Abstract
Aims
Plastic films efficiently control weed development in agriculture but may have environmental impacts, including alterations of the soil functioning and its microbiota. Canvases made of plant fibres are promising biodegradable alternatives showing uniform soil covering like plastic films, unlike straw mulching which is often laid unevenly on the ground. Hemp is particularly interesting for its resistance and possible effects on the soil microbiota. We tested the effect of several mulches differing in their biodegradability and homogeneity (uniform/uneven soil covering) on soil functioning and crop yield.
Methods
In greenhouse, we assessed the effects of different mulching on lettuce yields, soil properties (temperature, moisture, enzymatic activities) and the soil microbiota. We cropped lettuces either on bare soil (control), a homogeneous non-biodegradable mulch (plastic film), a biodegradable heterogeneous mulch (hemp straw) and a biodegradable and homogenous mulch (hemp canvas).
Results
Plastic film increased soil temperature, decreased most enzymatic activities, and altered the soil microbiota composition. The hemp canvas decreased fungal diversity, while increasing soil moisture, laccase activity, and the abundance of specific Ascomycota, Proteobacteria and Actinobacteria taxa. Plastic and hemp canvas gave similar lettuce yields.
Conclusions
Mulching with plastic films and hemp canvases changed soil functioning (C cycle enzymatic activities) and the soil microbiota. Although similar lettuce yields were obtained, effects of the plastic film were likely mediated by the increased soil temperature and accelerated organic matter degradation, while effects of the hemp canvas resulted from increased soil moisture and recalcitrant matter degradation, combined with the stimulation of potentially beneficial soil microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Weed control in farmed fields is a recurring issue in modern farming (Bagavathiannan et al. 2019). Weeds compete with crops for resources and may host pests and phytopathogens, resulting in yield losses (Chikowo et al. 2009). Mulching consists in covering a soil; it is an efficient solution to prevent weed germination and growth by creating a physical barrier that restricts access to light (El-Beltagi et al. 2022), and responds to the public demand to diminish herbicide use (Mogensen and Spliid 1995).
Mulching with plastic films is a popular and affordable way to control weeds (Mugalla et al. 1996; Steinmetz et al. 2016), especially in vegetable farms (Beriot et al. 2021) and organic farming (Bond and Grundy 2001). Amongst their known advantages besides efficient weed control, plastic films i) are robust toward weathering, so that perennial crops can be grown (Hablot et al. 2014), ii) increase soil surface temperature (Liu et al. 2010), iii) increase water retention when irrigation is applied below the mulch, while also reducing evaporation – and this improves water use efficiency (Li and Xiao 1992; Wang et al. 2009; Zhang et al. 2017) –, iv) accelerate plant maturation, harvest, yields, and sometimes quality (Ricotta and Masiunas 1991; Lament 1993; Laugale et al. 2015; Zhang et al. 2017; Li et al. 2022), and v) are potentially involved in pest damage reduction (Mac-Kenzie and Duncan 2001; Nottingham and Kuhar 2016). It is important to note that most of the advantages listed above are due to the fact that plastic films create a physical barrier that homogeneously covers soils. Therefore, homogeneous soil covering is a desired and important feature of mulching.
Plastic mulching also has disadvantages because i) it accelerates soil organic matter turnover and depletion (Lee et al. 2018), which may increase (Nan et al. 2016) or decrease (Zhao et al. 2022) CO2 emissions depending on the context, ii) it sorbs and releases agrochemicals and additives (Nerín et al. 1996; Wang et al. 2019), iii) it repels rainwater, which may increase runoff and soil erosion (Rice et al. 2004), iv) it induces costs associated to its removal, resulting in no net labour savings for farmers (Schonbeck 1999; Berger et al. 2013; Steinmetz et al. 2016), v) its incineration generates toxic greenhouse gases (Van Ruijven and Van Vuuren 2009), vi) the consequences of its direct incorporation into agricultural soils are unknown (e.g., biodegradable plastic mulches, Bandopadhyay et al. 2018), vii) it accumulates in the environment (Derraik 2002; Zhou et al. 2019) and is forecast to persist over the long term (Barnes et al. 2009), viii) its fragmentation into micro/nano-plastics may affect different trophic levels (Steinmetz et al. 2016; Bradney et al. 2019; Iqbal et al. 2020; Beriot et al. 2021), including the soil microbiota (Shen et al. 2016; Wang et al. 2016), and ix) it presents a potential risk for human health (Lehner et al. 2019). In Europe, a reduction of all “single-use” plastic-based products was announced in 2019 (European Parliament 2019), calling for soil mulching alternatives in agriculture.
Bio-sourced and biodegradable mulches are promising solutions for sustainable agriculture. In particular, canvases generated from woven plant fibres are solid, homogeneous and biodegradable mulching alternatives to plastic films (Tan et al. 2016). Amongst available plant materials, hemp (Cannabis sativa L.) is of prime interest (Schluttenhofer and Yuan 2017) due to i) its eco-friendly and sustainable cropping properties (e.g. weed control in crop rotations; Montford and Small 1999; Poisa and Adamovics 2010), ii) the resistance and durability of its high-quality fibres (Schluttenhofer and Yuan 2017) used to manufacture homogeneous canvases that fully cover the soil, and iii) its beneficial properties when composted and integrated in soils (Dresbøll and Jakob Magid 2006). Studies have suggested allelopathic effects of hemp fibres on soil microbes (Pudelko et al. 2014; Agnieszka et al. 2016) through the release of secondary metabolites (e.g., non-toxic phytocannabinoids, terpenes, and phenolic compounds, see Schluttenhofer and Yuan 2017). While the exact mechanisms are not fully understood, trophic and ecotoxicological effects of hemp biodegradation on soilborne living organisms (Radwan et al. 2008; Nissen et al. 2010; Frassinetti et al. 2018; Scott et al. 2018) and their activity (Van der Werf et al. 1995; Winston et al. 2014; Aubin et al. 2015; Ahmad et al. 2016) are often reported. However, no study has as yet compared soil mulching with plastic film versus hemp canvas during crop growth.
Considering the crucial role of microbes in agricultural soil functioning (Wall et al. 2012) and the effects of mulching practices on soil microbial activities (Li et al. 2022), our objective was to assess the effect of several mulches differing in their homogeneity and biodegradability on the soil microbiota, and explore potential connections with key abiotic (temperature, moisture) and biotic (enzymatic activities) soil functioning parameters and crop yield. For that purpose, we cropped lettuces in mesocosms under greenhouse conditions either on bare soil (control), a homogeneous non-biodegradable mulch (plastic film: the current reference agricultural practice), a biodegradable heterogeneous mulch (hemp straw: same density as the canvas) and a biodegradable and homogenous mulch (hemp canvas: made of woven fibres). We first hypothesized that plastic film and hemp canvas would have similar effects on soil properties because they are both homogeneous mulching methods (H1: homogeneity hypothesis). We also hypothesized that hemp straw and canvas will have similar effects on the soil properties because they are both biodegradable mulching methods (H2: biodegradability hypothesis). We also hypothesized that the hemp canvas would result in unique effects coming from the interaction between its homogeneity and biodegradability properties, that would distinguish it from the plastic and hemp straw mulches (H3: interaction hypothesis). We assumed that i) the plastic film and hemp canvas would have a physical effect due to their homogeneity and full covering of the soil, likely on the soil water content and temperature, ii) the hemp straw and canvas mulches would have a nutrient and/or allelopathic effect on the soil microbiota due to hemp biodegradability, and iii) an interaction between mulch homogeneity and biodegradability should be observed with the hemp canvas treatment. The effects of soil mulching were assessed on important agronomical traits such as lettuce yield and soil temperature and moisture, which are both important drivers of plant growth. Together with the analysis of the soil microbial community composition (bacteria and fungi), we measured important enzymatic activities of the carbon (labile vs recalcitrant sources), nitrogen, phosphate and sulphur cycles involved in organic matter decomposition, plant nutrient provision, and soil health (Sainju et al. 2022; Jian et al. 2016).
