Abstract
Aims
Late-successional trees have a relatively high growth rate at low temperature, which is important for their competitive advantage over mid-successional trees. However, we have a limited understanding of the underlying mechanism for such an advantage.
Methods
We transplanted two mid-successional tree seedlings (Castanopsis chinensis and Schima superba) and two late-successional species (Machilus chinensis and Cryptocarya chinensis) from Dinghushan (subtropical forest) to South China Normal University. The seedlings were incubated in pots with the same soil and routinely managed with regular watering and weeding. After 7 years of seedling culture, five pots of each tree species were selected for greenhouse cultivation to simulate the effect of low temperature (16 °C) on the studied tree species. A control treatment (ambient temperature) was also established.
Results
After 3 years, low temperatures changed the bacterial and diazotrophic communities, decreased the relative abundance of Actinobacteria in the mid-successional species Schima superba and Castanopsis chinensis, and increased the relative abundance of Rubrivivax in the late-successional species Machilus chinensis and Cryptocarya chinensis. In the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis increased N fixation and net N mineralization rates compared with those in mid-successional S. superba and Castanopsis chinensis. Meanwhile, the relative abundance of the fungal community changed insignificantly, indicating that bacteria play a major regulatory role in nutrient cycling at low temperatures.
Conclusions
Our results suggest that shifts in the bacterial community and changes in functional group structures may play a key role in soil nitrogen cycling during the succession of evergreen broadleaf forest ecosystems at low temperatures during the dry season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the mechanisms of forest community succession is crucial for predicting dynamic changes in vegetation and developing protection strategies (Clements 1916). The main factors promoting forest community succession are natural selection, environmental adaptability, and interactive effects (Chen et al. 2019). Environmental factors have a long-term impact on the community structure and composition of forests and may determine the direction and processes of plant succession (Mesquita et al. 2015). The major environmental factors affecting plant community succession in different regions include light, temperature, water, and nutrient supplies (Meiners et al. 2015; Yu et al. 2021; Zhou et al. 2013).
Temperature is a key driver of plant community dynamics (Gremer et al. 2012; Jiang et al. 2020; Midolo et al. 2019). Seasonal change is a periodic and continuous dynamic process by which plant growth is inevitably affected during summer and winter (Yu et al. 2023). Subtropical forests are particularly sensitive to low temperatures in winter (Cavanaugh et al. 2013; Huang et al. 2010) because they evolved within relatively narrow temperature regimes and are more highly vulnerable to temperature changes (Miller et al. 2021). However, subtropical regions frequently experience lower temperatures for several months in winter owing to their geographical location (Yan et al. 2013). Cold waves can damage some trees in tropical and subtropical forests, which are not adapted to low temperatures (Bojόrquez et al. 2021). Cold waves may cause stress in these subtropical plants, affecting their physiological and metabolic activities and affecting their growth and yield (Hilliard and West 1970; Hou et al. 2018). For example, low temperatures limit many neotropical species in northmost America that are found in northwestern Mexico (Bojόrquez et al. 2021). A short quiescent or inactive period in the wood formation of Chinese red pine was observed in the coldest month of the year in subtropical China (Huang et al. 2018). These findings indicate that low temperatures affect the growth and geographical distribution of plants (Allen and Ort 2001; Boyer 1982).
According to a previous study young leaves of late-successional trees have stronger and more flexible photoprotection strategies against low temperatures than those of mid-successional trees (Yu et al. 2023). When exposed to a low-temperature environment, the subtropical tree species of late-successional Acmena acuminatissima enhanced their photoprotection by accumulating more anthocyanin compared with mid-successional Castanopsis chinensis (Yu et al. 2020). This strongly suggests that the ability to maintain a relatively high growth rate at low temperatures is important for the competitive advantage of late-successional species over mid-successional species (Yu et al. 2023). However, we have a limited understanding of the underlying mechanism for such an advantage when exposed to low temperatures.
Under climate changes, subtropical forests occasionally experience increasing cold waves (Li et al. 2022a), which might alter the rate or trajectory of forest succession (Allen and Ort 2001; Liu et al. 2012). Low temperatures can indirectly limit the growth of many species by changing their root exudates, which affect microbially mediated nutrient transformations (Ahlawat et al. 1998; Liu et al. 2022). Nitrogen (N) plays a pivotal role in the growth of plants because of its intricate involvement in the biosynthesis of diverse biomolecules, including amino acids, nucleic acids, and chlorophyll (Luo et al. 2023). Ammonium (NH4+) and nitrate (NO3−) are the primary inorganic N forms that plants use (Andersen et al. 2017). Different plant species showed differential preferences for these two N forms (Bai et al. 2017). The use of N enhances plants’ ability to survive in adverse environmental conditions (Luo et al. 2023), such as low temperatures (Li et al. 2013). The preference for NH4+ over NO3− significantly increases with decreasing temperatures; below 5 °C, NH4+ uptake occurs and NO3− uptake ceases (Macduff and Jackson 1991). This preference indicates the elevated metabolic energy costs associated with NO3− absorption and assimilation relative to those of NH4+ (Luo et al. 2023). Alternatively, increased NH4+ uptake is related to multiple modifications in plants (e.g., changing membrane electrochemical potential) (Wu et al. 2019).
