Abstract
Background and aims
Nitrogen (N) deposition and native allelopathic plants may affect seed germination and growth of species through their effects on soil microbes and soil nutrient availability. However, our understanding of the interactions between N addition and allelopathic plants on the regeneration of alpine grasslands remains limited.
Methods
Here, we investigated the effects of N addition and the presence of the allelopathic plant Ligularia virgaurea (Maxim.) Mattf. on seed germination, survival, and growth of native herbaceous species (Elymus nutans, Delphinium kamaonense and Tibetia himalaica) on the Qinghai-Tibet Plateau. We used piecewise structural equation modelling to assess both the direct effects of N addition, allelopathic plants, and their interactions and indirect effects mediated by soil properties, soil microbial richness and diversity, and soil enzyme activity.
Results
We found that (1) L. virgaurea directly increased seed germination and early plant survival, and reduced plant root-to-shoot ratio; N addition directly increased early plant survival and biomass, (2) L. virgaurea indirectly increased plant biomass via bacterial richness, (3) N addition offsets the increase in seed germination promoted by the presence of L. virgaurea via soil acid phosphatase.
Conclusion
Our study suggests the importance of direct and indirect roles of allelopathic plants, N addition and their interaction on seed germination, survival and plant growth. Our results highlight the need to consider the interactions between environmental and biological factors as well as their direct and indirect effects to obtain reliable predictions and mechanistic understanding of the response of alpine plants to future climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystems around the globe are experiencing environmental change (Midolo et al. 2019). Economic development and increasing human activities, including the widespread deposition of nitrogen (N), in many terrestrial ecosystems are major drivers of environmental change (Xia and Wan 2008; Humbert et al. 2016). Increasing levels of soil nitrate and ammonium can increase the growth and dominance of some N-limited species, at the expense of other species (Bobbink et al. 2010). Increasing soil N can increase seed germination and lead to the depletion of seed banks (Plassmann et al. 2008; Basto et al. 2015). Additionally, N enrichment can lead to the range expansion of allelopathic plants, which can decrease forage quality (Shi et al. 2018). Nitrogen deposition and the expansion of allelopathic plants has a major impact on plant community structure and composition (Ade et al. 2021; Huang et al. 2021). A better understanding of seed germination, survival, and plant growth responses to changes in N and the presence of allelopathic plants is critical for predicting vegetation dynamics under future global change scenarios.
Nitrogen deposition significantly affects the conditions that are suitable for plants (Clark et al. 2007; Bird and Choi 2017). For example, increasing soil NO3− and NH4+ can result in increased seedling growth and survival (Fenner and Thompson 2005; Onipchenko et al. 2012). Nitrogen deposition also alters enzyme activity in plant roots and soil, and can induce metabolic changes (Wang et al. 2022). Strategies for N use vary across species and functional groups, which may lead to subsequent changes in plant community structure and composition (Niu et al. 2008; Duprè et al. 2010; Zhong et al. 2019). For example, N addition can increase grass density (Elymus nutans) and decrease forb density (Zhang et al. 2016). Soil inorganic nitrogen can also have effects on seed germination and mortality which are species-specific (Davis 2007). Thus, we expect N addition to have a positive overall effect on seed germination and plant growth of herbaceous species, but with different taxa experiencing distinct effects.
Allelopathic plants have dramatically increased in a number of ecosystems, and these allelopathic plants have displaced high-quality forage and affected seed germination and plant establishment of surrounding plants (Shi et al. 2018). Allelopathy is defined as the effect of one plant on the growth of another plant through the release of secondary substances (e.g., volatile terpenoids, flavonoids, phenols) into the soil (Rice 1984). Allelopathic plants can inhibit seed germination and plant growth of surrounding species and may alter competitive interactions (Zhang et al. 2021). For example, the effects of allelochemicals on plant germination and growth may occur through a variety of mechanisms, including reduced mitotic activity in roots and hypocotyls, inhibition of hormonal and enzymatic activity, inhibition of photosynthesis and respiration etc. (Rice 1974). However, some studies suggest that allelopathic plants may benefit species in grassland ecosystems (Zhang et al. 2020). For example, the toxic odor of allelopathic plants can drive away livestock and insects, thereby hindering the soil seed bank and adjacent plants from being eaten and trampled (Oesterheld and Oyarzábal 2004). Plants can alter the rhizosphere microbial diversity and community composition by producing a range of root exudates and litter (Vieira et al. 2020), and below-ground factors such as soil microorganisms are important regulators of seed germination and plant productivity (Van Der Heijden et al. 2008). Previous research has shown that soil microbial biomass and soil enzyme activities are higher in allelopathic plant patches than in gap areas (Shi et al. 2011), which may in turn alter plant community structure (Sun et al. 2009; Mishra and Nautiyal 2012). Allelopathic plants can also promote soil nutrient and soil moisture retention through their well-developed root systems (Zhang et al. 2020). Thus, we hypothesized that the presence of allelopathic plants increased seed germination and plant growth of herbaceous species by altering soil microbes and enzyme activity.