Materials and methods
Soil sampling, crop choice and greenhouse settings
The soil was sampled in an organic vegetable farm involved in the project that funded this research work. The soil was a sandy agricultural soil. It was sampled in May 2019 in Auxonne, France (47°11′08.1"N; 5°24′05.9"E) and brought to the Plant Phenotyping Platform for Plant-Microbe Interaction 4PMI greenhouse platform, INRAE Bourgogne Franche-Comté. The physical and chemical composition of the soil is provided in supporting Table S1. The preceding crop was tomato (Solanum lycopersicum L.). The soil was dried at room temperature, sieved to 2 mm, and stored in a sealed box. Twenty mesocosms (17 × 27x37cm) containing 17 kg of dry soil were set up (Fig. S1). The containers were pierced and placed on individual trays to allow sub-irrigation and drainage. The soil was rewetted to 80% of the relative water content (RWC). Four mulching treatments were applied in five biological replicates: i) no mulching (bare soil control); ii) plastic films (30 µm, ~ 29 g/m2, CELLOPLAST SA, Val-du-Maine, France); iii) hemp straw partially covering the soil surface; iv) hemp canvas fully covering the soil surface. Hemp straw and canvas were applied at the same density (hemp density 400 g/m2, Geochanvre F SA, Lézinnes, France, https://www.geochanvre.fr/). The mesocosms were randomly distributed on three tables and left to stabilise for two weeks. Four young lettuce seedlings (Lactuca sativa var. Isadora, Provence Plant SA, Tarascon, France) were transplanted in each corner of each mesocosm; the mulch was cut if necessary (Fig. S1). This lettuce variety is typically used and grown on this soil by the farmer. The lettuces were grown four weeks (16 h day light, 18 °C nighttime, 22 °C daytime). Watering was applied from the top every day by generous mist spraying above the lettuces, and from the bottom once a week by sub-irrigation to recover 80% RWC. The containers were randomised every week.
Soil parameter recording
During the growth period, one mesocosm of each mulching treatment was equipped with a probe buried ~ 10 cm in the soil (SD12, Campbell Scientific Ltd, Vincennes, France) to monitor relative humidity and temperature (Fig. S1). The probes were connected to a custom portable device for data acquisition, and the data were extracted and analysed with Rgui software (R Core Team 2021). Humidity was expressed as a percentage of the soil RWC, and presented as the overall mean calculated based on the averaged hourly records. As for soil temperature, day and night trends were similar. Consequently, data are showed as the overall mean calculated based on averaged daily day and night temperature records.
Lettuce yield
Lettuce shoots were collected at harvest, weighed (fresh shoot weight) and dried (50 °C, 48 h). Roots were extracted, washed thoroughly and dried (50 °C, 48 h) to obtain the dry weight. The dry shoot weight was divided by the dry root weight to obtain the shoot-root ratio.
Soil DNA extraction, amplicon preparation, sequencing and bioinformatic analysis
At harvest, 50 g of homogeneous bulk soil free from roots were sampled in the middle of each mesocosm and stored at -20 °C. Soil metagenomic DNA was extracted from 250 mg of soil using a DNeasy PowerSoil-htp 96 well DNA isolation kit (Qiagen, France). Bacterial and fungal diversity were obtained by sequencing the V3-V4 hypervariable regions of the bacterial 16S rRNA gene (small subunit of the ribosomal operon), using primers Pro341F and Pro805R (Baker et al. 2003; Herlemann et al. 2011) and the Internal Transcribed Spacer 2 (ITS2) region in the ribosomal operon of fungi, using primers ITS-3F and ITS-4R (White et al. 1990; Ihrmark et al. 2012) via Illumina Miseq 2 × 250 bp paired-end analysis. We analysed the 16S rRNA and ITS2 sequences using an OTU pipeline, as previously described (Jacquiod et al. 2022). For the sake of simplicity, the 16S rRNA gene and ITS amplicon datasets are referred to in the text as bacterial and fungal community datasets despite the presence of minor archaeal and protist sequences, respectively. More details on the sequencing procedure is provided in the supplementary file. The list and description of the sequenced samples is provided in Table S2. Raw sequences were submitted to the Sequence Read Archive (SRA) public repository (16S dataset: PRJNA999557; ITS2 dataset: PRJNA999582).
Soil enzymatic activities
At harvest, 500 g of rootless homogeneous fresh soil material were sampled in the middle of each mesocosm, placed in sealed plastic bags and shipped to Biochem-Env Platform (Versailles, France). A standardised ISO procedure was applied (ISO20130 2018; Cheviron et al. 2022), including an estimation of the total water content of each soil sample in order to standardise enzymatic activities based on dry soil weight. The following activities were measured: xylanase, cellulase, β-glucosidase for the C cycle; phosphatase, alkaline phosphatase, phosphodiesterase for the P cycle; arylamidase for the N cycle; and arylsulphatase for the S cycle. Laccase activity was measured to estimate recalcitrant organic matter degradation with an adapted protocol (Eichlerová et al. 2012). Soil respiration was used as a proxy of the global metabolic activity and monitored with the MicroResp™ method (Campbell et al. 2003).
Univariate statistical analysis
The effects of mulch homogeneity, mulch biodegradability and their potential interaction were tested on all recorded variables with the two-factor model “ ~ homogeneity*biodegradability” with the following attributes for the four treatments: bare soil control (homogeneous effect = No; biodegradation effect = No); ii) plastic film (homogeneous effect = Yes; biodegradation effect = No); iii) hemp straw mulch (homogeneous effect = No; biodegradation effect = Yes); iv) hemp canvas (homogeneous effect = Yes; biodegradation effect = Yes). Statistical analyses were performed with Rgui software (R Core Team 2021). Normality and variance homogeneity were verified using Shapiro and Bartlett tests, respectively. If normality was kept, significance was inferred from ANOVA under Tukey’s honestly significant difference (HSD) post-hoc test to compare the four mulching treatments (package agricolae, de Mendiburu 2019). If normality was rejected, significance was inferred from the non-parametric Scheirer-Ray-Hare test under Dunn’s post-hoc test to compare the mulching treatments. This method tests interactions between two factors and estimates variability partition (Sokal and Rohlf 1995; Jacquiod et al. 2022). Data was expressed as averaged values plus/minus the standard error of the mean (± SEM). The detailed variance analysis of all variables is presented in Table S3.
Multivariate analysis of the soil microbiota
Microbiota diversity coverage was assessed with rarefactions curves (Fig. S2). The samples were normalised by random resampling (n = 17,000 for the 16S rRNA gene, n = 3,000 for the ITS2 region), as recommended (Schöler et al. 2017). Alpha diversity indices were calculated with the ‘vegan’ package in R (Dixon 2003), using the following indices: observed richness (S), estimated Chao-1 richness, estimated abundance coverage estimator (ACE) richness, the Simpson reciprocal index (1/D, D = Dominance), the Shannon index (H), and equitability (H/ln(S)). Community beta diversity was estimated with the Bray-Curtis dissimilarity index and a PERMANOVA and distance-based redundancy analysis (Bray-Curtis dissimilarity ~ homogeneity*biodegradability, 10,000 group permutations, ‘adonis’ and ‘capscale’ functions, ‘vegan’ package, Dixon 2003). Discriminant OTUs whose abundance was changed by mulch homogeneity, biodegradability, and their interaction were identified from the raw unrarefied data with a likelihood ratio test under negative binomial distributions and generalised linear models (FDR-adjusted P < 0.01, fold-change > 2), as recommended (Mac-Murdie and Holmes 2014; Schöler et al. 2017). We tested the concordance between the microbial community structure and the collection of variables measured in the study (lettuce growth, enzymatic activities) using a sparse partial least square discriminant analysis with blocks (block sPLS-DA) implemented in the ‘mixOmics’ package (‘block.splsda’ function, Rohart et al. 2017). Two distinct models were generated – one for bacteria and the other for fungi. Only the correlations between microbial OTUs and the soil/lettuce parameters with a |strength|> 0.5 were considered.
Results
We first compared the effects of the four mulching treatments on a range of soil and plant properties. Second, we looked at the effect of mulching homogeneity (+ homogeneity: plastic film and hemp canvas;—homogeneity: bare soil control and hemp straw) and biodegradability (+ biodegradability: hemp straw and canvas;—biodegradability: bare soil control and plastic film) and their potential interaction (homogeneity*biodegradability).
Soil moisture and temperature
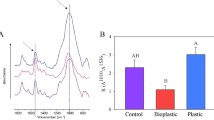
The daily soil RWC (Fig. 1A) was lowest under plastic mulch, and highest under the hemp canvas. The biodegradable mulch had a higher RWC than the other treatments (- biodegradable mulch: 53.31 ± 0.49%, + biodegradable much: 56.51 ± 0.45%, variance 26.30%, P < 0.001, Table S3). A significant interaction was detected (variance 41.75%, P < 0.001, Table S3), as the RWC was higher under the hemp canvas and lower under the plastic mulch compared to bare soil and hemp straw, respectively. Homogeneous mulching had a marginal effect (- homogeneous mulch: 55.29 ± 0.53%, + homogeneous mulch: 54.54 ± 0.40%, variance 2.10%, P = 0.05, Table S3).