Biological N fixation by microorganisms from the atmosphere is a crucial process for providing available N to plants and alleviating N limitation in many biomes (Xiao et al. 2023; Zheng et al. 2020). According to succession theory, the highest rates of biological N fixation occur in early- or mid-successional subtropical forests because N restricts plant growth. Conversely, soil N levels are high and biological N fixation rates decline in the late-successional stages (Batterman et al. 2013). Despite the abundance of soil N attributed to chronic N deposition in many subtropical forests, biological N fixation remains active and high N2 fixation rates are sustained (Reed et al. 2011; Zheng et al. 2020). However, the effects of low temperatures in winter on the biological N fixation of tree species at different community succession stages are unclear.
In this study, the experimental subjects consisted of two mid-successional species, Castanopsis chinensis Hance and Schima superba Gardn. et Champ., and two late-successional species, Machilus chinensis (Champ. ex Benth.) Hemsl. and Cryptocarya chinensis (Hance) Hemsl.. All the seedlings were collected from the subtropical evergreen broadleaf forest community of Dinghushan (Guangdong province, China). To explore the effects of low temperatures on diazotroph communities, we performed a controlled experiment with potted plants at the experimental base of South China Normal University. We also investigated the soil diazotroph community composition, soil physicochemical properties, and leaf photosynthetic N- and P-use efficiencies. N fixation rate increases throughout succession in subtropical forests (Zheng et al. 2020). Therefore, we hypothesized that under low temperatures, (1) the composition of soil diazotroph communities changes with different tree species in different forest succession stages; (2) the N fixation rate in late-successional dominant tree species is significantly higher than that in mid-successional dominant tree species mainly because late-successional trees have a relatively higher growth rate than the mid-successional dominant tree species under low temperature in winter; and (3) the leaf photosynthetic N-use efficiencies in late-successional dominant tree species are higher than those in mid-successional dominant tree species. These factors may be key clues to the gradual replacement of mid-successional species by late-successional species.
Materials and methods
Plant materials and growth conditions
Seedlings of mid-successional Castanopsis chinensis and S. superba and late-successional M. chinensis and Cryptocarya chinensis were obtained from the Dinghushan National Nature Reserve (112°30′39″-112°33′41″E, 23°09′21″-23°11′30″N) to South China Normal University (113°20′59″E, 23°8′22″N) in Guangzhou, Guangdong Province, in 2012. For each tree species, approximately 20 seedlings with an average height of approximately 1 m were selected. The plants were planted in pots (50 cm in diameter and 35 cm in depth) with the same soil, sampled from the biological garden of South China Normal University. The soil had an organic matter content of 33.39 g kg−1, total N of 1.11 g kg−1, NH4+_N of 0.84 mg kg−1, and NO3–-N of 4.74 mg kg−1. The climatic conditions at the planting sites mirrored those of the Dinghushan National Nature Reserve, which is characterized by the humid monsoon climate of low-altitude subtropical zones. The seedlings were managed with regular watering and weeding (Yu et al. 2021, 2023). The average day/night growth temperatures were 33 °C/24 °C in summer, and 20 °C/12 °C in winter.
The low-temperature experiments began seven years after seedling sowing. Five pots of each tree species were selected for greenhouse cultivation to simulate the effect of the low temperature in winter (16 °C) on the potential tree species (Fig. 1). Low-temperature cultivation of these plant species was conducted in a greenhouse at South China Normal University in January 2019. The plants and soil were sampled in July 2022, 3 years after the low-temperature treatment.
Soil sampling
Five cores were taken (0–10 cm depth) from each plot in July 2022, and the composite samples were sieved using a 2 mm mesh. A portion of the composite samples was stored at − 80 °C for soil microbial community analysis, and another was preserved at 4 °C to determine the NH4+-N and NO3–-N contents. The remaining soil was air-dried at room temperature for the analysis of chemical properties. The soil gravimetric moisture was tested by drying the soil at 105 °C for 24 h. Extraction from fresh soil filtered with 2 M KCl solution was used to determine soil dissolved organic carbon (DOC) and N (DON) concentrations using a total organic carbon and N analyzer (Multi N/C®2100(S), Analytik Jena AG, Germany). Soil extractable NH4+-N and NO3–-N were measured from 2 M KCl filtered extracts of fresh soil samples using a flow injection autoanalyzer (AA3, SEAL, Germany). The air-dried soils were digested with HF–HClO4 to determine total phosphorus (P). Available P was extracted with ammonium fluoride in hydrochloric acid and then measured using the molybdenum-blue method (Hedley et al. 1982). Soil total carbon and total N were determined using a TOC/TN analyzer (Elementar, varioMACRO cube, Germany).
