Abstract
Aim
To identify the main factors affecting Al mobilization in soils from different climate zones.
Methods
XRD and 27Al NMR were used to analyse clay minerals and the relative contents of four- and six-coordination Al. Soil acidification and Al mobilization were studied by constant pH automatic potentiometric titrator.
Results
The relative content of tetrahedral Al (AlIV) in the soils increased gradually from low to high latitude, and an opposite trend was observed for soil octahedral Al (AlVI). The larger organic carbon (OC) content in surface soils (0–20 cm) effectively inhibited both soil acidification and Al mobilization compared with subsurface soils (20–40 cm). The content of mobilized Al followed the order: Inceptisol > Alfisol > Mollisol > Ultisol in both surface and subsurface soils, which determined by soil CEC, the contents of OC and clay and relative contents of AlIV and AlVI. After removal of OC from soil colloids, the amounts of mobilized Al were consistent with the relative contents of AlIV in soil colloids and followed Mollisol > Inceptisol > Alfisol > Ultisol, suggesting that the solid Al in the soils from temperate and north subtropical regions was readily mobilized during soil acidification.
Conclusions
Al mobilization in different soils mainly depended on the CEC, the contents of OC and clay, and the coordination nature of Al in the soils. A larger CEC, a lower OC content, a greater clay content, and a higher content of AlIV led to a greater amount of mobilized Al during soil acidification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil acidification is the main factor restricting agricultural production and development all over the world, especially in tropical and subtropical areas. The trend of soil acidification has become more and more serious over the past few decades due to the anthropogenic activities such as excessive application of chemical nitrogen fertilizer (Bolan et al. 1991; Vonuexkull and Mutert 1995; Hajkowicz and Young 2005; Guo et al. 2010; Bian et al. 2013) and acid deposition (Reis et al. 2012; Yu et al. 2016). Soil acidification will lead to the mobilization of toxic and harmful heavy metals in soils, such as Cd, Cu, and Pb (Reddy et al. 1995; Blake and Goulding 2002; Yang et al. 2010; Kunhikrishnan et al. 2016). Microbial community structure and diversity in the soil can also be adversely affected (Schimel and Weintraub 2003; Zhang et al. 2015; Riggs and Hobbie 2016; Li et al. 2018). On the other hand, one of the most serious problems caused by soil acidification is the damage caused by toxic Al3+ to crop roots, resulting in the reduction of crop yields or even no harvest (Flores et al. 1988; Kopittke et al. 2016; Zhao et al. 2020a; Zhu et al. 2020). Soil acidification can be divided into natural acidification and the acidification caused by anthropogenic activities. Natural soil acidification is generally a process of continuous leaching loss of base cations (Ca2+, Mg2+, K+, and Na+) from soil cation exchange sites and increasing of exchangeable H+ and exchangeable Al3+ (Raza et al. 2020) under the condition that precipitation is greater than evapotranspiration (Slessarev et al. 2016; Fujii et al. 2021). All these reactions are natural processes accompanying the genesis and evolution of soils (Krug and Frink 1983; Kunhikrishnan et al. 2016). When a soil contains carbonate, the H+ ions entering the soil will first react with the carbonate (Sanderman 2012). When the soil pH is lower than 6.2 (Ulrich 1986), the carbonate in the soil is exhausted, and the H+ ions are mainly consumed in the weathering of phyllosilicate minerals and reacting with exchangeable base cations on the soil to produce exchangeable H+(Raza et al. 2020).
