climate change. Nat Clim Chang 11:710–715. https://doi.org/10.1038/s41558-021-01104-8
Dean R, Van KJ, Pretorius ZA et al (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Drechsler C (1936) A Fusarium-like species of Dactylella capturing and consuming testaceous rhizopods. J Wash Acad Sci 26:397–404
Ekelund F (1998) Enumeration and abundance of mycophagous protozoa in soil, with special emphasis on heterotrophic flagellates. Soil Biol Biochem 30:1343–1347. https://doi.org/10.1016/S0038-0717(97)00266-6
Flues S, Bass D, Bonkowski M (2017) Grazing of leaf-associated Cercomonads (Protists: Rhizaria: Cercozoa) structures bacterial community composition and function. Environ Microbiol 19:3297–3309. https://doi.org/10.1111/1462-2920.13824
Gao M, Xiong C, Tsui CKM, Cai L (2024) Pathogen invasion increases the abundance of predatory protists and their prey associations in the plant microbiome. Mol Ecol 33. https://doi.org/10.1111/mec.17228
Gao Z, Karlsson I, Geisen S et al (2019) Protists: Puppet masters of the rhizosphere microbiome. Trends Plant Sci 24:165–176. https://doi.org/10.1016/j.tplants.2018.10.011
Geisen S, Hu S, dela Cruz TEE, Veen GF (Ciska) (2021) Protists as catalyzers of microbial litter breakdown and carbon cycling at different temperature regimes. ISME J 15:618–621.https://doi.org/10.1038/s41396-020-00792-y
Geisen S, Koller R, Hünninghaus M et al (2016) The soil food web revisited: diverse and widespread mycophagous soil protists. Soil Biol Biochem 94:10–18. https://doi.org/10.1016/j.soilbio.2015.11.010
Geisen S, Mitchell EAD, Adl S et al (2018) Soil protists: a fertile frontier in soil biology research. FEMS Microbiol Rev 42:293–323. https://doi.org/10.1093/femsre/fuy006
Gourabathini P, Brandl MT, Redding KS et al (2008) Interactions between food-borne pathogens and protozoa isolated from lettuce and spinach. Appl Environ Microbiol 74:2518–2525. https://doi.org/10.1128/AEM.02709-07
Guo S, Jiao Z, Yan Z et al (2024) Predatory protists reduce bacteria wilt disease incidence in tomato plants. Nat Commun 15:829. https://doi.org/10.1038/s41467-024-45150-0
Guo S, Tao C, Jousset A et al (2022) Trophic interactions between predatory protists and pathogen-suppressive bacteria impact plant health. ISME J 16:1932–1943. https://doi.org/10.1038/s41396-022-01244-5
Jangir M, Sharma S, Sharma S (2019) Target and non-target effects of dual inoculation of biocontrol agents against Fusarium wilt in Solanum lycopersicum. Biol Control 138:104069. https://doi.org/10.1016/j.biocontrol.2019.104069
Jassey VEJ, Walcker R, Kardol P et al (2022) Contribution of soil algae to the global carbon cycle. New Phytol 234:64–76. https://doi.org/10.1111/nph.17950
Jones AK, Rhodes ME, Evans SC (1973) The use of antibiotics to obtain axenic cultures of algae. Brit Phycol J 8:185–196. https://doi.org/10.1080/00071617300650211
Jousset A, Bonkowski M (2010) The model predator Acanthamoeba castellanii induces the production of 2,4, DAPG by the biocontrol strain Pseudomonas fluorescens Q2–87. Soil Biol Biochem 42:1647–1649. https://doi.org/10.1016/j.soilbio.2010.05.018
Jousset A, Lara E, Wall LG, Valverde C (2006) Secondary metabolites help biocontrol strain Pseudomonas fluorescens cha0 to escape protozoan grazing. Appl Environ Microbiol 72:7083–7090. https://doi.org/10.1128/AEM.00557-06
Jousset A, Rochat L, Scheu S, Bonkowski M, Keel C (2010) Predator-prey chemical warfare determines theexpression of biocontrol genes by rhizosphere-associated Pseudomonas fluorescens. Appl Environ Microbiol 76(15):5263–5268. https://doi.org/10.1128/AEM.02941-09
Kaur R, Choudhary D, Bali S et al (2024) Pesticides: an alarming detrimental to health and environment. Sci Total Environ 915:170113. https://doi.org/10.1016/j.scitotenv.2024.170113