DNA was extracted from the frozen soil (0.5 g) using a Power Soil DNA Isolation Kit (MoBio, California, USA). The V3-V4 region of the 16S rRNA gene was amplified using the universal primers 338F (5′-ACTCCTACGGGAGGCAGCA -3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) for bacterial community analysis (Li et al. 2022b). The ITS region of the 18S rDNA gene was amplified using the primers ITS1F (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′) for fungal community analysis (Li et al. 2022b). Paired-end sequencing was conducted on an Illumina NovaSeq platform (Personal Biotechnology Co. Ltd., Shanghai, China). Demultiplexed FASTQ files corresponding to the DNA amplicons were processed for the quality-filtering, trimming, dereplication, and merging of paired-end reads into amplicon sequence variants, denoised, and chimera removed using the DADA2 software pipeline (Callahan et al. 2016). Non-singleton amplicon sequence variants (ASVs) were aligned with MAFFT (Katoh et al. 2002). The leveling depth of the total number of ASVs was set to 95% of the minimum sample sequence size using the QIIME feature-table rarefy of QIIME2. Bacterial and fungal ASVs were taxonomically classified with the SILVA Release 130 and UNITE Release 8.0, respectively, with a confidence threshold of 80%. The functional gene nifH of the diazotrophs was amplified using the following specific primer sets: nifH-F (AAA GGY GGW ATC GGY AAR TCC ACC AC) and nifH-R (TTG TTS GCS GCR TAC ATS GCC ATC AT) (Zhang et al. 2021). Raw sequences were subsequently subjected to de-multiplexing, filtration, quality assessment, and clustering following the Vsearch pipeline (Rognes et al. 2016). 16S and 18S rDNA sequence data were deposited in the NCBI Sequence Read Archive with accession numbers PRJNA1028249, 1,028,319, and 1,028,332, respectively.
Nitrogen fixation rates
Nitrogen fixation rates were determined following an acetylene reduction assay and measuring ethylene levels using gas chromatography (Shimadzu, GC-14, Kyoto, Japan) (Hardy et al. 1968). Fresh soil samples were placed in 250 mL glass bottles, with 10% of the headspace replaced with pure C2H2 (99.99%). The bottles were incubated in situ for 24 h at ambient temperature. After incubation, the headspace gas from each bottle was sampled and stored in a 12 mL evacuated exetainer for analysis. The concentration of background C2H4 naturally produced by the samples was also measured. The nitrogen fixation rates per unit mass were expressed as C2H4 production rates (nmol C2H2 g−1 dry weight h−1) (Hardy et al. 1968).
Nitrification rate
In brief, 15 g of soil from each sample was preincubated at room temperature for 1 week and then mixed with 100 mL of 1.5 mM ammonium sulfate. Following incubation for 12 and 24 h, 10-mL slurry samples were centrifuged, and the resulting supernatant was filtered through a membrane with a pore size of 0.45 μm. The NO3− concentration in the supernatant was promptly analyzed using the method described above for measuring KCl-extracted NO3−–N, and the nitrification rate was determined by calculating the increase in slurry NO3− concentration over time (Yao et al. 2011).
Net N mineralization rate
A total of 15 g of soil from each sample was subjected to aerobic incubation at a constant temperature (25 °C) in the dark. After a 7-d incubation, all soil samples were extracted with 2 M KCl. Net N mineralization rates were calculated as the changes in NO3−–N and NH4+-N before and after the 7-d incubation, respectively (Adams et al. 1989).
Plant trait measurements
Light-saturated photosynthetic rate (Pmax) was determined in the youngest fully expanded leaf using a Li-6400 Portable Photosynthesis System (Li-Cor, Lincoln, NE) with a photosynthetic photon flux density of 1500 μmol m−2 s−1, with carbon dioxide in the reference chamber at 400 μmol mol−1. After the stable values of Pmax were recorded, the sample leaves and leaves near them were collected. The leaves were oven-dried at 60 °C for 72 h, weighed, and ground finely. Nitrogen and P concentrations in the leaves were digested with H2SO4–H2O2. Total N was calculated using the Kjeldahl method, and total P was determined using the molybdenum blue method. Photosynthetic N- and P-use efficiencies were calculated as the ratio of Pmax to total N and P content in the leaves.
Statistical analyses
Statistical analyses were performed using SPSS v. 17.0 (SPSS Inc., Chicago, IL). Shapiro–Wilk and Levene tests were used to test for data normality and homogeneity, respectively, before analysis. If the data did not follow a normal distribution, we conducted non-parametric analyses on the soil microbial communities. The effects of succession and temperature and their interaction on soil microbial communities, N cycle, and plant leaf traits were assessed using general linear model analysis. The effects of trees on soil microbial communities, N cycle, and plant leaf traits under control and low-temperature conditions were analyzed using one-way analysis of variance and Tukey’s multiple comparison tests to determine significant results (p < 0.05). The effects of low temperatures on soil microbial communities, N cycle, and plant leaf traits were tested using independent sample t-tests (p < 0.05). Redundancy analysis (RDA) was performed using Canoco 4.5. Structural equation modeling (SEM) was performedusing AMOS 7.0 software to develop a causal understanding of the effects of low temperature and succession on the chemical and biotic properties of the soil. Model fit was assessed by χ2-test, Bentler-Bonnett normed fit index, and goodness-of-fit index.