With the progress of these reactions, the H+ ions adsorbed on the soil surface increase continuously, and these exchangeable H+ ions will further undergo the H/Al conversion processes to produce a large amount of exchangeable Al3+ (Yu 1997; Raza et al. 2020; Li et al. 2022a). This is the main mechanism for the production of exchangeable Al3+ in the natural soil acidification process. However, the detailed mechanisms for H/Al transformation during soil acidification remain elusive. Al mobilization means the process by which the Al releases from soil minerals into solution or soil exchangeable sites as soluble and exchangeable Al during soil acidification. Some soil properties may affect Al mobilization during soil acidification. Our recent study showed that the mobilization of Al during soil acidification was mainly affected by the cation exchange capacity (CEC) and content of organic matter (OM) (Li et al. 2022a). In addition, the coordination nature of the solid phase Al and hydroxyl groups on the mineral surfaces also have important effects on the mobilization of Al (Li et al. 2023a, b). Reducing the hydroxyl groups on the surfaces of minerals can effectively inhibit the mobilization of Al during acidification process (Li et al. 2023a). In minerals and soils, tetrahedral Al was more readily mobilized than octahedral Al (Li et al. 2023b). Soil acidification is slow under natural conditions. However, anthropogenic activities accelerate soil acidification greatly and thus increase the production of soil exchangeable Al3+. For example, the nitrification of ammonium in farmland soils under the condition of excessive application of ammonium nitrogen fertilizer can accelerate soil acidification (Chien et al. 2008; Guo et al. 2010; Kunhikrishnan et al. 2016). Acid deposition caused by industrial activities is another important human factor that accelerates soil acidification (Reis et al. 2012; Yu et al. 2016).
Climate plays an important role in soil formation. Climate can also affect soil fertility and other properties by affecting soil biological processes (Rozhkov 2009; Kozun et al. 2022). There are complex and diverse climate types and a significant continental monsoon climate, with tropical, subtropical, and temperate heat belts in China. Therefore, soil types in China also show various characteristics under the influence of complex and diverse climates. In the tropical and south subtropical regions with low latitudes, heavy rainfall, and high-temperature conditions lead to intense chemical weathering and leaching loss of solutes, and apparent desilication and allitization (Van Breemen and Buurman 2002). Some primary minerals such as feldspar and mica and 2:1 type secondary minerals undergo weathering to form kaolinite, goethite, gibbsite, and even lepidocrocite under wet conditions. Because the clay minerals in the soils in these areas are mainly kaolinite with good crystallization, soil CEC is low. Such soils are generally referred to as Ultisol or Oxisol (Soil Survey Staff 2022).
In the north subtropics and mid-latitudes of the warm temperate zones, warm and humid climates accelerate chemical weathering and eluviation of the soils in the regions. However, the rates of chemical weathering vary due to the wide range of temperature changes and the varying intensity of rainfall (Deepthy and Balakrishnan 2005; Stefaan et al. 2022). The clay minerals in the soils of the northern subtropics and warm temperate zones are mainly 2:1 type clay minerals such as hydromica, vermiculite, or chlorite, which are produced by the weathering of primary minerals (Xiong and Li 1987). Therefore, the CEC of the soils is greater and the nutrient retention ability of the soils is stronger because 2:1 type phyllosilicate minerals carry more negative charges due to more isomorphic substitutions in the minerals compared with 1:1 type kaolinite. This type of soil is commonly called Alfisol or Inceptisol. For the cold temperate zone with high latitude, the temperature of the region is low all year round, so the soil microbial activity is low, and soil OM is not easy to decompose. Because the content of soil OM in the region is usually high, the soil is often black, containing mainly hydromica and kaolinite, and the soil nutrient content is high (Xiong and Li 1987). Due to the phenomenon of isomorphic substitution, there are usually two kinds of solid phase Al with four (AlIV) and six (AlVI) coordination structures in minerals. The contents of two kinds of Al in soils are closely related to the type and quantity of clay minerals (Li et al. 2023b).
The characteristics of soil microbial community and composition under different climates with different latitudes (Zhao et al. 2016; Kozun et al. 2022) and physical and chemical properties of soils (Larionova et al. 2015; Wu et al. 2016; Zhang et al. 2019; Zhao et al. 2020b; Peng et al. 2022) have been investigated extensively. However, few studies have involved the characteristics of Al mobilization during the acidification of the soils from different climate zones. The acidification problem is particularly prominent in the subtropical and tropical regions in the south of China, and previous studies mainly focused on the acidification of Ultisols in these regions (Zhou et al. 2014; Zhang et al. 2016). In recent years, soil acidification was also observed for Alfisol, Inceptisol, and Mollisol in north subtropical and temperate regions (Liu et al. 2010; Zhang et al. 2020; Li et al. 2022b; Yin et al. 2023).