Soil relative water content (RWC) and temperature recorded throughout the experiment. A Average soil RWC and (B) average soil temperature recorded by the probe in the soil mesocosm of each treatment. The soil RWC values were calculated based on the averaged hourly records (mean ± SEM, n = 852). The soil temperature values were calculated based on the averaged day and night records of each day (mean ± SEM, n = 84). Different letters indicate statistically significant differences. Significance of the factors: * P < 0.05; ** P < 0.01; *** P < 0.001. w, with; w/o, without
The daily soil temperature (T°C, Fig. 1B) was lowest in bare soil, and highest under plastic mulch (+ 1.04 °C compared to the control). An interaction was detected (variance 23.91%, P = 7.88 × 10–3, Table S3): temperature increased under plastic mulch compared to bare soil, while remaining stable when biodegradable mulches were used. Homogeneous mulching had a marginal effect (- homogeneous mulch: 22.26 ± 0.16%, + homogeneous mulch: 22.73 ± 0.17%, variance 14.65%, P = 0.04, Table S3). Biodegradability did not impact temperature (P = 0.53, Table S3). In sum, the plastic film increased soil temperature but decreased the soil moisture, while the hemp canvas only increased soil moisture.
Lettuce yield
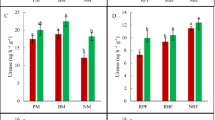
Lettuce fresh shoot, dry shoot and dry root weights were significantly higher when lettuce was grown with plastic mulch compared to hemp straw mulch (Fig. 2A–C). The fresh and dry shoot weights of lettuces grown with the hemp canvas and the plastic mulch were similar. The shoot–root ratio was not affected (Fig. 2D). A marginal trend was detected for mulch biodegradability (variance 14.65%, P = 0.04, Table 1). It impacted the fresh shoot weight by 70.90 g (- mulch: 306 ± 27.18 g, + mulch: 235.10 ± 28.76 g), the dry shoot weight by 7.8 g (- mulch: 36.00 ± 2.50 g, + mulch: 28.00 ± 2.82 g), and the dry root weight by 2.72 g (- mulch: 14.34 ± 1.04 g, + mulch: 11.62 ± 1.04 g). Mulch homogeneity had a strong effect (variance 47.11%, P = 2.00 × 10–3, Table 1): yields were higher with homogeneous mulch than in the absence of mulch, and impacted the fresh shoot weight by 125.10 g (+ homogeneous mulch: 333.10 ± 18.82 g,—homogeneous mulch: 208.00 ± 24.93 g), the dry shoot weight by 11.8 g (- homogeneous mulch: 26.20 ± 2.67 g, + homogeneous mulch: 38 ± 1.64 g), and the dry root weight by 4.87 g (- homogeneous mulch: 10.55 ± 0.76 g, + homogeneous mulch: 15.42 ± 0.82 g). No interaction was detected (P = 0.92, Table 1). In sum, yields were similar when lettuces were grown on the plastic film and the hemp canvas, highest on the plastic film and lowest on hemp straw.
Lettuce growth parameters recorded under each soil mulching modality at harvest. A Fresh shoot biomass, B dry shoot biomass, C dry root biomass, D shoot-to-root ratio. Mean ± SEM, n = 5. Different letters indicate statistically significant differences. Significance of the factors: * P < 0.05; ** P < 0.01; *** P < 0.001. w, with; w/o, without
Alpha diversity of the soil microbiota
All alpha diversity indices were analysed together by PERMANOVA (Table 1), and only the most significant ones were presented separately (Fig. 3). Bacterial richness was not affected (Fig. 3A). The bacterial Shannon index was significantly higher under plastic mulch than in bare soil, indicating an increased bacterial evenness (Fig. 3B). Despite a slight effect of homogeneity on the bacterial Shannon index, no overall effect was detected (P = 0.32). No effect of biodegradability was detected either (P = 0.52).
Alpha-diversity analysis of the soil bacterial and fungal communities. Panels A-F, diversity indices of the bacterial community (16S); panels G-L, diversity indices of the fungal community (ITS). Mean ± SEM, n = 5. Different letters indicate statistically significant differences. Significance of the factors: * P < 0.05; ** P < 0.01; *** P < 0.001. w, with; w/o, without
Fungal richness and the fungal Shannon index were significantly lower under the hemp canvas than under the plastic mulch and in bare soil (Fig. 3C–D). A strong biodegradability effect was observed (variance 38.07%, P < 0.001, Table 1), resulting in decreased richness and evenness with hemp-based mulch. A marginal effect of homogeneity was detected (variance 18.15%, P = 0.01). The interaction had no effect (P = 0.32). In sum, plastic mulch increased the soil bacterial evenness, and hemp biodegradability reduced fungal diversity, especially under the hemp canvas.
Beta diversity of the soil microbiota
The soil bacterial community was significantly structured by mulch homogeneity, mulch biodegradability and their interaction (variance 25.20%, P < 0.001, Fig. 4A). The bacterial community structure in bare soil showed a negative association with mulch homogeneity and biodegradability (constrained component 1 = 12.61%), with a reduction of Acidobacteria and increased Actinobacteria, Firmicutes and Cyanobacteria (Fig. 4C). The bacterial community structure in the soil mulched with the hemp canvas showed a positive association with the interaction effect (constrained component 2 = 6.83%), with a distinct community composition (Fig. 4C). Mulch homogeneity affected 22 bacterial OTUs – mainly Proteobacteria – that mostly decreased under homogeneous mulch (20/22, Table S4). Mulch biodegradability affected 33 bacterial OTUs – mainly Proteobacteria – that mostly increased in response to mulch biodegradability (Table S5). The interaction between mulch homogeneity and biodegradability altered 141 bacterial OTUs (Fig. S3) – mostly Actinobacteria and Firmicutes – whose abundance was reduced by mulching compared to bare soil. Two OTU clusters showed higher abundances under the hemp canvas: a Proteobacteria-driven cluster increased only under the hemp canvas, and the abundance of an Actinobacteria-driven cluster was partially maintained by the hemp canvas compared to bare soil, whereas it decreased under the plastic and hemp straw mulches (red boxes, Fig. S3).
Bacterial and fungal soil community structures and compositions. A Bacterial and (B) fungal community structures estimated by distance-based redundancy analysis based on the type of soil cover (PERMANOVA: community ~ cover homogeneity*cover biodegradability, Bray-Curtis dissimilarity). The relevance of the constrained models was tested using 10,000 free permutations, and the first two constrained components (CAP: Canonical Analysis of Principal Coordinates) where shown. The vectors represent the direction of the effects and their significance. * P < 0.05; ** P < 0.01; *** P < 0.001. Relative abundance of (C) bacterial and (D) fungal phyla, and their grouping according to taxonomy
The soil fungal community structure was also significantly impacted by mulch homogeneity, mulch biodegradability and their interaction (variance 21.38%, P < 0.001, Fig. 4B). In soil mulched with the hemp canvas, the fungal community was positively associated with the interaction effect (constrained component 1 = 9.48%), with a decrease of Ascomycota and increased unclassified fungi (Fig. 4D). The hemp mulch showed a positive association with the biodegradability effect, segregating away from the bare soil control (constrained component 2 = 7.01%), with distinct community compositions (Fig. 4D). Then, we extracted fungal OTUs responding to mulch properties. Mulch homogeneity affected 16 OTUs – mainly Ascomycota. Half of them increased and the other half decreased as a result of homogeneous mulching (Table S4). Mulch biodegradability affected 16 OTUs – mainly Ascomycota again – that mostly increased as a result of mulch biodegradability (12/16, Table S5). The interaction effect altered 41 OTUs – mostly Ascomycota – including some enriched by the hemp canvas, belonging to unclassified Sordariomycetes, and Stachybotrys chartarum (red square, Fig. S4). In sum, the soil microbiota structure under the hemp canvas was associated with the interaction effect between mulch homogeneity and biodegradability, resulting in the enrichment of a specific set of microbial OTUs.
Soil enzymatic activities
The soil enzymatic activities are presented in Fig. 5 (C cycle: panels A-D; N cycle: panel E; S cycle: panel F; P cycle: panels G-I). The xylanase and cellulase activities were significantly lower under plastic mulch than under the other treatments (Fig. 5A–B). Laccase activity was significantly different in all treatments, and ranked as follows: hemp canvas > plastic mulch > bare soil > hemp straw (Fig. 5C). β-glucosidase activity was significantly lower under the hemp canvas and the plastic mulch than in bare soil (Fig. 5D). Arylamidase activity was significantly lower under the plastic mulch than in bare soil and under the hemp canvas (Fig. 5E). Arylsulphatase activity was significantly lower under the plastic mulch than in bare soil and under the hemp mulch (Fig. 5F). Alkaline phosphatase activity was significantly lower under the plastic mulch than under the hemp canvas (Fig. 5G). No difference was observed for the phosphatase and phosphodiesterase activities (Fig. 5H–I), or for soil respiration (Fig. S5).