Ligularia virgaurea (Maxim.) Mattf (hereafter L. virgaurea) is a perennial herb in the Asteraceae family. L. virgaurea can become dominant in grazed alpine communities due to its toxicity to grazers. The allelochemicals released by L. virgaurea (Wu et al. 2011) induce changes in alpine plant and soil microbial communities, affecting soil nutrients and forming positive feedback effects (Zhang et al. 2020; Ade et al. 2021). Seed germination and early seedling survival are considered to be two key stages of plant life history and may be impacted by factors associated with global climate change (An et al. 2020; Ganjurjav et al. 2020). Many studies have attempted to shed light on how N addition and allelopathic plants alter natural plant community composition and diversity (Broadbent et al. 2018; Midolo et al. 2019). However, few studies have examined the effects of N deposition and allelopathic plants and their interactions on seed germination, early plant survival, and growth (Cai et al. 2023). We aimed to explore how N addition and the presence of an allelopathic plant (L. virgaurea) would affect seed germination and growth of herbaceous species and to elucidate the potential mechanisms. In this study, we examined the following questions: (a) Do allelopathic plants and N addition (and the interaction between these factors) affect seed germination, early survival, and growth of plants on the Qinghai-Tibet Plateau? (b) What mechanisms drive the responses of seed germination, early survival, and growth to allelopathic plants and N addition?
Method
Study site
We performed our experiments at the Gannan Grassland Ecosystem National Observation and Research Station (101°51′ E, 33°40′ N) at 3550 m.a.s.l, Gansu Province, China. The average annual rainfall is 672 mm (mainly in summer and autumn), and the annual average temperature is 2.2 °C. The specific climate data during the experiment are shown in Table S1. The growing season generally begins in late April and ends in late October. The study site has alpine meadow soil. Total soil nitrogen is 5.28 g kg−1, total soil phosphorus 1.31 g kg−1, soil organic carbon 54.71 g kg−1 and soil pH 7.06. The grassland types are mainly alpine meadows, which are dominated by Cyperaceae, Fabaceae, Poacea and Ranunculaceae.
Experimental design
The experiment was conducted during the plant growth season of 2021 (June to September), we selected a flat and homogeneous ungrazed site (fenced with 60 × 60 m barbed wire). L. virgaurea was abundant in the site. We used two factorial designs with the presence/absence of the L. virgaurea fully crossed with N addition (with/without). We used polyvinyl chloride (PVC) pipes (20 cm diameter, 30 cm height) to create a microenvironment for all treatments. The PVC tubes were inserted in the soil by hammering into the soil and kept it level with the ground. There were eight replicates for each treatment. Thus, a total of 32 microenvironments were arranged in a completely randomized block containing all treatments (i.e., 8 blocks). A 5 m buffer zone was maintained between blocks, and the buffer zone was left untreated. The schematic diagram of our experimental site is shown in Fig. S1.
Nitrogen deposition is projected to increase to 40 g N m−2 yr−1 by 2050 (Zong et al. 2016), but 8 g N m−2 yr−1 has already led to soil nitrogen saturation in the current Qinghai-Tibet Plateau region (Xiao et al. 2020). In this experiment, the amount of nitrogen applied was 10 g N m−2 yr−1 and the N addition treatments were applied by dissolving 4 g of urea in 160 mL of water each time and applying it evenly to the microhabitat of each N treatment. Nitrogen was added once in June, July and early August, where each addition was one-third of the planned additive amount for the year. For treatments without added N, only equal amounts of water were added at the same time of N addition. For the L. virgaurea treatment, two evenly sized and well grown L. virgaurea seedlings were selected in the meadow, the PVC tubes were added surrounding these already established seedlings of L. virgaurea. We expect that the soil already contains allelochemicals. We did not choose to plant or transplant L. virgaurea, because we had a low survival after several attempts. For the without L. virgaurea treatment, we selected plots without the presence of L. virgaurea and framed gap soil where other native plants have been removed with PVC tubes embedded in the soil. In all treatments, we removed other native plants that were excluded from natural growth in the microenvironment (in the L. virgaurea treatment we only left L. virgaurea). A spade was used to obtain 0–5 cm of soil from each microenvironment and a sieve (0.2 cm pore size) was used to remove plant and fine root debris. To prevent the seed bank at the bottom of the microenvironment from interfering with our experiments, we laid down a layer of gauze. Finally, we filled the screened soil on top of the gauze. We sowed a one-time surface mixture of seeds from three species commonly found in alpine grasslands in the microenvironments, including Elymus nutans Griseb. (Poaceae), Delphinium kamaonense Huth var.glabrescens (Ranunculaceae), and Tibetia himalaica (Baker) H. P. Tsui (Fabaceae), thereafter referred to by their genus names. To prevent the seeds from being blown away by the wind, we cover the surface with a small amount of soil.
All seeds used in this study were collected in the autumn of 2020 in alpine meadows on the Tibetan Plateau and stored at room temperature. Before sowing, the seeds were cut to check their viability, which was between 95% and 100% depending on the species. From each species, 50 mature seeds were selected and sown in each microenvironment, and the number of seed germination was recorded every 15 days. At the start of September, above-biomass and below-biomass of plant were harvested. The biomass in each pot was separated by species, and all soil was washed from the roots and dried at 65 °C for 72 h. In each PVC tube (microenvironment), soil from the topsoil was collected with a soil auger (4 cm diameter). At each sampling, the shovel was cleaned, scrubbed with 75% alcohol wipes and air dried. Soil samples were packed in polyethylene bags and dispensed in 5 ml PE tubes, immediately stored in coolers with ice packs and transported to the laboratory. Soil samples were sieved (2 mm) and all visible roots, debris and stones were removed. Soil samples packed in polyethylene bags were stored at 4◦C for measuring the biogeochemical properties and enzymes of the soil, and soil dispensed in PE tubes stored at -80◦C for soil DNA extraction.