In this study, four typical soils were collected from different climate zones at low, middle, and high latitudes in China: Ultisol from Qiyang, Hunan Province; Alfisol from Zhenjiang, Jiangsu Province; Inceptisol from Weihai, Shandong Province; and Mollisol from Nenjiang, Heilongjiang Province. Soil Al mobilization is so sensitive to pH change that we need to accurately control the pH of the system to compare the differences in the amount of Al mobilization. The soils were acidified using a constant pH automatic potentiometric titrator and then Al mobilization was investigated to (1) clarify the differences of acid consumption and Al mobilization among different soils and (2) identify the factors affecting soil acidification and Al mobilization in different soils. The results obtained in the present study will provide useful references for the inhibition of soil acidification and Al mobilization as well as the amelioration of acidic soils in various climate zones.
Materials and methods
Soil sampling and analysis
Four typical soils were collected from different climate zones and used in this study. The Ultisol was collected from a field of long-term field experiment in Qiyang County, Yongzhou City, Hunan Province (26° 45′ N, 111° 52′ E) and the Alfisol was collected from Jurong County, Zhenjiang City, Jiangsu Province (32° 10′ N, 119° 11′ E). The Inceptisol was collected from Tianfu Mountain, Weihai City, Shandong Province (37° 14′ N, 122° 12′ E), while the Mollisol was collected from Nenjiang County, Heihe City, Heilongjiang Province (48° 57′ N, 125° 14′ E). The surface (0–20 cm) and subsurface (20–40 cm) soils were sampled from each site. The four soils above were derived from Quaternary red earth, Xiashu loess, granite, and diluvial loess, respectively.
The soil samples were air dried, finely ground, passed through 2-mm sieve for the determination of soil pH and dissolved organic carbon (DOC), 0.85-mm sieve for the determination of CEC, and 0.25-mm sieve for the determination of Dithionite-Citrate-Bicarbonate (DCB)-extractable Fe/Al oxides and oxalate-extractable Fe/Al oxides as well as soil clay content. Soil pH was determined using an Orion A211 pH meter with a composite glass electrode in a suspension with a soil/water ratio of 1:2.5 (Pansu and Gautheyrou 2006). Soil organic carbon (OC) was determined by the potassium dichromate method (Pansu and Gautheyrou 2006). Soil DOC was determined using the method of Zhang et al. (2007). Briefly, 10.0 g of soil was mixed with 30 mL of distilled water in a 50 mL polyethylene plastic centrifuge tube, shaken on an end-over-end shaker at approximately 230 rpm for 30 min, and then centrifuged at 8000 rpm for 20 min. The supernatant was filtered through a 0.45 μm membrane filter for DOC analysis. The DOC in the extracts was determined by a TOC analyser (Multi NC 3100, Jena AG, Germany). Soil CEC was determined with the ammonium acetate method at pH 7.0. The DCB-extractable Fe/Al (Pansu and Gautheyrou 2006) and oxalate-extractable Fe/Al (Pansu and Gautheyrou 2006) were determined by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) (VISTA-MPX, Varian, USA). The former included crystalline Fe oxides, non-crystalline Fe oxides, Fe and Al organic complexes as well as some crystalline Al oxides, and the latter included Fe and Al organic complexes as well as hydrated oxides of Fe and Al (Pansu and Gautheyrou 2006). The soils’ basic properties are listed in Table 1.
X-ray diffraction (XRD)
The clay fractions with particle size <2 μm (soil colloids) were separated from soil samples by pipette method. The pipette method is based on sedimentation of the particles by gravity according to the law of Stokes. Recovery of the aliquot at a given depth and a given time makes it possible to identify a specific class of particles when all the particles bigger than the selected diameter have been eliminated (Pansu and Gautheyrou 2006). Then DCB method was used to remove free iron oxides from soil colloids, and the H2O2 oxidation method was used to remove soil organic matter (Li et al. 2022a). The treated soil colloid samples were dried in an oven at 40 °C and then finely ground and passed through a 0.074-mm sieve for XRD determination. A part of soil colloids was taken to treat with H2O2 and used for the later Al mobilization experiment to further verify the effect of the coordination nature of solid Al on Al mobilization.