Soil parameters measured under the cover modalities at harvest. A xylanase, B cellulase, C laccase, D β-glucosidase, E arylamidase, F arylsulphatase, G alkaline phosphatase, H phosphatase, and (I) phosphodiesterase activities. Enzymatic activities were all measured at standardised temperature and soil humidity. Mean ± SEM, n = 5. Different letters indicate statistically significant differences. Significance of the factors: * P < 0.05; ** P < 0.01; *** P < 0.001. w, with; w/o, without
The PERMANOVA revealed a significant effect of mulch homogeneity (variance 30.47%, P < 0.001, Table 1), mainly driven by the high laccase activity and low β-glucosidase activity under homogeneous mulch (P < 0.001, Fig. 5C–D). A significant interaction was detected (variance 14.32%, P = 0.02) because of the differences between the ‘plastic film’ and ‘hemp canvas’ activities (P < 0.05, Fig. 5A, B, E, G). Despite significant trends, no overall effect of mulch biodegradability was detected (P = 0.21). In sum, soil mulched with the plastic film harboured the lowest enzymatic activities. Activities were similar under the other treatments, except for laccase and β-glucosidase whose activities were higher and lower in soil mulched with the hemp canvas, respectively.
Block sPLS-DA of the soil microbiota, soil enzymatic activities and lettuce yields
A block sPLS-DA was done to test the level of correlation between the microbial datasets and the soil/lettuce variables in response to the mulch treatment. The analysis revealed a strong correlation between the bacterial community and the soil/lettuce variables (arrow plots, R2 = 0.71, P < 0.001, Fig. S6). Likewise, a strong correlation was found for the fungal community (arrow plots, R2 = 0.74, P < 0.001, Fig. S6). Circle correlation plots detected bacterial and fungal OTUs whose abundance was correlated with enzymatic activities and lettuce growth (Fig. 6A–B). Most enzymatic activities correlated with each other, except those of β-glucosidase and laccase. Lettuce growth was negatively correlated with β-glucosidase activity, but slightly positively correlated with laccase activity. Using the list of discriminant taxa, we detected eight bacterial OTUs whose abundances were significantly enriched by the hemp canvas (Fig. S3, red dots in the dendrogram of the Proteobacteria-driven cluster), and showing a positive correlation with laccase activity and the lettuce yield (yellow stars, strength > 0.5, Fig. 6A). They included five Alphaproteobacteria (four unclassified Sphingomonadales – Erythrobacteraceae, Sphingomonadaceae, Novosphingobium, Kaistobacter – and one unclassified Caulobacteracea), one Gammaproteobacterium (unclassified Xanthomonadacea), one Betaproteobacterium (unclassified Ellin_6067), and one Actinobacterium (unclassified Acidimicrobiale). Only one unclassified fungal OTU (significantly enriched by the hemp canvas, Fig. S4) was positively associated with laccase activity, but not with the lettuce yield (yellow stars, strength > 0.5, Fig. 6B).
Circle correlation plots of the sPLS-DA between the microbial datasets and the soil/lettuce dataset. The plots show the correlations of bacterial and fungal OTUs with soil enzymatic activities and lettuce yield. The x and y axes show the correlation strength. Only variables with strong correlations are shown (Pearson |rho|> 0.5). Microbial OTUs are in pale red, while enzymatic activities (Bglu, β-glucosidase; xyl, xylanase; cell, cellulase; lac, laccase; arylA, arylamidase; arylS, arylsulphatase; alkP, alkaline phosphatase; phos, phosphatase) and lettuce yield (shoot_fresh) are in purple. Yellow stars, microbial OTUs significantly enriched by the hemp canvas
Discussion
We aimed to describe the effects of mulch homogeneity, mulch biodegradability and their interaction on soil functioning, the soil microbiota and crop yield. We hypothesized that hemp-based canvas, which is both homogeneous and biodegradable, would have similar and specific effects on soil functioning and plant growth compared to plastic film and hemp straw mulches. We discussed our results by considering the effects of i) mulch homogeneity, ii) mulch biodegradability, and iii) their interaction effect.
Effects of mulch homogeneity (H1)
We confirmed our first hypothesis (H1) that the hemp canvas presented similarities with the plastic mulch due to their common homogeneity property. Mulch homogeneity had the highest number of significant effects found in this study (n = 20, Table S3), indicating that homogeneous mulches such as plastic film and hemp canvas have similar effects when compared to non-homogeneous mulch (hemp straw) or bare soil (control). However, several effects were weak (0.01 < P < 0.05), e.g., changes in the soil climatic parameters. While the physical barrier effect could explain this because the heat that radiates from the soil can be trapped (Liu et al. 2010) and water run-off can be increased (Rice et al. 2004), the variability resulting from the difference in effect intensity between the plastic mulch and the hemp canvas also explained these weak effects. However, mulch homogeneity was beneficial for plant growth: yields were equal and highest when lettuces were grown with the hemp canvas and the plastic mulch. The higher soil temperature under homogeneous mulch may partly explain this: higher temperature can accelerate plant growth (Wilcox and Pfeiffer 1990), especially lettuce growth (Salomez and Hofman 2007; Gruda 2008). While this may be true with plastic mulch, other mechanisms are likely at play with the hemp canvas, as discussed later.
Mulch homogeneity had marked effects on the activity of C cycle enzymes. β-glucosidase activity (which targets labile carbon sources) and laccase activity (which targets recalcitrant sources) were decreased and boosted by mulch homogeneity, respectively. The higher soil temperature under homogeneous mulch may have enhanced microbial activity (Pietikäinen et al. 2005), especially toward more accessible carbon sources. Lower β-glucosidase activity potentially indicated the exhaustion of accessible nutrients at harvest, hence a shift toward the use of recalcitrant substrates by the microbial community via increased laccase activity. Despite these changes, the soil respiration was stable, indicating maintained global microbial activities. Therefore, soil functioning was likely shifted/restructured by mulching. This was supported by changes in the soil microbiota associated with mulch homogeneity, e.g., higher bacterial evenness denoting the reduction of dominance effects. Consistent patterns were observed amongst the taxa impacted by mulch homogeneity. The autotrophic Cyanobacterium Acutodesmus obliquus decreased under homogeneous mulch, likely due to the lack of/insufficient light. Taxa involved in C cycling were increased by mulch homogeneity, e.g., Sordariomycetes taxa (Su et al. 2020), and S. chartarum involved in recalcitrant matter decomposition via its laccase activity (Mander et al. 2006). Agronomically important species were also affected, like the phytopathogens Septoria cretae (Quaedvlieg et al. 2013) and Olpidium brassicae (Lot et al. 2002), which were increased and decreased by mulch homogeneity, respectively. The pesticide-remover species Acinetobacter lwoffii DNS32 (Tao et al. 2019) was also decreased by mulch homogeneity. In sum, homogeneous mulches had an effect on soil functioning and increased lettuce yield and temperature, altered the soil microbiota, and favoured enzymatic activities involved in the use of recalcitrant carbon sources.
Effect of mulch biodegradability (H2)
We confirmed our second hypothesis (H2) that the hemp canvas had similarities with the hemp straw mulch due to their common biodegradability property. Mulch biodegradability had the second highest number of significant effects (n = 18, Table S3), indicating that the biodegradable hemp mulches had similar effects when compared to the other non-biodegradable treatments. Mulch biodegradability increased the soil RWC, likely due to water retention by the mulched plant residues (Tuure et al. 2021; Wang et al. 2021) and hemp permeability (compared to plastic and to the dry topsoil crust observed on bare soil). However, the lowest lettuce yields were observed for the hemp straw treatment, where the degraded hemp fibres maintained high soil surface moisture. Lettuce growth appears to be favoured when soil water is not in excess (Dessureault-Rompré et al. 2020). Alternatively, the lower lettuce yields in the presence of biodegradable mulches could be linked to the timing of organic matter degradation and nitrogen immobilization by microbes (Chen et al. 2014). A transient disequilibrium of the C/N ratio may have occurred when hemp carbon was incorporated to the soil, and this may have favoured microbial growth and nitrogen uptake to the detriment of lettuces. This is supported by the higher soil enzymatic activities involved in the C (cellulase), N (arylamidase) and P (alkaline phosphatase) cycles observed in the soils covered with the biodegradable mulches.