Determination of physical and chemical properties of soils
The soil water content (WC) used gravimetric analysis, i.e., 30 g of fresh soil was weighed in an oven at 105◦C for 48 h until a constant weight was reached. Soil pH was measured using a calibrated pH meter (PHS-3 C) with a fresh soil to water ratio of 1:5. The electrical conductance (EC) is determined by the electrode method using a conductivity meter. Soil organic carbon (SOC) was determined using the potassium dichromate volumetric method (Bao 2000). Soil total nitrogen (TN) content was determined by the semi-micro Kjeldahl method (Bao 2000). Soil total phosphorus (TP) was determined by the alkali fusion-Mo-Sb Anti spectrophotometric method (Bao 2000). The determination of soil fast-acting nitrogen (nitrate, NO3−; nitrate ammonium, NH4+) was performed by KCl leaching and indophenol blue colorimetric method. The content of fast total nitrogen, total phosphorus and fast-acting nitrogen was determined using a flow analyzer (Smartchem 200). Soil available phosphorus (AP) was determined using a 0.5 mol L−1 NaHCO3 leaching-molybdenum antimony colorimetric method (Wang et al. 2022).
Determination of soil microorganisms and enzymes
Each soil sample was weighed at 0.25 g and soil DNA was extracted using the PowerSoil® DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA). Bacterial DNA was extracted using primers 515FB/806RB (GTGYCAGCMGCCGCGGTAA / GGACTACNVGGGTWTCTAAT) (Podell et al. 2019) and fungal DNA was amplified using primers ITS1F/ITS2 (CTTGGTCATTTAGAGGAAGTAA / GCTGCGTTCTTCATCGATGC) (Coince et al. 2013), respectively. The PCR products were then paired-end sequenced using the Illumina Novaseq PE250 platform. The double-end sequences were de-hybridized separately using Trimmomatic software: the sequences below 100 bp in length were finally removed. High-quality double-end sequences were concatenated using flash software with a minimum overlap region of 10 bp and a maximum mismatch rate of 0.2, and sequences containing ambiguous base N were removed. The quality-controlled double-end sequences were noise reduced using the dada2 plug-in of QIIME software, and then the valid sequences were clustered into Amplicon Sequence Variants (ASVs) for each sample based on the 97% sequence similarity. Finally, after sparsifying the rest of the samples based on the lowest ASV number among all samples, the resulting data tables can be used for subsequent analysis. The bacterial and fungal classifications were analyzed against SILVA (Pruesse et al. 2007) and UNITE (Kõljalg et al. 2005), respectively, and this process yielded a total of 27,354 bacterial OTUs and 3617 fungal OTUs.
In this study, three hydrolytic enzymes were measured, namely β-glucosidase related to carbon metabolism, N-acetyl-β-D-glucosaminidase related to N metabolism, and acid phosphatase related to phosphorus metabolism. The determination was performed by the methods supplied by the kit manufacturer (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China). Then we measured the light density of the samples by colorimetric method. The enzyme activity was shown in µmol day−1g−1 dry soil.
Data analysis
The experiment used a block design, thus we specified block as a random effect in all models. Allelopathic plants, N addition and their interactions were considered as fixed factors. Seed germination, early plant survival, and relative abundance of soil microbes were analyzed using generalized linear mixed models with a binomial distribution. Microbial richness was analyzed using generalized linear mixed models with a negative binomial distribution. We also used linear mixed-effects models to assess effects of treatments on plant biomass, root-to-shoot ratio, soil properties, soil enzyme activity, bacterial and fungal Shannon diversity. In addition, for species-level analyses, we also considered the number of neighbouring species nested within block as a random effect. We used testUniformity function and testDispersion function to diagnose the model. For the residuals of the linear mixed models that did not satisfy the assumption of uniformity, we performed a log transformation of the response variable. For the residuals of generalized linear mixed models with over dispersion, we used the glmmTMB function with a beta binomial distribution. P < 0.05 was significant and p < 0.1 was marginally significant.
We conducted a categorical random forest analysis to determine the relative importance of soil physical variables, microbial community and enzyme activity in explaining seed germination, plant survival, biomass and root-shoot ratio. Prediction accuracy was averaged across all trees (10,000) to produce a final importance measure (Breiman 2001), and then we selected the most important attributes to construct piecewise structural equation modelling (piecewise SEM). Subsequently, piecewise SEM were used to synthesize the direct and indirect relationships of allelopathic plants (L. virgaurea), N addition and their interactions on seed germination, plant survival, biomass and root to shoot ratio via biotic and abiotic pathways. Adequacy of piecewise SEM was assessed by Shipley’s d-separation test with Fisher’s C-statistic (Shipley 2009).
All data were analyzed with R version 4.1.0 (R Core Team 2021). We used the freq.calc function in the R package ‘spaa’ to calculate relative abundance of microbes. We used the specnumber and diversity functions in the R package ‘vegan’ to calculate richness and Shannon diversity, respectively. The functions of the diagnostic model were derived from the ‘DHARMa’ package (Hartig 2019) and the ‘glmmTMB’ package was used to assess the significance of the model (Brooks et al. 2017). The ‘lme4’ package was used for conducting mixed-effects models. The ‘emmeans’ package was used to compute least squares means and standard errors (Lenth et al. 2020), the ‘piecewiseSEM’ package was used to compute piecewise SEM (Lefcheck 2016), and the ‘ggplot2’ package (Wickham 2008) was used to plot figures.
Results
Soil properties, microbial community composition, and enzyme activity
The results of our linear mixed model showed that N addition increased soil NH4+ (Table 1; Table S2). While the pair-wise test showed no significant differences in soil EC and WC between different treatments, there was a trend for N addition decreased soil EC and L. virgaurea increased soil WC (Table 1; Table S2). The negative effects of the presence of L. virgaurea on soil pH and BD tended to be reduced by N addition, and the positive effects of the presence of L. virgaurea on the soil TP tended to be reduced by N addition (Table 1; Table S2). There was no significant effect of N addition and the presence of L. virgaurea on SOC, NO3−, TN and the AP (Table 1).