The XRD patterns of soil colloids were obtained using an Ultima IV diffractometer, Cu target, Kα radiation source (λ = 0.154 nm), and graphite filter. The test conditions were as follows: the tube voltage was 40 kV, the tube current was 40 mA, the scanning speed was 1° (2θ) min−1, and the scanning step was 0.02°. Semi-quantitative estimation of the mineral proportion was calculated by X’Pert high score, comparing with the standard samples (Jiang et al. 2011).
Solid-state nuclear magnetic resonance (NMR)
The soil colloids were ground through 0.149-mm sieve for the determination of NMR. The determination of solid-state NMR was carried out with the instrument Bruker AVANCE II 400 MHz. The test conditions of the 27Al magic-angle spinning (MAS) NMR were as follows: the 27Al chemical shift reference was Al nitrate (Al(NO3)3) solution, the resonance frequency was 104.3 Mhz, the sample speed was 17 kHz, the pulse delay was 2.5 s. The scan number was 2048 and the probe was 4 mm. The relative content of AlIV and AlVI was obtained from the relative ratio of peak area for AlIV/that for AlVI in 27Al NMR spectra (Badreddine et al. 2002).
Al release from soils and their colloids during their acidification
1.0 g soil or colloid with OC partially removed sample was weighed in a 50 mL beaker and 30 mL of deionized water was added. Then, a 902 constant pH automatic potentiometric titrator (Swiss Watone) was used to titrate the mixture to a constant pH and maintained for 2 h afterward. During this time, the mixture was under constant magnetic stirring and 0. 1 M HCl was added into the suspension through the titrator to adjust pH to the objective value. After that, a 50 mL polyethylene plastic centrifuge tube was weighed as W1 (g). The soil suspension in the 50 mL beaker was poured into the centrifuge tube and centrifuged at 4500 rpm for 5 min. The supernatant was filtered with a 0.45 μm membrane filter and used to determine the content of soluble Al. Then, the centrifugal tube was weighed together with its content as W2 (g). The soil sample in the centrifugal tube was then washed to a filter paper in a glass funnel with 1.0 M KCl and the exchangeable Al of the soils was extracted by leaching with 1.0 M KCl solution (Pansu and Gautheyrou 2006). The leachate was collected in a 50 mL volumetric flask. The 8-hydroxyquinoline colorimetric method (pH 8.3) was used to determine the contents of Al in the extractants and supernatants (Xu and Ji 1998). The soluble and exchangeable Al of each system were calculated by eqs. (1) and (2). Each treatment was repeated twice.
where [Al]Equ represents soluble Al; [Al]Ex is the exchangeable Al; V1 is the volume of hydrochloric acid consumed (mL); V2 represents the volume of 1.0 M KCl (50 mL); [Al]KCl represents the measured concentration of Al in KCl extract (mM); C represents the concentration of Al in the supernatant after centrifugation (mM), and ρ is the density of pure water (1 g mL−1). All data were expressed as mean ± standard deviation of 2 repetitions. Excel 2016 and Origin 2017 were used for data analyses.
Results
XRD and 27Al NMR analyses of four soils
The XRD patterns of the colloids of subsurface soils (20–40 cm) after the removal of free iron oxides and organic matter are shown in Fig. 1. The main clay minerals contained in these soils are vermiculite (PDF#76–0847), chlorite (PDF#29–0701), hydromica (PDF#80–0742), and kaolinite (PDF#06–0221). However, the proportions of various clay minerals in the soils differed. The percentage of clay minerals in each soil is listed in Table 2. Accordingly, Ultisol contained the highest content of kaolinite, Alfisol contained more hydromica, Inceptisol contained more chlorite and vermiculite, and Mollisol mainly contained hydromica, chlorite, and kaolinite as dominant clay minerals. It can be seen that the proportion of major clay minerals contained in the four soils differed greatly due to differences in soil development degree caused by different climatic conditions (Winkler et al. 2002; Deepthy and Balakrishnan 2005; Locsey et al. 2012).