Results
Soil properties
As illustrated in Table 1, succession significantly affected soil total N (F = 25.5, p < 0.001), total C (F = 11.5, p = 0.002), C:N (F = 35.2, p < 0.001), NH4+–N (F = 27.3, p < 0.001), NO3−–N (F = 20.6, p < 0.001), total P (F = 34.6, p < 0.001), DOC (F = 4.2, p = 0.047), and DON (F = 14.6, p = 0.001). Temperature significantly affected soil C:N (F = 11.9, p = 0.001), NH4+–N (F = 37.9, p < 0.001), NO3−–N (F = 4.6, p = 0.040), available P (F = 6.5, p = 0.015), and DON (F = 12.5, p = 0.001) (Table 1). A significant interaction effect was observed between succession and temperature on soil NH4+–N (F = 33.2, p < 0.001), NO3−–N (F = 10.1, p = 0.003), and DON (F = 24.6, p < 0.001).
In the control treatment, mid-successional S. superba had higher soil total N, NO3−–N, DON, and total P than late-successional M. chinensis and Cryptocarya chinensis (Table 1). However, in the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis had higher soil C:N, NH4+–N and DON than mid-successional S. superba and Castanopsis chinensis (Table 1). Therefore, low-temperatures significantly increased soil NH4+–N and DON in late-successional M. chinensis and Cryptocarya chinensis compared with those in the control.
Soil microbial community
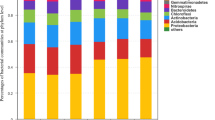
In the bacterial community, succession affected the relative abundances of Proteobacteria (F = 15.6, p < 0.001), Actinobacteria (F = 39.3, p < 0.001), Acidobacteria (F = 9.2, p = 0.004), Bacteroidetes (F = 11.0, p = 0.002),Gemmatimonadetes (F = 4.4, p = 0.043), Firmicutes (F = 7.4, p = 0.010), and Rokubacteria (F = 11.0, p = 0.002). Temperature influenced the relative abundances of Proteobacteria (F = 20.6, p < 0.001), Actinobacteria (F = 13.0, p = 0.001), Chloroflexi (F = 11.7, p = 0.002), Gemmatimonadetes (F = 6.5, p = 0.015), and Rokubacteria (F = 7.1, p = 0.011). An interaction effect was observed between succession and temperature on the relative abundances of Proteobacteria (F = 4.3, p = 0.045), Actinobacteria (F = 12.3, p = 0.001), and Patescibacteria (F = 4.1, p = 0.049).In the control treatment, late-successional M. chinensis increased the relative abundance of Actinobacteria compared with mid-successional S. superba (Fig. 2A). However, in the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis increased the relative abundance of Actinobacteria compared with mid-successional S. superba and Castanopsis chinensis (Fig. 2A). Low temperatures decreased the relative abundance of Actinobacteria in mid-successional S. superba and Castanopsis chinensis but did not affect the relative abundance of Actinobacteria in late-successional M. chinensis and Cryptocarya chinensis. The C:N ratio and total carbon were positively correlated with the relative abundance of Actinobacteria but negatively correlated with total N and P (Fig. 3A).
The taxonomic compositions of bacterial communities (A), fungal communities (B) and diazotroph communities (C) in different treatments. Different uppercase letters indicate significant differences among low temperature (16℃) treatments; different lowercase letters indicate significant differences among control treatment. Asterisks indicate significant differences between the control and low temperature treatments (ns no significant, * p < 0.05, *** p < 0.001)
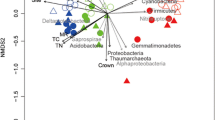
Redundancy analysis (RDA) of the bacterial communities and soil environmental variables (A) and diazotroph communities and soil environmental variables (B). SWC, soil water content; TP, total P; AP, available phosphorus; DOC, dissolved organic carbon; DON, dissolved organic nitrogen; TN, total nitrogen; TC, Total carbon; C:N, total carbon: total nitrogen
In the fungal community, succession affected the relative abundances of Mucoromycota (F = 14.5, p = 0.001) and Calcarisporiellomycota (F = 4.6, p = 0.039). Temperature influences the relative abundances of Basidiomycota (F = 7.1, p = 0.011), Mortierellomycota (F = 12.6, p = 0.001), and Mucoromycota (F = 34.3, p < 0.001). An interaction effect was observed between succession and temperature on the relative abundance of Mucoromycota species (F = 15.9, p < 0.001). Low temperatures did not change the fungal community during the mid-and late-succession stages (Fig. 2B).
In the diazotroph community, succession affected the relative abundances of Solidesulfovibrio (F = 7.8, p = 0.008), Rubrivivax (F = 49.7, p < 0.001), Geobacter (F = 14.8, p < 0.001), Azoarcus (F = 4.4, p = 0.042) and Azohydromonas (F = 11.1, p = 0.002). Temperatures affected the relative abundance of Rubrivivax (F = 4.4, p = 0.042), Methylocystis (F = 6.1, p = 0.019), and Azohydromonas (F = 6.5, p = 0.015). An interaction was observed between succession and temperature on the relative abundances of Rubrivivax (F = 17.2, p < 0.001) and Azohydromonas (F = 6.9, p = 0.013).