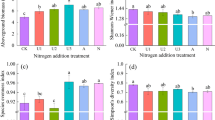
We found that the presence of L. virgaurea increased soil bacterial richness (Fig. 1a; Table 2). Bacterial Shannon diversity, soil fungal richness, and fungal Shannon diversity were not significantly affected by the treatments (Fig. 1b-d; Table 2). The relative abundance of bacteria belonged mostly to eight different phyla and the relative abundance of fungi belonged mostly to three different phyla (Table 1). We found that there was a trend for the presence of L. virgaurea increased the relative abundance of Actinobacteriota (Table 1; Table S2). Besides, there was an interactive effect between N addition and the presence of L. virgaurea on the relative abundance of Proteobacteria and Acidobacteriota (Table 1). Specifically, the negative effect of the presence of L. virgaurea on the relative abundance of Proteobacteria was reversed by N addition, and the positive effect of the presence of L. virgaurea on the relative abundance of Acidobacteriota was decreased by N addition (Table S2).
Effects of L. virgaurea, nitrogen addition and their interactions on (a) bacteria richness, (b) bacteria Shannon diversity, (c) fungal richness, (d) fungal Shannon diversity. The different lowercases show significant between treatments (P < 0.05). Error bars represent means ± standard error. Table 2 for details of models’ outputs
We found that the presence of L. virgaurea increased soil acid phosphatase by 4.7% (Fig. 2c; Table 2), while N addition reduced soil acid phosphatase by 6.8%. In addition, the interaction between N addition and L. virgaurea on soil acid phosphatase was significant (Fig. 2c; Table 2). Specifically, the increase in soil acid phosphatase occurred only in the presence of L. virgaurea, and the N addition was able to counterbalance this effect. There was no significant effect of treatments on soil N-acetyl-β-glucosaminidase and soil β-glucosidase (Fig. 2a, b; Table 2).
Effects of L. virgaurea, nitrogen addition and their interactions on (a) N-acetyl-β-glucosaminidase (S-NAG), (b) soil β-glucosidase(S-β-GC), (c) soil acid phosphatase (S-ACP). The different lowercases show significant between treatments (P < 0.05). Error bars represent means ± standard error. Table 2 for details of models’ outputs
Seed germination, early plant survival, and growth
The presence of L. virgaurea increased the germination by 46.9%, plant survival by 36%, and biomass by 190.8%, respectively (Fig. 3a-c; Table 3). The presence of L. virgaurea decreased root–shoot ratio by 46.9% (Fig. 3d; Table 3). Nitrogen addition increased early plant survival by 30.7% and increased biomass by 143.4% (Fig. 3b-c; Table 3). Moreover, the interaction between N addition and L. virgaurea had a significant effect on seed germination: the positive effect of the presence of L. virgaurea on seed germination was offset by N addition (Fig. 3a; Table 3). At the species level, our results showed that the presence of L. virgaurea increased the germination of Elymus, Delphinium, and Tibetia (Fig. S2a-c). There was a marginally significant interaction effect on germination of Delphinium (Fig. S2b). The presence of L. virgaurea increased early survival of Elymus (Fig. S3a). There was a trend of increased early survival of Delphinium with N addition (Fig. S3b). The presence of L. virgaurea alone and the addition of N alone increased biomass of Elymus (Fig. S4a). Moreover, the presence of L. virgaurea alone and N addition alone positively affected the root-shoot ratio of Tibetia (Fig. S5c). We found that seed germination, biomass, and root–shoot ratio were all significantly affected by species identity, except for early survival (Table S3).
Effects of L. virgaurea, nitrogen addition and their interactions on (a) total seed germination, (b) total plant early survival, (c) total plant biomass, and (d) total root-shoot ratio for all species (Elymus, Delphinium, and Tibetia). The different lowercases show significant between treatments (P < 0.05). Error bars represent means ± standard error. Table 3 details of models’ outputs
Our SEM explained 61%, 36%, 40%, 37% of the variation in the germination, survival, biomass, and root–shoot ratio, respectively (Fig. 4a-d). Specifically, the SEM analysis suggested that the presence of L. virgaurea had a direct positive effect on seed germination. In addition, the presence of L. virgaurea had an indirect positive effect on germination via acid phosphatases. Nitrogen addition had an indirect positive effect on germination via NH4+ and WC, but N addition also indirectly negatively affected germination via acid phosphatases (Fig. 4a). This may mean that there was no significant effect of N addition on seed germination by positive and negative offsetting each other (Fig. S6a). The interaction between N addition and the presence of L. virgaurea negatively affected germination via acid phosphatases (Fig. 4a). The presence of L. virgaurea alone or N addition alone had a direct positive effect on survival (Fig. 4b). Nitrogen addition had a direct positive effect on biomass; the presence of L. virgaurea had an indirect positive effect on biomass via bacterial richness (Fig. 4c). Moreover, there was a direct negative effect of L. virgaurea on root-shoot ratio; L. virgaurea also positively affected the root-shoot ratio indirectly through bacterial richness and Shannon diversity (Fig. 4d). However, the total effect of L. virgaurea was a negative effect on the root-shoot ratio (Fig. S6d).