The clay content of four subsurface soils was in the order of Ultisol > Alfisol > Mollisol > Inceptisol, which reflected the clay content in the surface layer of the soils. Table 1 presents that the CEC of Mollisol was the largest (Table 1) and mainly depended on the type and content of clay minerals. The CEC of the four soils was in the order of Mollisol > Alfisol >Ultisol > Inceptisol. In general, the CEC of 2:1 type phyllosilicate minerals such as chlorite, vermiculite, and hydromica is significantly greater than that of 1:1 type phyllosilicate minerals such as kaolinite (Bhattacharyya and Gupta 2008).
The 27Al solid-state NMR spectra of the colloids of subsurface soils are shown in Fig. 2. The four soils all contained both tetrahedral and octahedral Al (Sanz et al. 1986; Badreddine et al. 2002; Ejeckam and Sherriff 2005; Di Pietro et al. 2022), which indicated that the isomorphic substitution of Si4+ by Al3+ occurred in Si-O tetrahedron of the clay minerals of all four soils, but the degree of isomorphic substitution varied with soil type. The quantitative analysis results based on the peak area of NMR spectra showed that the relative contents of tetrahedral and octahedral Al also varied with soil type (Table 3). Also, the content of tetrahedral Al gradually increased, while the content of octahedral Al gradually decreased with the increase of latitude of soil sampling sites. In other words, the content of tetrahedral Al followed Mollisol > Inceptisol > Alfisol > Ultisol, and the content of octahedral Al followed Ultisol > Alfisol > Inceptisol > Mollisol .
The composition and content of soil clay minerals determined the contents of tetrahedral and octahedral Al in the soils (Fig. 1 and Table 2). According to our recent study (Li et al. 2023b), almost all solid Al in kaolinite exists as octahedral Al, while the 2:1 phyllosilicate minerals such as hydromica, vermiculite, and chlorite contain more tetrahedral Al. It can be noted from Fig. 2 that the chemical shifts of tetrahedral Al in Ultisol, Alfisol, and Inceptisol were all around 70 ppm, while that in Mollisol was different from these in the other three soils (near 57 ppm). This indicates that the tetrahedral Al in Mollisol was more symmetrical than that in the other three soils in terms of nuclear magnetic resonance (Woessner 1989). The high tetrahedral Al content in Mollisol also provided evidence for the above inference (Table 3).
Soil acidification and Al mobilization in different sampling depths of soils
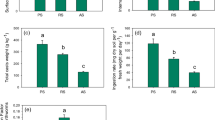
Figures 3, 4, 5 and 6 show the results of acid consumption and Al mobilization during the acidification process of four typical soils at different sampling depths. For the Ultisol, when pH is under an acidic condition (pH < 5.5), the exchangeable hydrogen ions adsorbed on the soil exchangeable sites begin to be gradually converted into exchangeable Al. During this process, the protonation reactions between soil exchangeable H+ and hydroxyl groups on soil mineral surfaces occur at first, which enhances the release of Al from Al-O tetrahedron and octahedron of soil minerals (Li et al. 2023a), and then the released Al3+ ions are adsorbed by soils to form exchangeable Al3+ (Li et al. 2023b). A part of exchangeable Al3+ is released into the solution to become soluble Al. With the decrease in pH, the consumption of HCl, the contents of exchangeable Al, and soluble Al in Ultisol gradually increased (Fig. 3). The HCl consumption by surface soil (0–20 cm) was greater than that by subsurface soil when the soil was acidified to the same pH. For example, at pH 4.3, the HCl consumption by surface soil was 50.1 mmol kg−1, while that of the latter was only 28.5 mmol kg−1. For exchangeable Al, the trend was opposite to that of HCl consumption, that is, the exchangeable Al content in surface soil was lower than that in subsurface soil at various acidic pH values. Specifically, at pH 4.3, the exchangeable Al content in surface soil was 1.49 mmol kg−1 and 1.83 mmol kg−1 in subsurface soil. On the other hand, the variation of soluble Al was consistent with the consumption of HCl. That is, the content of soluble Al produced in surface soil was higher than that in subsurface soil at the same pH (Fig. 3). At pH 4.3, the contents of soluble Al produced in surface and subsurface soils were 0.51 and 0.20 mmol kg−1, respectively.