In the control treatment, late-successional Cryptocarya chinensis increased the relative abundance of Rubrivivax compared with that of mid-successional S. superba (Fig. 2C). In the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis increased the relative abundance of Rubrivivax compared with mid-successional S. superba and Castanopsis chinensis (Fig. 2C). The C:N ratio, DOC, and total carbon were positively correlated with the relative abundance of Rubrivivax, but negatively correlated with total N and P (Fig. 3B).
Soil N cycle
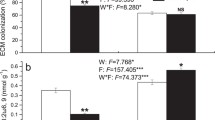
Succession influenced N fixation (F = 8.8, p = 0.005) and net N mineralization rates (F = 16.5, p < 0.001). Furthermore, low temperatures affected N fixation (F = 7.4, p = 0.010), nitrification (F = 16.1, p < 0.001), and net N mineralization rates (F = 17.3, p = 0.000) (Fig. 4). In the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis increased N fixation and net N mineralization rates compared with those in mid-successional S. superba and Castanopsis chinensis (Fig. 4). Soil C:N and DON were the main factors affecting N fixation and net N mineralization rates (Fig. 5a).
The N fixation rates, net N mineralization rate and nitrification rate in different treatments. Different uppercase letters indicate significant differences among low temperature (16℃) treatments; different lowercase letters indicate significant differences among control treatment. Asterisks indicate significant differences between the control and low temperature treatments (** p < 0.01)
Structural equation model showing the effects of succession and low temperature on soil chemical properties, biological process and plant performance. Numbers on arrows are standardized regression coefficients. (a) χ2 = 10.593, df = 10, p = 0.390, NFI = 0.944, GFI = 0.938, RMSEA = 0.039. Arrow represent positive (green solid line) significant path coefficients. The thickness represents the magnitude of the path coefficients. *p < 0.05, **p < 0.05, ***p < 0.001. (b) χ2 = 12.054, df = 6, p = 0.061, NFI = 0.934, GFI = 0.928, RMSEA = 0.161. Arrow represent positive (green solid line) significant path coefficients. The thickness represents the magnitude of the path coefficients. The compositions of diazotroph and bacterial community were represented by the first component of principal component analysis. *p < 0.05, **p < 0.05, ***p < 0.001
Plant trait measurements
Succession affected N (F = 27.5, p < 0.001) and P concentrations (F = 8.3, p = 0.007), as well as Pmax (F = 44.8, p < 0.001) in leaves. Temperature influenced N (F = 11.7, p = 0.002) and P concentrations (F = 19.0, p < 0.001), Pmax (F = 59.8, p < 0.001), and photosynthetic N-use efficiency (PNUE) (F = 122.3, p < 0.001) in leaves. An interaction effect was observed between succession and temperature on P concentration (F = 9.357, p = 0.004), Pmax (F = 24.100, p < 0.001), PNUE (F = 16.8, p < 0.001), and photosynthetic P-use efficiency (PPUE) (F = 8.4, p = 0.006) in leaves (Fig. 6).
Differences in (A) nitrogen (N) and (B) phosphorus (P) concentration, (C) leaf light-saturated photosynthetic rate (P max), (D) photosynthetic N-use efficiencies (PNUE) and (E) photosynthetic P-use efficiencies (PPUE) in different treatments. Different uppercase letters indicate significant differences among low temperature (16℃) treatments; different lowercase letters indicate significant differences among control treatment. Asterisks indicate significant differences between the control and low temperature treatments (* p < 0.05, ** p < 0.01, *** p < 0.001)
The N contents in the leaves of mid-successional plants were higher than those of the late-successional M. chinensis in the control treatment (Fig. 6). In the low-temperature treatment, the N and P concentrations in S. superba were higher than those in the other trees (Fig. 6). In the control treatment, mid-successional S. superba and Castanopsis chinensis had higher Pmax than late-successional M. chinensis and Cryptocarya chinensis. However, insignificant differences in Pmax were observed among these trees in the low-temperature treatments (Fig. 6).
In the control treatment, mid-successional Castanopsis chinensis significantly increased PNUE compared with late-successional M. chinensis and Cryptocarya chinensis. However, in the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis had higher PNUE than mid-successional S. superba and Castanopsis chinensis. Diazotroph composition positively affected N fixation rate and PNUE (Fig. 5b). In particular, late-successional Cryptocarya chinensis had higher PPUE than mid-successional S. superba (Fig. 6).
Discussion
Effect of low temperature on the soil microbial community
Low-temperature experiments predicted the impact of winter on tree growth at different successional stages in subtropical forests. Changes in aboveground plants cause variations in soil environmental factors, resulting in changes in bacterial and fungal communities (Jiang et al. 2021). In this study, low temperatures significantly decreased the relative abundance of Actinobacteria in mid-successional S. superba and Castanopsis chinensis. However, the relative abundance of Actinobacteria in late-successional M. chinensis and Cryptocarya chinensis was not a effected significantly (Fig. 2). These results are mainly attributed to late-successional trees have a high level of dissolved organic N and exhibiting a positive effect on Actinobacteria (Fig. 3). Alternatively, the relative abundance of Actinobacteria was significantly and positively correlated with soil C:N, supporting the conclusion that the C:N ratio is positively related to the abundance of Actinobacteria (Liu et al. 2020; Wu et al. 2023). Actinobacteria are Gram-positive bacteria that are widely distributed in the soil and play vital roles in organic matter turnover and N cycling (Boubekri et al. 2022). Actinobacteria are adaptable to environmental changes, leading to their wide distribution in the biosphere (including extremophiles) (Zenova et al. 2012). Moreover, they can grow and metabolize under low-temperature conditions (Manucharova et al. 2007). These properties may have provided the phylum with a superior adaptation mechanism to low-temperature environments during late-successional stages.