Results of the SEM analyses indicating effects of L. virgaurea, nitrogen addition and their interactions on (a) seeds germination (b) plant early survival, (c) plant biomass, and (d) root-shoot ratio. Only marginally significant and significant pathways were shown. Blue and red solid arrows indicate significant positive and negative effects (at the level P < 0.05), respectively. While the dashed arrows indicate marginally significant effect (at level P < 0.1). Arrow width corresponds directly to the standardized path coefficient. R2 values associated with response variables indicate the proportion of explained variation by relationships with other variables. Values associated with arrows represent standardized path coefficients. (*): P < 0.1, *: P < 0.05, **: P < 0.01, ***: P < 0.001
Discussion
Positive effects of L. virgaurea on seed germination, early survival, and biomass, and negative effects on plant root-to-shoot ratio
We found that the presence of L. virgaurea significantly increased seed germination, early survival and biomass of alpine plants. Several previous studies found that some plants secrete allelochemicals that exert negative effects on neighboring plants and exhibit negative effects on the seed germination and plant performance (Callaway and Ridenour 2004; Inderjit et al. 2011; Kim and Lee 2011). In our study, the observed effects may be because L. virgaurea densities are not high enough to have a negative impact. It has been found that at low densities, L. virgaurea has a positive effect on the maintenance of the productivity and diversity of grasslands (Wang et al. 2008). We found that aboveground biomass was higher than belowground biomass in the presence of L. virgaurea, which may be related to the shade of L. virgaurea. For example, Reich (2014) proposed that leaf traits are closely related to plant ecological processes, and in alpine meadow plant communities, L. virgaurea has a relatively large leaf area that favors high light interception, can lead to the growth of above-ground parts of plants in its lower layers to compete for light resources. In the absence of light, plants allocate more biomass to aboveground parts to compete for space and light for their own growth and reproduction (Mokany et al. 2006). However, light interception data was not measured in this study to reflect the shading effect of L. virgaurea. Therefore, future studies need to be refined in order to obtain a more comprehensive and reasonable explanation.
Our piecewise SEM results showed that L. virgaurea increased seed germination both directly, and indirectly through increasing soil acid phosphatase. Shi et al. (2011) had previously showed an increased phosphatase activity under L. virgaurea patches compared with normal grassland, which was associated with increased soil microbial biomass through the quality and quantity of litter and exudates. Soil acid phosphatase has a crucial catalytic function in promoting seed germination (Seneviratne et al. 2019) and we were able to detect a trend confirming this effect. Moreover, we found that L. virgaurea increased plant biomass via soil bacterial community richness. It has been previously shown that an allelopathic plant (Acacia dealbata) alters soil microbial community structure and increases bacterial richness (Lorenzo et al. 2010). Our results suggest that the increase in bacterial richness promoted by the presence of L. virgaurea influenced nutrient cycling in the soil and provided an enhanced soil environment for plant growth. In addition, L. virgaurea had no significant effect on fungal communities. This result may be due to the fact that soil fungi are more stable than bacteria and less affected by allelochemicals, litter and other mechanisms (Agnelli et al. 2004; Lorenzo et al. 2013). We found that L. virgaurea was associated with an increase in bacterial richness, which in turn affected Shannon diversity indirectly affecting the root-to-shoot ratio. A recent study showed significant changes in the structure and diversity of soil microbial communities in plant communities dominated by L. virgaurea and L. sagitta (Ade et al. 2021). In our study, L. virgaurea had a tendency to increase the relative abundance of Actinomycetes. Actinomycetes can protect seeds and plants using enzymes and anti-fungus compounds to inhibit the growth of potential pathogens (Jones et al. 2017).
Positive effects of N addition on early survival and plant biomass
Our results showed that N addition increased seedling survival and biomass. Alpine meadows are distributed in high altitude areas all over the world, and are a common ecosystem in the Qinghai-Tibet Plateau. Despite high soil N stores, N limitation is frequent because of slow mineralization of soil organic matter under low temperature and water deficiency in alpine meadow ecosystems (Baumann et al. 2009; Chen et al. 2013). Nitrogen addition can increase the photosynthetic efficiency of plants, thereby increasing plant survival. In addition, plants can accumulate more above-ground biomass when soil N resources are unrestricted (Wang et al. 2022). Therefore, increasing N inputs can increase the amount of available N in the soil, eliminate or alleviate N limitation, improve seedling survival, and promote plant growth (Meng et al. 2021; Zhao et al. 2021; Cai et al. 2023).
Different functional groups respond differently to N addition: we found a significant effect of N addition only on the biomass of Elymus (grass), but not on that of the other two species, Delphinium (forb) and Tibetia (legume). Grasses are tall plants in alpine herb communities and have fibrous root systems. Thus, Elymus (a dominant grass in these communities) may compete rapidly and effectively for water, nutrients and light in alpine communities (Hautier et al. 2009), while Delphinium and Tibetia are mainly found in the understory. Although N addition can alleviate the nutrient limitation of herbaceous growth (Alvarez-Clare et al. 2013), the lack of effect on Delphinium and Tibetia could be due to the enhanced shading effect of the tall and fast growing Elymus forming the upper canopy .Based on the SEM results, we found that ammonia nitrogen and water content associated with N addition had an indirect positive effect on germination, but N addition had a negative effect by affecting acid phosphatase. These effects may counteract each other, resulting in no significant effect of N addition on germination.