Mulch biodegradability had a strong effect on fungal diversity by reducing their richness and evenness, hence a potential allelopathic/trophic effect of hemp. Several fungal OTUs were stimulated by the biodegradable mulches, including unclassified Sordariomycetes and S. chartarum linked to organic matter decomposition (Mander et al. 2006; Su et al. 2020). Some unclassified protist OTUs belonging to Ciliphora were also stimulated, in line with the reported increase in protists following soil organic matter addition under anaerobic conditions (Randall et al. 2020). The higher soil moisture may have induced partially anoxic conditions favouring protists. Biodegradable mulch also influenced the abundance of phytopathogens by decreasing the abundance of S. cretae (Quaedvlieg et al. 2013) and increasing the abundance of Phialophora cyclaminis (Williams 1991), but also decreasing the abundance of members of the Basidiomycota yeast Kondoa linked with increased crop yields (Stefan et al. 2021). Several bacterial OTUs were also stimulated by mulch biodegradability, including members of families known to host species involved in nutrient cycling and to have beneficial effects on plants like Oxalobacteraceae (Janthinobacterium: Yin et al. 2021, Massilia: Xiao et al. 2022), Sphingomonadaceae (Kaistobacter: Ji et al. 2021) and Xanthomonadaceae (Luteimonas mephitis and Thermomonas: Lee et al. 2022, Xie et al. 2022; Lysobacter: Xiao et al. 2022). Some play pivotal roles in decomposing soil recalcitrant organic matter like lignin (Geobacter: Merino et al. 2021; Prosthecobacter: Zhu et al. 2020). Agronomically important taxa were also increased, like potential Erwinia pathogens, whose abundance in the soil is linked to the C/N ratio (Xie et al. 2022), Azoarcus members potentially involved in soil pesticide degradation (Lian et al. 2022), and potential plastic-degrading Exiguobacteria (Maroof et al. 2022). In sum, mulch biodegradability increased the soil water content and stimulated several microbial taxa and soil enzymatic activities. Hemp had a strong effect on fungal diversity, suggesting a selection mechanism.
Interaction between mulch homogeneity and biodegradability (H3)
We confirmed our third hypothesis (H3) that the hemp canvas had specific effects on soil functioning, due to the combination of its homogeneity and biodegradability properties (n = 12, Table S3). Thus, weaving hemp fibres into a canvas had non-additive effects diverging from those of the homogeneous and biodegradable properties observed with the plastic film and hemp straw, respectively. This was exemplified by the diverging soil water contents of the plastic film and the hemp canvas, as well as the soil temperature that remained stable under the hemp-based mulches but increased under the plastic film. Higher temperature likely enhanced lettuce growth, but also likely increased transpiration rates; this may explain the converse patterns of soil temperature and moisture observed with the plastic film. The higher temperature under the plastic mulch could also be explained by the low albedo of the black films and its insulating effect, which both trapped calories. Despite these differences, plastic and hemp canvas mulching had similar effects on lettuce yields, implying that other mechanisms were at play with the hemp canvas.
Interaction effects were also seen on the soil microbiota: very distinct bacterial and fungal assemblages were observed under the hemp canvas compared to the other treatments. This was particularly true for the fungal community. The hemp canvas had a strong effect on fungal diversity and evenness, indicating a potential selection mechanism. Several fungal OTUs belonging to Ascomycota were enriched by the hemp canvas, including some with known activity toward organic matter decomposition (e.g., some Sordariomycetes, Su et al. 2020; S. chartarum, Mander et al. 2006). Other trophic levels were likely stimulated by the hemp canvas: a member of Orbiliaceae – a family specialised in nematode predation (Li et al. 2006) – was also enriched. Two clusters of soil bacterial OTUs displayed remarkable abundance signatures associated to the hemp canvas. The first one was driven by a few Proteobacteria, little abundant in bare soil and at an intermediate level under the plastic and hemp mulches, but at high abundances under the hemp canvas. Several taxa from this cluster are involved in plant growth promotion and nutrient cycling, such as some Erythrobacteraceae (Tang et al. 2019), Sphingomonadaceae (Luo et al. 2019a), and Caulobacteraceae (Luo et al. 2019b). These taxa were enriched by the hemp canvas and positively associated with laccase activity and lettuce growth, in line with previous reports. The second cluster was driven by Nocardiaceae (Actinobacteria), which strongly decreased under the plastic and hemp straw mulches compared to bare soil, but not under the hemp canvas. Soil Nocardiceae are involved in organic matter decomposition (Jacquiod et al. 2013) and bioremediation (Pathom-Aree et al. 2021). Therefore, our results support a stimulation of potentially beneficial soil microbes induced by the hemp canvas. This enrichment of specific microbial groups strongly correlated with important functional changes, as evidenced by the block sPLS-DA.
The soil enzymatic activities were also altered by the interaction effect. The lowest levels were recorded under the plastic mulch across all cycles: the C cycle (xylanase and cellulase activities), the N cycle (arylamidase), the S cycle (arylsulphatase) and the P cycle (alkaline phosphatase). This suggests nutrient depletion under the plastic mulch (Lee et al. 2018), probably resulting from higher soil temperature and microbial activities that provided the nutrients that sustained better lettuce growth. Laccase and arylamidase activities increased under the hemp canvas. This has to be discussed in light of the microbial stimulation induced by the hemp canvas, featuring key microbial members involved in nutrient cycling. Therefore, our results indicate that soil mulching with a hemp canvas changed the soil activity and functioning. This could result from combined effects on the climatic variable (stable soil temperature and a higher moisture) and the carbon source (hemp biodegradation). These effects likely acted synergistically by modifying soil ecological factors in a favourable way for potentially beneficial microbes and their activities, leading to a similar lettuce yield to that of the plastic mulch. Thus, our results indicate that plant-soil-microbial feedbacks seemed to be altered by the soil mulching modality. Plant-soil-microbial feedbacks were identified as a crucial factor for better understanding the discrepancies observed between laboratory and field trials (Chen et al. 2023).
Mulching with hemp canvases seems a good alternative to plastic mulch, especially owing to its positive effect on specific soil microorganisms and enzymatic activities. The interaction between hemp canvas homogeneity and biodegradability affected abiotic and biotic soil properties, but did not affect lettuce yields. Our study suggests that hemp canvas has similar performances to plastic mulch as regards plant yield and is more protective of soil functioning than plastic mulch. One could even expect higher plant yields with hemp canvases dyed black with natural pigments to reduce their albedo and increase soil temperature, in a similar manner to plastic mulch, while keeping the advantage of a biodegradable mulch. Further experiments including field trials are needed to confirm our observations done under controlled greenhouse conditions. Indeed, important gaps between laboratory and field-based studies are often observed, and plant–soil–microbial feedbacks may be altered by environmental factors and their interactions in the field (Chen et al. 2023).
Data availability
The data supporting this study is currently being prepared for deposition in the Sequence Read Archive (SRA) database.