Figure 4 shows the results of Al mobilization during soil acidification at different depths in Alfisol. The trends of HCl consumption, exchangeable Al, and soluble Al contents in surface and subsurface samples were similar to those of the Ultisol. In other words, the HCl consumption and soluble Al content in subsurface soil were lower than those in surface soil, while the exchangeable Al content of the former was higher than that of the latter. For instance, the amounts of HCl consumed by surface and subsurface soils were 23.8 and 18.4 mmol kg−1 at pH 4.3, respectively. The exchangeable Al content in surface soil was 2.88 mmol kg−1, and 3.23 mmol kg−1 in subsurface soil at pH 4.3. The contents of soluble Al produced in surface and subsurface soils were 0.09 and 0.04 mmol kg−1, respectively.
Figure 5 shows the results of Al mobilization in different sampling depths of the Inceptisol during soil acidification. Opposite trends for acid consumption and Al mobilization in the Inceptisol were observed compared with those in the Ultisol and Alfisol as mentioned above. Specifically, the consumption of acid by subsurface soil was greater than that of surface soil when the Inceptisol was acidified to the same pH. For example, the HCl consumption by surface soil was 20.6 mmol kg−1, while that of subsurface soil was 29.7 mmol kg−1 when the soil was acidified to pH 4.3. The content of exchangeable Al produced in surface soil was greater than that in subsurface soil while the content of soluble Al in surface soil was slightly higher than that in subsurface soil at the same pH. The exchangeable Al produced in surface soil was 5.55 mmol kg−1, and 5.32 mmol kg−1 in subsurface soil at pH 4.3. The contents of soluble Al in surface and subsurface soils were 0.17 and 0.18 mmol kg−1, respectively.
Figure 6 shows the trends of Al mobilization in different sampling depths of the Mollisol during soil acidification. When surface and subsurface soils were acidified to the same pH, the consumption of HCl was almost the same, and the content of exchangeable Al in subsurface soil was higher than that in surface soil (Fig. 6B). At pH 5.5, the contents of exchangeable Al in surface and subsurface soils were 0.23 and 0.73 mmol kg−1, respectively. When pH was decreased from 5.5 to 4.3, the exchangeable Al of the corresponding soils was increased by 1.53 and 1.35 mmol kg−1, respectively. In the range of 4.9 < pH < 5.5, the content of soluble Al in the subsurface soil was greater than that in the surface soil (Fig. 6C). In the range of 4.3 < pH < 4.9, the content of soluble Al in surface was almost the same with that in subsurface soil. When soil pH was decreased from 5.5 to 4.3, soluble Al in the surface and subsurface soils were increased by 73% and 24%, respectively.
Al mobilization in different soils
When different soils were compared, it was found that soil acidification and Al mobilization varied with soil type (Figs. 3, 4, 5 and 6). The HCl consumption followed the order Mollisol > Ultisol > Alfisol > Inceptisol for the surface soils and Mollisol > Inceptisol > Ultisol > Alfisol for the subsurface soils when the soils were acidified to pH 4.3. The variation in acid consumption among different soils was mainly related to their acid buffer capacity, which was mainly determined by soil OC content and CEC (Li et al. 2022a). The OC contents and CEC values of both surface and subsurface layers of the Mollisol were the largest among the soils (Table 1), so the acid buffer capacity and acid consumption of the soil were also the greatest. Additionally, the content of OC in the surface layer of the Ultisol was higher than that of Alfisol and Inceptisol, but these surface soils had a similar CEC, which was responsible for the greater acid consumption of the soil than Alfisol and Inceptisol due to its greater acid buffer capacity than the other two soils. Similarly, higher content of OC in the sub-layer of the Inceptisol than those of Ultisol and Alfisol led to greater acid buffering capacity and acid consumption of the soil than those in sub-layers of Ultisol and Alfisol.