Negative effects of interaction between L. virgaurea and N addition on seed germination
We found that N addition counterbalanced the increase in seed germination in the presence of L. virgaurea by exerting an opposing negative effect on acid phosphatase activity. Acid phosphatase is mainly produced by plant roots (Speir and Cowling 1991; Susanne KraÈmer and Green 2000). We observed that N addition inhibited the positive effect of L. virgaurea on acid phosphatase. We speculated that with increased N, plants may reduce the allocation of belowground resources by reducing root yield and using more nutrients for aboveground growth and reproduction (Wallenstein et al. 2006; Kiær et al. 2013). That altered allocation strategies of allelopathic plants have potential negative effects on the synthesis and activity of soil acid phosphatase. Thus, the simultaneous presence of allelopathic plants and N addition is unfavourable for seed germination. In addition, our results showed that the coverage of L. virgaurea was significantly higher with the N addition. This was perhaps due to the fact that N addition increases N availability, which stimulates L. virgaurea growth and canopy cover, which can increase its capacity to intercept light. Therefore, with N addition, light availability may be lower under canopy of L. virgaurea. Light is one of the key factors for seed germination, light can also regulate the levels of many enzymes and hormones that affect seed respiration and metabolism, as well as the transport of substances (Li et al. 2020). Thus, the interaction between N addition and L. virgaurea reduces seed germination, suggesting that it is necessary to consider the potential interaction of multiple factors for seed germination and early seedling establishment.
Our results showed that there was an interaction between N addition and L. virgaurea on the relative abundance of Proteobacteria. In particular, nitrogen addition counteracted the negative effect of L. virgaurea on the relative abundance of Proteobacteria, possibly because Proteobacteria are eutrophic bacteria that thrive in nutrient-rich environments (Dai et al. 2018). Consequently, N addition may be beneficial for Proteobacteria colonization. Previous studies have shown a positive correlation between Proteobacteria abundance and plant growth under shrubs in soil ecosystems (Lozano et al. 2017), however, we did not observe such an association in our study. Perhaps the duration of our experiment was relatively short, and future studies should take into account whether temporal changes in microbial abundance can exert significant impacts on plant growth.
Conclusion
Our results indicate that the presence of allelopathic plants and N addition may not only individually affect the regenerative stages of alpine plant communities on the Tibetan Plateau but also mutually modulate the effects of each other. We found a positive effect of allelopathic plants alone and/or N addition alone on seed germination, seedling survival and growth. However, the positive effect of allelopathic plants on seed germination can be cancelled out by N addition. Such phenomena may be attributed to the decrease of soil acid phosphatase associated with allelopathic plants by N addition. Overall, our study highlights the need to understand the interactions between factors associated with climate change (e.g., N deposition) and the presence of allelopathic plants to better predict plant community dynamics and whether management and conservation measures succeed or fail in the alpine grasslands of the Tibetan Plateau.
Data availability
The datasets are available from corresponding authors upon reasonable request.
References
Ade L, Millner JP, Hou F (2021) The dominance of Ligularia spp. related to significant changes in soil microenvironment. Ecol Indic 131:108183. https://doi.org/10.1016/j.ecolind.2021.108183
Agnelli A, Ascher J, Corti G et al (2004) Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol Biochem 36:859–868. https://doi.org/10.1016/j.soilbio.2004.02.004
Alvarez-Clare S, Mack MC, Brooks M (2013) A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 94:1540–1551. https://doi.org/10.1890/12-2128.1
An H, Zhao Y, Ma M (2020) Precipitation controls seed bank size and its role in alpine meadow community regeneration with increasing altitude. Glob Chang Biol 26:5767–5777. https://doi.org/10.1111/gcb.15260
Bao S (2000) Soil agro-chemistrical analysis. Chinese Agricultural Press, Beijing in Chinese
Basto S, Thompson K, Phoenix G et al (2015) Long-term nitrogen deposition depletes grassland seed banks. Nat Commun 6:1–6. https://doi.org/10.1038/ncomms7185
Baumann F, He JS, Schmidt K et al (2009) Pedogenesis, permafrost, and soil moisture as controlling factors for soil nitrogen and carbon contents across the Tibetan Plateau. Glob Chang Biol 15:3001–3017. https://doi.org/10.1111/j.1365-2486.2009.01953.x
Bird EJ, Choi YD (2017) Response of native plants to elevated soil nitrogen in the sand dunes of Lake Michigan, USA. Biol Conserv 212:398–405. https://doi.org/10.1016/j.biocon.2016.12.001
Bobbink R, Hicks K, Galloway J et al (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59. https://doi.org/10.1890/08-1140.1
Breiman L (2001) Random forest. Mach Learn 45:5–32
Broadbent A, Stevens CJ, Peltzer DA et al (2018) Belowground competition drives invasive plant impact on native species regardless of nitrogen availability. Oecologia 186:577–587. https://doi.org/10.1007/s00442-017-4039-5
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glm-mTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/rj-2017-066
Cai J, Fu J, Liu H et al (2023) Divergent responses of leaf mineral nutrient concentrations among plant families and functional groups to nitrogen addition and irrigation in a semi-arid grassland. Plant Soil. https://doi.org/10.1007/s11104-023-05904-z