In this study, the fungal community was not affected by succession, which is inconsistent with a previous finding stating that the fungal community responds to changes in the succession of boreal forests (Jiang et al. 2021). Fungi may play a major regulatory role in N cycling during succession (Jiang et al. 2021). In our study, the N cycle was mainly regulated by bacteria. In the low-temperature treatment, late-successional M. chinensis and Cryptocarya chinensis increased the relative abundance of Rubrivivax compared with mid-successional S. superba and Castanopsis chinensis (Fig. 2C). This result is consistent with the first hypothesis that the community composition of soil diazotrophs varies under low temperatures with different forest succession stages. The C:N ratio, DOC, and total carbon were positively correlated with the relative abundance of Rubrivivax (Fig. 3). Thus, the soil diazotrophic community was controlled by soil C:N, which is consistent with a previous study finding that N fixation was controlled by substrate C:N stoichiometry (Zheng et al. 2020). Ecological stoichiometry is important in explaining soil N fixation and forest succession.
Effect of low temperature on soil N fixation rates
Consistent with our hypothesis, we found that N fixation rates increased during the late-succession stage at low temperatures. This finding also agrees with a previous study showing that N fixation remains active in N-rich forests (Menge et al. 2009). At low temperatures, N fixation rates in late-successional plants were higher than those in mid-successional plants, which is consistent with a previous finding stating N fixation rates were the highest in late-successional forests (Zheng et al. 2020). This phenomenon might be due to the high C:N ratio at the late-successional stages, as confirmed using structural equation modeling (Fig. 5). The results are the same as those of a previous study, in which the N fixation rate was significantly positively correlated with C:N in forest soils (Li et al. 2023).
Several species of Actinobacteria are free-living diazotrophs that play a substantial role in nitrogen supply to ecosystems (Boubekri et al. 2022). At low temperatures, the relative abundance of Actinobacteria higher in late-successional forests than in mid-successional habitats. A previous study suggested that actinorhizal symbioses between nonleguminous plants and Actinomycete bacteria are common in temperate and boreal forests (Menge et al. 2009). Our results suggest that the relative abundance of Actinobacteria was positively correlated with N fixation rates (r = 0.469, p = 0.002), indicating that bacteria may play a major regulatory role in N cycling during successional processes at low temperatures. Thus, late-successional trees could acquire additional soil nitrogen. Our findings are inconsistent with those of a previous study, in which the relative abundance of the bacterial functional groups participating in the nitrogen cycle did not show significant variation during the succession of boreal forests (Jiang et al. 2021). In addition, fungi play a major regulatory role in N cycling during the succession process because of the high carbon content in boreal forests (Jiang et al. 2021).
In the soil diazotrophic community, the key diazotrophic genus, Rubrivivax, significantly affected the N fixation rate (r = 0.558, p = 0.000). Rubrivivax is a photosynthetic species mediated primarily by nitrogenase (Fu et al. 2023), implying its critical role in maintaining the structure and function of ecological communities.
Effect of low temperature on plant traits and succession
Under control conditions, the mid-successional trees had a higher Pmax, which may have contributed to their high relative growth rate (Miller et al. 2021). However, low temperatures significantly decreased their Pmax, possibly due to low temperature-induced photoinhibition and decreased photosynthetic efficiency (Zhang and Scheller 2004). Low temperatures had a strong negative net effect on photosynthetic nutrient use efficiency in the species because tropical forests evolved within relatively narrow temperature regimes, making them potentially vulnerable to temperature changes (Miller et al. 2021). However, in the low-temperature treatment, the leaves of late-successional trees had higher PNUE than those of mid-successional trees. To our knowledge, this study is the first to report that the efficiency of photosynthetic nutrient use increases with succession at low temperatures. These traits may facilitate late-successional tree acclimation to the low temperatures in winter. Our results indicate that late-successional trees have high leaf photosynthetic N-use efficiencies, contributing to forest succession at low temperatures.
Conclusion
The effect of low temperatures on forest community succession can be explained in two ways. First, at low temperatures, late-successional trees produce belowground feedback that enhances their growth potential by accelerating the cycling of soil nutrients, particularly nitrogen, primarily through alterations in the community structure of functional microorganisms, such as free-living N fixation bacteria. Second, late-successional trees exhibit high leaf photosynthetic N-use efficiencies at low temperatures, contributing to forest succession. Competitive advantage under low temperatures plays an important role in determining subtropical forest succession from mid- to late-succession. Given that the litter layer is an essential component that bridges vegetation and soils, nitrogen fixation by plant litter could contribute effectively to the soil nutrient pool. Further studies are warranted to understand the effects of plant litter on nitrogen fixation and soil NH4+–N during forest succession.