Abbreviations
- RNA:
-
Ribonucleic acid
- ITS:
-
Internal transcribed spacer
- ANOVA:
-
Analysis of variance
- PERMANOVA:
-
Permutational analysis of variance
- OTU:
-
Operational taxonomic unit
- ISO:
-
International organization for standardization
- SEM:
-
Standard error of the mean
- sPLS-DA:
-
Sparse partial least square discriminant analysis
- FDR:
-
False discovery rate
- ACE:
-
Abundance coverage estimator
- RWC:
-
Relative water content
References
Agnieszka S, Magdalena R, Jan B, Katarzyna W, Malgorzata B, Krzysztof H, Kalemba D (2016) Phytotoxic effect of fiber hemp essential oil on germination of some weeds and crops. J Essent Oil Bear Plants 19:262–276. https://doi.org/10.1080/0972060X.2015.1137236
Ahmad R, Tehsin Z, Malik ST, Asad SA, Shahzad M, Bilal M, Shah MM, Khan SA (2016) Phytoremediation potential of hemp (Cannabis Sativa L.): identification and characterization of heavy metals responsive genes. Clean-Soil Air Water 44:195–201. https://doi.org/10.1002/clen.201500117
Aubin MP, Seguin P, Vanasse A, Tremblay GF, Mustafa AF, Charron JB (2015) Industrial hemp response to nitrogen, phosphorus, and potassium fertilization. CFTM 1:1–10. https://doi.org/10.2134/cftm2015.0159
Bagavathiannan MV, Graham S, Ma Z, Barney JN, Coutts SR, Caicedo AL, De Clerck-Floate R, West NM, Blank L, Metcalf AL, Lacoste M, Moreno CR, Evans JA, Burke I, Beckie H (2019) Considering weed management as a social dilemma bridges individual and collective interests. Nat Plants 5:343–351. https://doi.org/10.1038/s41477-019-0395-y
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555. https://doi.org/10.1016/j.mimet.2003.08.009
Bandopadhyay S, Martin-Closas L, Pelacho AM, DeBruyn JM (2018) Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions. Front Microbiol 9:819. https://doi.org/10.3389/fmicb.2018.00819
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond B Biol Sci 364:1985–1998. https://doi.org/10.1098/rstb.2008.0205
Berger S, Kim Y, Kettering J, Gebauer G (2013) Plastic mulching in agriculture - friend or foe of N2O emissions? Agric Ecosyst Environ 167:43–51. https://doi.org/10.1016/j.agee.2013.01.010
Beriot N, Peek J, Zornoza R, Geissen V, Lwanga EH (2021) Low density-microplastics detected in sheep faeces and soil: a case study from the intensive vegetable farming in Southeast Spain. Sci Total Environ 755:142653. https://doi.org/10.1016/j.scitotenv.2020.142653
Bond WA, Grundy C (2001) Non-chemical weed management in organic farming systems. Weed Res 41:383–405. https://doi.org/10.1046/j.1365-3180.2001.00246.x
Bradney L, Wijesekara H, Palansooriya KN, Obadamudalige N, Bolan NS, Ok YS, Rinklebe J, Kim KH, Kirkham MB (2019) Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ Int 131:104937. https://doi.org/10.1016/j.envint.2019.104937
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599. https://doi.org/10.1128/AEM.69.6.3593-3599.2003
Chen B, Liu E, Tian Q, Yan C, Zhang Y (2014) Soil nitrogen dynamics and crop residues. A review. Agron Sustain Dev 34:429–442. https://doi.org/10.1007/s13593-014-0207-8
Chen J, Zhang Y, Kuzyakov Y, Wang D, Olesen JE (2023) Challenges in upscaling laboratory studies to ecosystems in soil microbiology research. Glob Chang Biol 9:569–574. https://doi.org/10.1111/gcb.16537
Cheviron N, Grondin V, Marrauld C, Poiroux F, Bertrand I, Abadie J, Pandard P, Riah-Anglet W, Dubois C, Malý S, Marques CR, Valverde Asenjo I, Alonso A, Marquina Díaz D, Mougin C (2022) Inter-laboratory validation of an ISO test method for measuring enzyme activities in soil samples using colorimetric substrates. Environ Sci Pollut Res 29:29348–29357. https://doi.org/10.1007/s11356-021-17173-3
Chikowo R, Faloya V, Peti S, Munier-Jolain NM (2009) Integrated Weed Management systems allow reduced reliance on herbicides and long-term weed control. Agric Ecosyst Environ 132:237–242. https://doi.org/10.1016/j.agee.2009.04.009
de Mendiburu F (2019) Agricolae: Statistical Procedures for Agricultural Research. R Package version 1.3–1. https://CRAN.R-project.org/package=agricolae. Accessed June 2021
Derraik JG (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
Dessureault-Rompré J, Caron J, Plamondon L, Gaudreau L, Jutras S, Lafond JA (2020) Growth and water-use characteristics of Romaine lettuce cultivated in Histosol as affected by irrigation management, compaction, and seeding type. Can J Soil Sci 100:278–288. https://doi.org/10.1139/cjss-2019-0123
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x
Dresbøll DB, Jakob Magid J (2006) Structural changes of plant residues during decomposition in a compost environment. Bioresour Technol 97:973–981. https://doi.org/10.1016/j.biortech.2005.05.003
Eichlerová I, Šnajdr J, Baldrian P (2012) Laccase activity in soils: considerations for the measurement of enzyme activity. Chemosphere 88:1154–1160. https://doi.org/10.1016/j.chemosphere.2012.03.019
El-Beltagi HS, Basit A, Mohamed HI, Ali I, Ullah S, Kamel EAR, Shalaby TA, Ramadan KMA, Alkhateeb AA, Ghazzawy HS (2022) Mulching as a sustainable water and soil saving practice in agriculture: a review. Agronomy 12:1881. https://doi.org/10.3390/agronomy12081881
European Parliament (2019) https://www.europarl.europa.eu/news/en/press-room/20190321IPR32111/parliament-seals-ban-on-throwaway-plastics-by-2021. Accessed Dec 2023
Frassinetti S, Moccia E, Caltavuturo L, Gabriele M, Longo V, Bellani L, Giorgi G, Giorgetti L (2018) Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem 262:56–66. https://doi.org/10.1016/j.foodchem.2018.04.078
Gruda N (2008) The effect of wood fiber mulch on water retention, soil temperature and growth of vegetable plants. J Sustain Agric 32:629–643. https://doi.org/10.1080/10440040802395049
Hablot E, Dharmalingam S, Hayes DG, Wadsworth LC, Blazy C, Narayan R (2014) Effect of simulated weathering on physicochemical properties and inherent biodegradation of PLA/PHA nonwoven mulches. J Polym Environ 22:417–429. https://doi.org/10.1007/s10924-014-0697-0
Herlemann DP, Labrenz M, Jurgens K, Bertilsson S, Waniek J, Andersson AF (2011) Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J 5:1571–1579. https://doi.org/10.1038/ismej.2011.41
Ihrmark K, Bödeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandström-Durling M, Clemmensen KE, Lindahl BD (2012) New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677. https://doi.org/10.1111/j.1574-6941.2012.01437.x
Iqbal S, Xu J, Allen SD, Khan S, Nadir S, Arif MS, Yasmeen T (2020) Unraveling consequences of soil micro- and nano-plastic pollution on soil-plant system: implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 260:127578. https://doi.org/10.1016/j.chemosphere.2020.127578
ISO20130 (2018) Soil quality: Measurement of enzyme activity patterns in soil samples using colorimetric substrates in micro-well plates 1:29. https://www.iso.org/standard/67074.html. Accessed Dec 2023
Jacquiod S, Franqueville L, Cécillon S, Vogel TM, Simonet P (2013) Soil bacterial community shifts after chitin enrichment: an integrative metagenomic approach. PLoS ONE 8:e79699. https://doi.org/10.1371/journal.pone.0079699
Jacquiod S, Raynaud T, Pimet E, Ducourtieux C, Casieri L, Wipf D, Blouin M (2022) Wheat rhizosphere microbiota respond to changes in plant genotype, chemical inputs, and plant phenotypic plasticity. Front Ecol Evol 10:903008. https://doi.org/10.3389/fevo.2022.903008
Ji L, Nasir F, Tian L, Chang J, Sun Y, Zhang J, Li X, Tian C (2021) Outbreaks of root rot disease in different aged American ginseng plants are associated with field microbial dynamics. Front Microbiol 12:676880. https://doi.org/10.3389/fmicb.2021.676880
Jian S, Li J, Chen J, Wang G, Mayes MA, Dzantor KE, Hui D, Luo Y (2016) Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: a meta-analysis. Soil Biol Biochem 101:32–43. https://doi.org/10.1016/j.soilbio.2016.07.003
Lament WJ (1993) Plastic mulches for the production of vegetable crops. HortTechnology 3:35–39. https://doi.org/10.21273/HORTTECH.3.1.35
Laugale VS, Strautina S, Krasnova I, Seglina D, Kampuss K (2015) The influence of cultivation system on biochemical content of strawberry fruits. J Hortic Res 22:85–92. https://doi.org/10.2478/johr-2014-0025