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443. https://doi.org/10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
Chen H, Zhu Q, Peng C et al (2013) The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob Chang Biol 19:2940–2955. https://doi.org/10.1111/gcb.12277
Clark CM, Cleland EE, Collins SL et al (2007) Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol Lett 10:596–607. https://doi.org/10.1111/j.1461-0248.2007.01053.x
Coince A, Caël O, Bach C et al (2013) Below-ground fine-scale distribution and soil versus fine root detection of fungal and soil oomycete communities in a French beech forest. Fungal Ecol 6:223–235. https://doi.org/10.1016/j.funeco.2013.01.002
Dai Z, Su W, Chen H et al (2018) Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob Chang Biol 24:3452–3461. https://doi.org/10.1111/gcb.14163
Davis AS (2007) Nitrogen Fertilizer and Crop Residue effects on seed mortality and germination of eight Annual Weed species. Weed Sci 55:123–128. https://doi.org/10.1614/ws-06-133.1
Duprè C, Stevens CJ, Ranke T et al (2010) Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Glob Chang Biol 16:344–357. https://doi.org/10.1111/j.1365-2486.2009.01982.x
Fenner M, Thompson K (2005) The ecology of seeds. Cam-bridge University Press, Cambridg
Ganjurjav H, Gornish ES, Hu G et al (2020) Warming and precipitation addition interact to affect plant spring phenology in alpine meadows on the central Qinghai-Tibetan Plateau. Agric for Meteorol 287:107943. https://doi.org/10.1016/j.agrformet.2020.107943
Hartig F (2019) DHARMa: Residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.2.4. https://CRAN.R-project.org/package=DHARMa. Assessed 24 Aug 2020
Hautier Y, Niklaus PA, Hector A (2009) Competition for light causes plant biodiversity loss after eutrophication. Sci (80-) 324:636–638. https://doi.org/10.1126/science.1169640
Huang K, Kardol P, Yan X et al (2021) Plant–soil biota interactions explain shifts in plant community composition under global change. Funct Ecol 35:2778–2788. https://doi.org/10.1111/1365-2435.13940
Humbert JY, Dwyer JM, Andrey A, Arlettaz R (2016) Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: a systematic review. Glob Chang Biol 22:110–120. https://doi.org/10.1111/gcb.12986
Inderjit EH, Crocoll C et al (2011) Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 92:316–324. https://doi.org/10.1890/10-0400.1
Jones SE, Ho L, Rees CA et al (2017) Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife 6:1–21. https://doi.org/10.7554/eLife.21738
Kiær LP, Weisbach AN, Weiner J (2013) Root and shoot competition: a meta-analysis. J Ecol 101:1298–1312. https://doi.org/10.1111/1365-2745.12129
Kim YO, Lee EJ (2011) Comparison of phenolic compounds and the effects of invasive and native species in East Asia: support for the novel weapons hypothesis. Ecol Res 26:87–94. https://doi.org/10.1007/s11284-010-0762-7
Kõljalg U, Larsson KH, Abarenkov K et al (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1068. https://doi.org/10.1111/j.1469-8137.2005.01376.x
Lefcheck JS (2016) piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579. https://doi.org/10.1111/2041-210X.12512
Lenth R, Singmann H, Love J, Buerkner P, Herve M et al (2020) Emmeans: estimated marginal means, aka least-squares means. Available at: https://CRAN.R-project.org/package=emmeans. Accessed 16 Oct 2021
Li S, Liu C, Tan X et al (2020) Interactive effects of light and nitrogen on pakchoi (Brassica chinensis l.) growth and soil enzyme activity in an underground environment. Agronomy 10:1772. https://doi.org/10.3390/agronomy10111772
Lorenzo P, Rodríguez-Echeverría S, González L, Freitas H (2010) Effect of invasive Acacia dealbata link on soil microorganisms as determined by PCR-DGGE. Appl Soil Ecol 44:245–251. https://doi.org/10.1016/j.apsoil.2010.01.001
Lorenzo P, Pereira CS, Rodríguez-Echeverría S (2013) Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata link. Soil Biol Biochem 57:156–163. https://doi.org/10.1016/j.soilbio.2012.08.018
Lozano YM, Armas C, Hortal S et al (2017) Disentangling above- and below-ground facilitation drivers in arid environments: the role of soil microorganisms, soil properties and microhabitat. New Phytol 216:1236–1246. https://doi.org/10.1111/nph.14499
Meng B, Li J, Maurer GE et al (2021) Nitrogen addition amplifies the nonlinear drought response of grassland productivity to extended growing-season droughts. Ecology 102:1–10. https://doi.org/10.1002/ecy.3483
Midolo G, Alkemade R, Schipper AM et al (2019) Impacts of nitrogen addition on plant species richness and abundance: a global meta-analysis. Glob Ecol Biogeogr 28:398–413. https://doi.org/10.1111/geb.12856
Mishra S, Nautiyal CS (2012) Reducing the allelopathic effect of Parthenium hysterophorus L. on wheat (Triticumaestivum L.) by Pseudomonas putida. Plant Growth Regul 66:155–165. https://doi.org/10.1007/s10725-011-9639-1
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Glob Chang Biol 12:84–96. https://doi.org/10.1111/j.1365-2486.2005.001043.x
Niu K, Luo Y, Choler P, Du G (2008) The role of biomass allocation strategy in diversity loss due to fertilization. Basic Appl Ecol 9:485–493. https://doi.org/10.1016/j.baae.2007.06.015
Oesterheld M, Oyarzábal M (2004) Grass-to-grass protection from grazing in a semi-arid steppe. Facilitation, competition, and mass effect. Oikos 107:576–582. https://doi.org/10.1111/j.0030-1299.2004.13442.x
Onipchenko VG, Makarov MI, Akhmetzhanova AA et al (2012) Alpine plant functional group responses to fertiliser addition depend on abiotic regime and community composition. Plant Soil 357:103–115. https://doi.org/10.1007/s11104-012-1146-2
Plassmann K, Brown N, Laurence JM, Edwards-Jones G (2008) Can atmospheric input of nitrogen affect seed bank dynamics in habitats of conservation interest? The case of dune slacks. Appl Veg Sci 11:413–420. https://doi.org/10.3170/2008-7-18498