Data availability
The data will be made available on request.
References
Adams MA, Polglase PJ, Attiwill PM, Weston CJ (1989) In situ studies of nitrogen mineralization and uptake in forest soils: some comments on methodology. Soil Biol Biochem 21:423–429
Ahlawat A, Jain V, Nainawatee HS (1998) Effect of low temperature and rhizospheric application of naringenin on pea-Rhizobium leguminosarum biovar viciae symbiosis. J Plant Biochem Biot 7:35–38
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6:36–42
Andersen KM, Mayor JR, Turner BL (2017) Plasticity in nitrogen uptake among plant species with contrasting nutrient acquisition strategies in a tropical forest. Ecology 98:1388–1398
Bai J, Jia J, Huang C, Wang Q, Wang W, Zhang G, Cui B, Liu X (2017) Selective uptake of nitrogen by Suaeda salsa under drought and salt stresses and nitrogen fertilization using 15 N. Ecol Eng 102:542–545
Batterman SA, Hedin LO, van Breugel M, Ransijn J, Craven DJ, Hall JS (2013) Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502:224–227
Bojόrquez A, Martínez-Yrízar A, Alvarez-Yépiz JC (2021) A landscape assessment of frost damage in the northmost Neotropical dry forest. Agr Forest Meteorol 308–309:108562
Boubekri K, Soumare A, Mardad I, Lyamlouli K, Ouhdouch Y, Hafidi M, Kouisni L (2022) Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol res 261:127059
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Cavanaugh KC, Kellner JR, Forde AJ, Gruner DS, Parker JD, Rodriguez W, Feller IC (2013) Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. P Natl Acad Sci USA 111:723–727
Chen L, Swenson NG, Ji NN, Mi XC, Ren HB, Guo LD, Ma KP (2019) Differential soil fungus accumulation and density dependence of trees in a subtropical forest. Science 366:124–128
Clements FE (1916) Plant succession: an analysis of the development of vegetation. Carnegie Institution of Washington Publishing, Washington
Fu X, Ma Y, Wang D, Zhan L, Guo Z, Fan K, Yang T, Chu H (2023) Long-term chemical fertilization results in a loss of temporal dynamics of diazotrophic communities in the wheat rhizosphere. Sci Total Environ 875:162663
Gremer JR, Kimball S, Angert AL, Venable DL, Huxman TE (2012) Variation in photosynthetic response to temperature in a guild of winter annual plants. Ecology 93:2693–2704
Hardy RW, Holsten R, Jackson E, Burns R (1968) The acetyleneethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Hilliard JH, West SH (1970) Starch accumulation associated with growth reduction at low temperatures in a tropical plant. Science 168:494–496
Hou YP, Zhang MF, Liu SR, Sun PS, Yin LH, Yang TL, Li YD, Li Q, Wei XH (2018) The hydrological impact of extreme weather-induced forest disturbances in a tropical experimental watershed in South China. Forest 9:734
Huang JG, Guo X, Rossi S, Zhai L, Yu B, Zhang S, Zhang M (2018) Intra-annual wood formation of subtropical Chinese red pine shows better growth in dry season than wet season. Tree Physiol 38:1225–1236
Huang W, Zhang SB, Cao KF (2010) The different effects of chilling stress under moderate light intensity on photosystem II compared with photosystem I and subsequent recovery in tropical tree species. Photosynth Res 103:175–182
Jiang C, Ryu Y, Wang H, Keenan TF (2020) An optimality-based model explains seasonal variation in C3 plant photosynthetic capacity. Glob Chang Biol 26:6493–6510
Jiang S, Xing Y, Liu G, Hu C, Wang X, Yan G, Wang Q (2021) Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol Biochem 161:108393
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Li N, Chang R, Jiang H, Tariq A, Sardans J, Peñuelas J, Sun F, Zhou X (2022a) Combined livestock grazing-exclusion and global warming decreases nitrogen mineralization by changing soil microbial community in a Tibetan alpine meadow. Catena 219:106589
Li X, Luo X, Wang A, Chen W, Huang Q (2023) Control factors of soil diazotrophic community assembly and nitrogen fixation rate across eastern China. Geoderma 432:116410
Li X, Zhang YJ, Gao H, Ding T (2022b) Extreme cold wave in early November 2021 in China and the influences from the meridional pressure gradient over East Asia. Adv Clim Chang Res 13:797–802
Liu C, Gong X, Dang K, Li J, Yang P, Gao X, Deng X, Feng B (2020) Linkages between nutrient ratio and the microbial community in rhizosphere soil following fertilizer management. Environ Res 184:109261
Li SX, Wang ZH, Stewart BA (2013) Responses of crop plants to ammonium and nitrate N. Adv Agron 118:205–397
Liu Y, Evans SE, Friesen ML, Tiemann LK (2022) Root exudates shift how N mineralization and N fixation contribute to the plant-available N supply in low fertility soils. Soil Biology Biochem 165:108541
Liu YF, Qi MF, Li TL (2012) Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery. Plant Sci 196:8–17
Luo L, Zhao C, Zheng D, Wang E, Liang J, Yin C (2023) Nitrogen uptake preference and allocation in Populus cathayana in response to drought stress. Environ Exp Bot 213:105415