Lee JG, Young H, Mun H, Park H, Hoon C, Pil L, Kim J (2018) Depletion of soil organic carbon stocks are larger under plastic film mulching for maize. Eur J Soil Sci 70:807–818. https://doi.org/10.1111/ejss.12757
Lee SK, Chiang MS, Hseu ZY, Kuo CH, Liu CT (2022) A photosynthetic bacterial inoculant exerts beneficial effects on the yield and quality of tomato and affects bacterial community structure in an organic field. Front Microbiol 13:959080. https://doi.org/10.3389/fmicb.2022.959080
Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B (2019) Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol 53:1748–1765. https://doi.org/10.1021/acs.est.8b05512
Li SX, Xiao L (1992) Distribution and management of drylands in the People’s Republic of China. Adv Soil Sci 18:148–302. https://doi.org/10.1007/978-1-4612-2844-8_4
Li Y, Jeewon R, Hyde KD, Mo MH, Zhang KQ (2006) Two new species of nematode-trapping fungi: relationships inferred from morphology, rDNA and protein gene sequence analyses. Mycol Res 110:790–800. https://doi.org/10.1016/j.mycres.2006.04.011
Li Y, Chen J, Dong Q, Feng H, Siddique KHM (2022) Plastic mulching significantly improves soil enzyme and microbial activities without mitigating gaseous N emissions in winter wheat-summer maize rotations. Field Crop Res 286:108630. https://doi.org/10.1016/j.fcr.2022.108630
Lian L, Xing Y, Zhang N, Jiang B (2022) Identification of chlorpyrifos-degrading microorganisms in farmland soils via cultivation-independent and -dependent approaches. Environ Sci Process Impacts 24:1050–1059. https://doi.org/10.3389/fmicb.2017.00518
Liu Y, Yang SJ, Li SQ, Chen XP, Chen F (2010) Growth and development of maize (Zea mays L.) in response to different field water management practices: resource capture and use efficiency. Agric For Meteorol 150:606–613. https://doi.org/10.1016/j.agrformet.2010.02.003
Lot H, Campbell RN, Souche S, Milne RG, Roggero P (2002) Transmission by Olpidium brassicae of Mirafiori lettuce virus and Lettuce big-vein virus, and their roles in lettuce big-vein etiology. Phytopathology 92:288–293. https://doi.org/10.1094/PHYTO.2002.92.3.288
Luo Y, Wang F, Huang Y, Zhou M, Gao J, Yan T, Sheng H, An L (2019a) Sphingomonas sp. Cra20 increases plant growth rate and alters rhizosphere microbial community structure of arabidopsis thaliana under drought stress. Front Microbiol 10:1221. https://doi.org/10.3389/fmicb.2019.01221
Luo D, Langendries S, Garcia Mendez S, De Ryck J, Liu D, Beirinckx S, Willems A, Russinova E, Debode J, Goormachtig S (2019b) Plant growth promotion driven by a novel Caulobacter strain. Mol Plant Microbe Interact 32:1162–1174. https://doi.org/10.1094/MPMI-12-18-0347-R
Mac-Kenzie C, Duncan LW (2001) Landscape fabric as a physical barrier to neonate Diaprepes abbreviatus (Coleoptera: Curculionidae). Fla Entomol 84:721–722. https://doi.org/10.2307/3496409
Mac-Murdie PJ, Holmes S (2014) Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10:e1003531. https://doi.org/10.1371/journal.pcbi.1003531
Mander GJ, Huaming W, Elizabeth B, Jens W, Kay V, Vinuesa C, Foster C, Leeder AC, Allen G, Hamill V, Janssen GG, Dunn-Coleman N, Karos M, Lemaire HG, Subkowski T, Bollschweiler C, Turner G, Nüsslein B, Fischer R (2006) Use of laccase as a novel, versatile reporter system in filamentous fungi. Appl Environ Microbiol 72:5020–5026. https://doi.org/10.1128/AEM.00060-06
Maroof L, Khan I, Hassan H, Azam S, Khan W (2022) Microbial degradation of lowdensity polyethylene by Exiguobacterium sp. strain LM-IK2 isolated from plastic dumped soil. World J Microbiol Biotechnol 38:197. https://doi.org/10.1007/s11274-022-03389-z
Merino C, Kuzyakov Y, Godoy K, Jofré I, Nájera F, Matus F (2021) Iron-reducing bacteria decompose lignin by electron transfer from soil organic matter. Sci Total Environ 761:143194. https://doi.org/10.1016/j.scitotenv.2020.143194
Mogensen BB, Spliid NH (1995) Pesticides in Danish watercourses: occurrence and effects. Chemosphere 31:3977–3990. https://doi.org/10.1016/0045-6535(95)00270-I
Montford SE, Small E (1999) A comparison of the biodiversity friendliness of crops with special reference to hemp (Cannabis sativa L.). J Int Hemp Assoc 6:53–63
Mugalla CI, Jolly CM, Martin NR (1996) Profitability of black plastic mulch for limited resource farmers. J Prod Agric 9:283–288. https://doi.org/10.2134/jpa1996.283
Nan W, Yue S, Huang H, Li S, Shen Y (2016) Effects of plastic film mulching on soil greenhouse gases (CO2, CH4 and N2O) concentration within soil profiles in maize fields on the Loess Plateau China. J Integr Agric 15:451–464. https://doi.org/10.1016/S2095-3119(15)61106-6
Nerín C, Tornés AR, Domeño C, Cacho J (1996) Absorption of pesticides on plastic films used as agricultural soil covers. J Agric Food Chem 44:4009–4014. https://doi.org/10.1021/jf960326k
Nissen L, Zatta A, Stefanini I, Grandi S, Sgorbati B, Biavati B, Monti A (2010) Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81:413–419. https://doi.org/10.1016/j.fitote.2009.11.010
Nottingham LB, Kuhar TP (2016) Reflective polyethylene mulch reduces Mexican Bean Beetle (Coleoptera: Coccinellidae) densities and damage in snap beans. J Econ Entomol 109:1785–1792. https://doi.org/10.1093/jee/tow144
Pathom-Aree W, Matako A, Rangseekaew P, Seesuriyachan P, Srinuanpan S (2021) Performance of Actinobacteria isolated from rhizosphere soils on plant growth promotion under cadmium toxicity. Int J Phytoremediation 23:1497–1505. https://doi.org/10.1080/15226514.2021.1913992
Pietikäinen J, Pettersson M, Bååth E (2005) Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol 52:49–58. https://doi.org/10.1016/j.femsec.2004.10.002
Poisa L, Adamovics A (2010) Hemp (Cannabis Sativa L.) as an environmentally friendly Energyplant. Environ Clim Technol 5:80–85. https://doi.org/10.2478/v10145-010-0038-z
Pudelko K, Majchrzak L, Narozna D (2014) Allelopathic effect of fibre hemp (Cannabis sativa L.) on monocot and dicot plant species. Ind Crops Prod 56:191–199. https://doi.org/10.1016/j.indcrop.2014.02.028
Quaedvlieg W, Verkley GJM, Shin HD, Barreto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW (2013) Sizing up Septoria. Stud Mycol 75:307–390. https://doi.org/10.3114/sim0017
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed June 2021
Radwan MM, ElSohly MA, Slade D, Ahmed SA, Wilson L, El-Alfy AT, Khan IA, Ross SA (2008) Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry 69:2627–2633. https://doi.org/10.1016/j.phytochem.2008.07.010
Randall TE, Fernandez-Bayo JD, Harrold DR, Achmon Y, Hestmark KV, Gordon TR, Stapleton JJ, Simmons CW, Vander Gheynst JS (2020) Changes of Fusarium oxysporum f.sp. lactucae levels and soil microbial community during soil biosolarization using chitin as soil amendment. PLoS One 15:e0232662. https://doi.org/10.1371/journal.pone.0232662
Rice PJ, Harman-Fetcho JA, Teasdale JR, Sadeghi AM, McConnell LL, Coffman CB, Herbert RR, Heighton LP, Hapeman CJ (2004) Use of vegetative furrows to mitigate copper loads and soil loss in runoff from polyethylene (plastic) mulch vegetable production systems. Environ Toxicol Chem 23:719–725. https://doi.org/10.1897/03-14
Ricotta JA, Masiunas JB (1991) The effects of black plastic mulch and weed control strategies on herb yield. Hort Sci 26:539–541. https://doi.org/10.21273/HORTSCI.26.5.539
Rohart F, Gautier B, Singh A, Lê Cao KA (2017) mixOmics: an R package for ‘omics feature selection and multiple data integration. PLOS Comput Biol 13:e1005752. https://doi.org/10.1371/journal.pcbi.1005752
Sainju UM, Liptzin D, Dangi S (2022) Enzyme activities as soil health indicators in relation to soil characteristics and crop production. Agrosyst Geosci Environ 5:1–10. https://doi.org/10.1002/agg2.20297
Salomez J, Hofman G (2007) A soil temperature/short-wave radiation growth model for butterhead lettuce under protected cultivation in flanders. J Plant Nutr 30:397–410. https://doi.org/10.1080/01904160601171629
Schluttenhofer C, Yuan L (2017) Challenges towards revitalizing hemp: a multifaceted crop. Trends Plant Sci 22:917–929. https://doi.org/10.1016/j.tplants.2017.08.004