Podell S, Blanton JM, Neu A et al (2019) Pangenomic comparison of globally distributed Poribacteria associated with sponge hosts and marine particles. ISME J 13:468–481. https://doi.org/10.1038/s41396-018-0292-9
Pruesse E, Quast C, Knittel K et al (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. https://doi.org/10.1093/nar/gkm864
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Reich PB (2014) The world-wide fast-slow plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Rice EL (1974) Allelopathy. Academic Press, New York, p 353
Rice EL (1984) Allelopathy. Academic Press, Orlando, p 422
Seneviratne M, Rajakaruna N, Rizwan M et al (2019) Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review. Environ Geochem Health 41:1813–1831. https://doi.org/10.1007/s10653-017-0005-8
Shi XM, Li XG, Wu RM et al (2011) Changes in soil biochemical properties associated with Ligularia Virgaurea spreading in grazed alpine meadows. Plant Soil 347:65–78. https://doi.org/10.1007/s11104-011-0818-7
Shi GX, Wang WY, Jiang SJ et al (2018) Effects of the spreading of Ligularia Virgaurea on soil physicochemical property and microbial functional diversity. Chin J Plant Ecol 42:126–132. https://doi.org/10.17521/cjpe.2017.0111
Shipley B (2009) Confirmatory path analysis in a generalized multilevel context. Ecology 90:363–368. https://doi.org/10.1890/08-1034.1
Speir TW, Cowling JC (1991) Phosphatase activities of pasture plants and soils: relationship with plant productivity and soil P fertility indices. Biol Fertil Soils 12:189–194. https://doi.org/10.1007/BF00337200
Sun G, Luo P, Wu N et al (2009) Stellera Chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern tibetan Plateau of China. Soil Biol Biochem 41:86–91. https://doi.org/10.1016/j.soilbio.2008.09.022
Susanne KraÈmer DMG, Green DM (2000) Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol Biochem 32:179–188
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Vieira S, Sikorski J, Dietz S et al (2020) Drivers of the composition of active rhizosphere bacterial communities in temperate grasslands. ISME J 14:463–475. https://doi.org/10.1038/s41396-019-0543-4
Wallenstein MD, McNulty S, Fernandez IJ et al (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manage 222:459–468. https://doi.org/10.1016/j.foreco.2005.11.002
Wang X, Yan X, Huang K et al (2022) Nitrogen enrichment and warming shift community functional composition via distinct mechanisms: the role of intraspecific trait variability and species turnover. Funct Ecol 36:1230–1242. https://doi.org/10.1111/1365-2435.14012
Wang MT, Zhao ZG, Du GZ, He YL (2008) Effects of light on the growth and clonal reproduction of Ligularia Virgaurea. J Integr Plant Biol 50:1015–1023. https://doi.org/10.1111/j.1744-7909.2008.00645.x
Wickham H (2008) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wu GL, Ren GH, Shi ZH (2011) Phytotoxic effects of a dominant weed Ligularia Virgaurea on seed germination of Bromus inermis in an alpine meadow community. Plant Ecol Evol 144:275–280. https://doi.org/10.5091/plecevo.2011.459
Xia J, Wan S (2008) Global response patterns of terrestrial plant species to nitrogen addition. New Phytol 179:428–439. https://doi.org/10.1111/j.1469-8137.2008.02488.x
Xiao Y, Li C, Yang Y et al (2020) Soil fungal community composition, not assembly process, was altered by nitrogen addition and precipitation changes at an alpine steppe. Front Microbiol 11:1–12. https://doi.org/10.3389/fmicb.2020.579072
Zhang Y, Loreau M, Lü X et al (2016) Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Glob Chang Biol 22:1445–1455. https://doi.org/10.1111/gcb.13140
Zhang Z, Liu Y, Yuan L et al (2021) Effect of allelopathy on plant performance: a meta-analysis. Ecol Lett 24:348–362
Zhang Z, Sun J, Liu M et al (2020) Don ’ t judge toxic weeds on whether they are native but on their ecological effects. Ecol Evol 9014–9025. https://doi.org/10.1002/ece3.6609
Zhao DD, Ma HY, Wang L et al (2021) Effects of water and nitrogen addition on the seed yield and germination characteristics of the Perennial Grass Leymus chinensis (trin.) Tzvel. Front Environ Sci 9:1–8. https://doi.org/10.3389/fenvs.2021.704097
Zhong M, Miao Y, Han S, Wang D (2019) Nitrogen addition decreases seed germination in a temperate steppe. Ecol Evol 9:8441–8449. https://doi.org/10.1002/ece3.5151
Zong N, Shi P, Song M et al (2016) Nitrogen critical loads for an Alpine Meadow Ecosystem on the Tibetan Plateau. Environ Manage 57:531–542. https://doi.org/10.1007/s00267-015-0626-6
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41830321, 32071532, 31870412), Joint Funds of National Natural Science Foundation of China (U21A20186), the “111 Project” (BP0719040), the Natural Science Foundation of Gansu Province (22JR5RA402), the Second Tibetan Plateau Scientific Expedition and Research:Program (2019QZKK0302) and Core Facility of School of Life Sciences, Lanzhou University. We thank Yanhu Li and Yaya Chen for helpful comments on this work. We are also grateful to the editors and anonymous reviewers for many valuable comments on our manuscript.
Author information
Authors and Affiliations
Contributions
S.X. and J.W. designed the experiments; K.L., Z.L., X.J., J.L., J.C., H.C., L.A., H.S., Z.Y., J.W. and Y.W. collected field and laboratorial data. J.W. and S.X. performed statistical analysis and made the figures. J.W. drafted the manuscript. S.C., S.P.B. and S.X. revised the manuscript. All authors contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Roberta L C Dayrell.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 0.98 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Liu, K., Bonser, S.P. et al. Nitrogen addition reduces the positive effect of Ligularia virgaurea on seed germination of alpine species on the Tibetan Plateau. Plant Soil 501, 307–321 (2024). https://doi.org/10.1007/s11104-024-06517-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-024-06517-w