Macduff JH, Jackson SB (1991) Growth and preferences for ammonium or nitrate uptake by barley in relation to root termperature. J Exp Bot 42:521–530
Manucharova NA, Vlasenko AN, Stepanov AL (2007) Temperature as an autoecological factor of chitinolytic microbial complex formation in soils. Biol Bull 34:163–169
Meiners SJ, Cadotte MW, Fridley JD, Pickett STA, Walker LR, Thompson K (2015) Is successional research nearing its climax? New approaches for understanding dynamic communities. Funct Ecol 29:154–164
Menge Duncan NL, Levin Simon A, Hedin Lars O (2009) Facultative versus obligate nitrogen fixation strategies and their ecosystem consequences. Am Nat 174:465–477
Mesquita RdCG, Massoca PEdS, Jakovac CC, Bentos TV, Williamson GB (2015) Amazon rain forest succession: stochasticity or land-use legacy? Bioscience 65:849–861
Midolo G, De Frenne P, Holzel N, Wellstein C (2019) Global patterns of intraspecific leaf trait responses to elevation. Glob Chang Biol 25:2485–2498
Miller BD, Carter KR, Reed SC, Wood TE, Cavaleri MA (2021) Only sun-lit leaves of the uppermost canopy exceed both air temperature and photosynthetic thermal optima in a wet tropical forest. Agr Forest Meteorol 301–302:108347
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol S 42:489–512
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. Peer J 4:e2584
Wu X, Liu T, Zhang Y, Duan F, Neuhäuser B, Ludewig U, Schulze WX, Yuan L (2019) Ammonium and nitrate regulate NH4+ uptake activity of Arabidopsis ammonium transporter AtAMT1;3 via phosphorylation at multiple C-terminal sites. J Exp Bot 70:4919–4930
Wu X, Xing H, Wang X, Yang J, Chen J, Liu X, Dai D, Zhang M, Yang Q, Dong S, Liu Y (2023) Changes in soil microbial communities are linked to metal elements in a subtropical forest. Appl Soil Ecol 188:104919
Xiao D, He X, Xu Z, Bai SH, Zhang W, Hu P, Chen M, Wang K (2023) Strong cooperations among diazotroph and arbuscular mycorrhizal fungi taxa promote free-living nitrogen fixation at soil-rock mixing layer. Geoderma 437:116600
Yan JH, Zhang YP, Yu GR, Zhou GY, Zhang LM, Li K, Tan ZH, Sha LQ (2013) Seasonal and inter-annual variations in net ecosystem exchange of two old-growth forests in southern China. Agri Forest Meteorol 182–183:257–265
Yao H, Gao Y, Nicol GW, Campbell CD, Prosser JI, Zhang L, Han W, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microb 77:4618–4625
Yu ZC, Lin W, Zheng XT, Chow WS, Luo YN, Cai ML, Peng CL (2020) The relationship between anthocyanin accumulation and photoprotection in young leaves of two dominant tree species in subtropical forests in different seasons. Photosynth Res 149:41–55
Yu ZC, Zheng XT, Lin W, Yan GZ, He W, Luo YN, Lin XL, Zhu H, Peng CL (2023) Differences in leaf photoprotection strategies of tree species at different successional stages in subtropical forests under seasonal climate change and their relationship to construction cost strategies. Environ Exp Bot 207:105230
Yu ZC, Lin W, Zheng XT, Cai ML, Zhang TJ, Luo YN, Peng CL (2021) Interpretation of the difference in shade tolerance of two subtropical forest tree species of different successional stages at the transcriptome and physiological levels. Tree Physiol 41:1669–1684
Zenova GM, Kozhevin PA, Manucharova NA, Dubrova MS, Zvyagintsev DG (2012) Temperature as a factor of development of psychrotolerant mycelial bacteria complexes in soils of north regions. Biol Bull 39:416–422
Zhang J, Zheng M, Zhang Y, Wang J, Shen H, Lin Y, Tang X, Hui D, Lambers H, Sardans J, Peñuelas J, Liu Z (2021) Soil phosphorus availability affects diazotroph communities during vegetation succession in lowland subtropical forests. Appl Soil Ecol 166:104009
Zhang S, Scheller HV (2004) Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol 45:1595–1602
Zheng M, Chen H, Li D, Luo Y, Mo J (2020) Substrate stoichiometry determines nitrogen fixation throughout succession in southern Chinese forests. Ecol Lett 23:336–347
Zhou G, Peng C, Li Y, Liu S, Zhang Q, Tang X, Liu J, Yan J, Zhang D, Chu G (2013) A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob Chang Biol 19:1197–1210
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32171493).
Author information
Authors and Affiliations
Contributions
CL Peng conceived the ideas and designed methodology; F Sun collected the data, analyzed the data and led the writing of the manuscript; W Lin collected the data, review & editing. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Stavros D. Veresoglou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, F., Lin, W., Deng, G. et al. Low temperature-mediated changes in soil free-living nitrogen fixation functional groups in tree species at different successional stages in subtropical forests. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06724-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06724-5