Schöler A, Jacquiod S, Vestergaard G, Schulz S, Schloter M (2017) Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol Fertil 53:485. https://doi.org/10.1007/s00374-017-1205-1
Schonbeck MW (1999) Weed suppression and labor costs associated with organic, plastic, and paper mulches in small-scale vegetable production. J Sustain Agric 13:13–33. https://doi.org/10.1300/J064v13n02_04
Scott M, Rani M, Samsatly J, Charron JB, Jabaji S (2018) Endophytes of industrial hemp (Cannabis sativa L.) cultivars: identification of culturable bacteria and fungi in leaves, petioles, and seeds. Can J Microbiol 64:664–680. https://doi.org/10.1139/cjm-2018-0108
Shen Y, Chen Y, Li S (2016) Microbial functional diversity, biomass and activity as affected by soil surface mulching in a semiarid farmland. PLoS ONE 11:e0159144. https://doi.org/10.1371/journal.pone.0159144
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman, New York
Stefan L, Hartmann M, Engbersen N, Six J, Schöb C (2021) Positive effects of crop diversity on productivity driven by changes in soil microbial composition. Front Microbiol 12:660749. https://doi.org/10.3389/fmicb.2021.660749
Steinmetz Z, Wollmann C, Schaefer M, Buchmann C, David J, Tröger J, Muñoz K, Frör O, Schaumann GE (2016) Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci Total Environ 550:690–705. https://doi.org/10.1016/j.scitotenv.2016.01.153
Su Y, He Z, Yang Y, Jia S, Yu M, Chen X, Shen A (2020) Linking soil microbial community dynamics to straw-carbon distribution in soil organic carbon. Sci Rep 10:5526. https://doi.org/10.1038/s41598-020-62198-2
Tan Z, Yi Y, Wang H, Zhou W, Yang Y, Wang C (2016) Physical and degradable properties of mulching films prepared from natural fibers and biodegradable polymers. Appl Sci 6:147–151. https://doi.org/10.3390/app6050147
Tang T, Sun X, Dong Y, Liu Q (2019) Erythrobacter aureus sp. nov., a plant growth-promoting bacterium isolated from sediment in the Yellow Sea, China. 3 Biotech 9:430. https://doi.org/10.1007/s13205-019-1958-3
Tao Y, Hu S, Han S, Shi H, Yang Y, Li H, Yaqi J, Qi Z, Modupe SA, Mingyuan J, Zhaobo C, Ying Z (2019) Efficient removal of atrazine by iron-modified biochar loaded Acinetobacter lwoffii DNS32. Sci Total Environ 682:59–69. https://doi.org/10.1016/j.scitotenv.2019.05.134
Tuure J, Räsänen M, Hautala M, Pellikka P, Mäkelä PSA, Alakukku L (2021) Plant residue mulch increases measured and modelled soil moisture content in the effective root zone of maize in semi-arid Kenya. Soil Tillage Res 209:104945. https://doi.org/10.1016/j.still.2021.104945
Van der Werf H, Van Geel W, Wijlhuizen M (1995) Agronomic research on hemp (Cannabis sativa L.) in the Netherlands, 1987–1993. JIHA 2:14–17
Van Ruijven B, Van Vuuren DP (2009) Oil and natural gas prices and greenhouse gas emission mitigation. Energy Policy 37:4797–4808. https://doi.org/10.1016/j.enpol.2009.06.037
Wall DH, Bardgett RD, Behan-Pelletier V, Herrick JE, Jones H, Ritz K, Six J, Strong DR, Van der Putten WH (eds) (2012) Soil ecology and ecosystem services. Oxford Univ. Press
Wang Y, Xie Z, Malhi SS, Vera CL, Zhang Y, Wang J (2009) Effects of rainfall harvesting and mulching technologies on water use efficiency and crop yield in the semi-arid Loess Plateau. China Agric Water Manage 96:374–382. https://doi.org/10.1016/j.agwat.2008.09.012
Wang J, Lv S, Zhang M, Chen G, Zhu T, Zhang S, Teng Y, Christie P, Luo Y (2016) Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 151:171–177. https://doi.org/10.1016/j.chemosphere.2016.02.076
Wang J, Liu X, Li Y, Powell T, Wang X, Wang G, Zhang P (2019) Microplastics as contaminants in the soil environment: a mini-review. Sci Total Environ 691:848–857. https://doi.org/10.1016/j.scitotenv.2019.07.209
Wang B, Niu J, Berndtsson R, Zhang L, Chen X, Li X, Zhu Z (2021) Efficient organic mulch thickness for soil and water conservation in urban areas. Sci Rep 11:6259. https://doi.org/10.1038/s41598-021-85343-x
White T, Bruns T, Lee S, Taylor F, White TJ, Lee SH, Taylor L, Taylor JS (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (ed. 18). Academic Press, London, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Wilcox GE, Pfeiffer CL (1990) Temperature effect on seed germination, seedling root development and growth of several vegetables. J Plant Nutr 13:1393–1403. https://doi.org/10.1080/01904169009364161
Williams MAJ (1991) Phialophora cyclaminis: (I.M.I. Descriptions of pathogenic fungi and bacteria CMI descriptions of pathenogenic fungi and bacteria sheet 1086). https://bioinfo.org.uk/html/b152999.htm. Accessed Dec 2023
Winston ME, Hampton-Marcell J, Zarraonaindia I, Owens SM, Moreau CS, Gilbert JA, Hartsel JA, Kennedy SJ, Gibbons SM (2014) Understanding cultivar-specificity and soil determinants of the cannabis microbiome. PLoS ONE 9:e99641. https://doi.org/10.1371/journal.pone.0099641
Xiao X, Li J, Lyu J, Feng Z, Zhang G, Yang H, Gao C, Jin L, Yu J (2022) Chemical fertilizer reduction combined with bio-organic fertilizers increases cauliflower yield via regulation of soil biochemical properties and bacterial communities in Northwest China. Front Microbiol 13:922149. https://doi.org/10.3389/fmicb.2022.922149
Xie Y, Zhou L, Dai J, Chen J, Yang X, Wang X, Wang Z, Feng L (2022) Effects of the C/N ratio on the microbial community and lignocellulose degradation, during branch waste composting. Bioprocess Biosyst Eng 45:1163–1174. https://doi.org/10.1007/s00449-022-02732-w
Yin C, Casa-Vargas JM, Schlatter DC, Hagerty CH, Hulbert SH, Paulitz TC (2021) Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 9:86. https://doi.org/10.1186/s40168-020-00997-5
Zhang P, Wei T, Cai T, Ali S, Han Q, Ren X, Jia Z (2017) Plastic-film mulching for enhanced water-use efficiency and economic returns from maize fields in semiarid China. Front Plant Sci 8:512. https://doi.org/10.3389/fpls.2017.00512
Zhao Z, Shi F, Guan F (2022) Effects of plastic mulching on soil CO2 efflux in a cotton field in northwestern China. Sci Rep 12:4969. https://doi.org/10.1038/s41598-022-08793-x
Zhou B, Wang J, Zhang H, Shi H, Fei Y, Huang S, Tong Y, Wen D, Luo Y, Barceló D (2019) Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: multiple sources other than plastic mulching film. J Hazard Mater 388:121814. https://doi.org/10.1016/j.jhazmat.2019.121814
Zhu L, Wei Z, Yang T, Zhao X, Dang Q, Chen X, Wu J, Zhao Y (2020) Core microorganisms promote the transformation of DOM fractions with different molecular weights to improve the stability during composting. Bioresour Technol 299:122575. https://doi.org/10.1016/j.biortech.2019.122575
Acknowledgements
We thank the members of the 4PMI platform for their expertise and help during plant phenotyping (For Plant and Microbe Interaction, INRAE Bourgogne Franche-Comté, France, https://www6.dijon.inra.fr/umragroecologie/Plateformes/Serres-PPHD). We thank the members of Biochem-Env (https://doi.org/10.15454/HA6V6Y), a service of the “Investissement d’Avenir” infrastructure AnaEE-France, overseen by the French National Research Agency (ANR) (ANR-11-INBS-0001). We thank Madam Sandrine Boudier for her help during the project. We are also grateful to Madam Annie BUCHWALTER for the edition of our manuscript.
Funding
This study was supported by the French “Programme d’Investissement d’Avenir” PIA3 via the “Mulch-Catalyse” project and the University of Bourgogne Franche-Comté via an ISITE-BFC International Junior Fellowship award (grant number: AAP3:RA19028.AEC.IS).
Author information
Authors and Affiliations
Contributions
Funding (FR, FML), Equipment (FR, FML), Experimental design (SJ, MB, FML), Experiments (SJ, EB, AC), Data acquisition (SJ, EB, NC, CM, AC), Statistical analysis (SJ, AC), Writing (SJ, MB, FML), Text revisions (NC, CM, MB, FML).
Corresponding author
Ethics declarations
Financial interests
The authors of this study agreed on the content and have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Hans Lambers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jacquiod, S., Bouchard, E., Beguet, J. et al. Effect of plastic film and hemp canvas mulching on soil properties, microbial diversity and lettuce yield. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06589-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06589-8