Mahé F, de Vargas C, Bass D et al (2017) Parasites dominate hyperdiverse soil protist communities in Neotropical rainforests. Nat Ecol Evol 1:0091. https://doi.org/10.1038/s41559-017-0091
Mansfield J, Genin S, Magori S et al (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Matz C, Kjelleberg S (2005) Off the hook – how bacteria survive protozoan grazing. Trends Microbiol 13:302–307. https://doi.org/10.1016/j.tim.2005.05.009
Murase J, Asiloglu R (2023) Protists: the hidden ecosystem players in a wetland rice field soil. Biol Fertil Soils. https://doi.org/10.1007/s00374-023-01705-9
Neff RJ (1958) Mechanisms of purifying amoebae by migration on Agar surfaces. J Protozool 5:226–231. https://doi.org/10.1111/j.1550-7408.1958.tb02557.x
Old KM, Darbyshire JF (1978) Soil fungi as food for giant amoebae. Soil Biol Biochem 10:93–100. https://doi.org/10.1016/0038-0717(78)90077-9
Old KM, Patrick ZA (1979) Giant soil amoebae, potential biocontrol agents. Soil-Borne Plant Pathogens. Academic Press, London, pp 617–628
Page FC (1988) A new key to freshwater and soil gymnamoebae: with instructions for culture. Freshwater Biological Association, Ambleside, p 121. https://www.semanticscholar.org/paper/A-New-Key-to-Freshwater-and-Soil-Gymnamoebae%2C-with-Schönborn/628edaebf15752ad61724a2e3f24e1a965a3292f
Petz W, Foissner W, Adam H (1985) Culture, food selection and growth rate in the mycophagous ciliate Grossglockneria acuta Foissner, 1980: first evidence of autochthonous soil ciliates. Soil Biol Biochem 17:871–875. https://doi.org/10.1016/0038-0717(85)90149-X
Ren P, Sun A, Jiao X et al (2023) Predatory protists play predominant roles in suppressing soil-borne fungal pathogens under organic fertilization regimes. Sci Total Environ 863:160986. https://doi.org/10.1016/j.scitotenv.2022.160986
Sacharow J, Salehi-Mobarakeh E, Ratering S et al (2023) Control of Blumeria graminis f. sp. hordei on barley leaves by treatment with fungi-consuming protist isolates. Curr Microbiol 80:384. https://doi.org/10.1007/s00284-023-03497-5
Santos JC, Enninga J (2016) At the crossroads: communication of bacteria-containing vacuoles with host organelles. Cell Microbiol 18:330–339. https://doi.org/10.1111/cmi.12567
Snelling WJ, Moore JE, McKenna JP et al (2006) Bacterial–protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect 8:578–587. https://doi.org/10.1016/j.micinf.2005.09.001
Thambugala KM, Daranagama DA, Phillips AJL et al (2020) Fungi vs. fungi in biocontrol: an overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infect Microbiol 10:. https://doi.org/10.3389/fcimb.2020.604923
Trivedi P, Leach JE, Tringe SG et al (2020) Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18:607–621. https://doi.org/10.1038/s41579-020-0412-1
Vaerewijck MJM, Baré J, Lambrecht E et al (2014) Interactions of foodborne pathogens with free-living protozoa: potential consequences for food safety. Compr Rev Food Sci Food Saf 13:924–944. https://doi.org/10.1111/1541-4337.12100
Xiao Y, Tang S (2008) The effect of initial density and parasitoid intergenerational survival rate on classical biological control. Chaos Solitons Fractals 37:1048–1058. https://doi.org/10.1016/j.chaos.2006.10.002
Xiong W, Song Y, Yang K et al (2020) Rhizosphere protists are key determinants of plant health. Microbiome 8:1–9. https://doi.org/10.1186/s40168-020-00799-9
Funding
This research was funded by the Japan Society for the Promotion of Science (JSPS) to Asiloglu R (Grant No. JP22K14804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Xinqi Huang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujino, M., Bodur, S.O., Harada, N. et al. Guardians of plant health: roles of predatory protists in the pathogen suppression. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06846-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06846-w