The contents of exchangeable Al followed Inceptisol > Alfisol > Mollisol > Ultisol for both surface and subsurface soils (Figs. 3, 4, 5 and 6) and the OC content, CEC, and composition of minerals were the main factors to affect soil exchangeable Al. Greater CEC enhanced the production of soil exchangeable Al during soil acidification due to the larger amount of negative charge can make the soil adsorb more exchangeable Al. However, higher OC content inhibited this process because SOC absorbed more H+ ions powerfully instead of transforming to exchangeable Al3+ (Li et al. 2022a). The soluble Al followed Ultisol > Mollisol > Inceptisol > Alfisol in surface layers and Mollisol > Ultisol > Inceptisol > Alfisol in sub-layers (Figs. 3, 4, 5 and 6).
Al mobilization in four soil colloids with organic matter partially removed
To avoid the interference of differences of clay content among different soils on the effect of the coordination nature of solid Al on Al mobilization, the colloids were separated from sub-surface layers of the soils and used in the study. The exchangeable and soluble Al of four soil colloids with organic matter partially removed at pH 4.3 are presented in Fig. 7. We observed that the exchangeable Al was increased from 4.0 to 5.41 mmol kg−1 and increased by 35%, while soluble Al was increased from 1.79 to 11.66 and increased by 4.8 times in the colloids of Ultisol after SOC was removed. Similarly, the exchangeable Al was increased from 4.24 to 13.58 mmol kg−1 (3.2 times), while soluble Al was increased from 1.79 to 11.66 mmol kg−1 (6.5 times) in the colloids of Mollisol. These results confirmed that soil OC significantly inhibited Al mobilization from soil minerals and was also consistent with previous observations (Li et al. 2022a). As mentioned above, SOC inhibited mobilization of soil Al through physical mask and association of organic anions of SOC with H+, in which SOC inhibited the reaction between H+ ions and minerals and hindered the H/Al conversion reactions (Yu 1997). Given that Mollisol contained the highest OC content, more OC was removed from its colloids with H2O2 treatment (Table 1), which led to greater increases in both exchangeable and soluble Al.
After OC was partially removed from soil colloids, the total amount of Al (sum of exchangeable and soluble Al) mobilized in soil colloids at pH 4.3 followed the order Mollisol > Inceptisol > Alfisol > Ultisol, which was consistent with the order of relative content of tetrahedral Al in these colloids (Table 3). This further suggested that the Al in Al-O tetrahedrons of soil minerals was more readily mobilized than Al in Al-O octahedrons during soil acidification. As observed by Li et al. (2023b), 2:1 type phyllosilicate minerals of illite and vermiculite contain Al-O tetrahedrons, while kaolinite does not contain Al-O tetrahedrons, and this influences Al mobilization behaviors in these minerals.
Discussion
Effects of OC on soil acidification and Al mobilization in different sampling depths of soils
Soil OC content and CEC are two important factors in determining soil acid buffer capacity (Aitken 1992; Nelson and Su 2010; Xu et al. 2012; Li et al. 2022a). For Ultisol, under the condition of a small difference in CEC between surface and subsurface soils, the higher OC content in surface soil led to more amounts of acid consumed by the soil compared with subsurface soil, when soil pH was decreased to the same value (Table 1, Fig. 3A). On the other hand, soil OC can significantly inhibit the H/Al conversion during the acidification process and thus decrease the production of exchangeable Al, which was also responsible for greater exchangeable Al in subsurface soil than in surface soil of Ultisol and Alfisol (Figs. 3 and 4). This is consistent with the observation presented in a previous study (Li et al. 2022a). The OC covering on soil minerals protected the minerals against attacking of H+. In addition, the organic anions of soil OC associated with H+ to form neutral molecules and thus consumed more H+ ions during soil acidification (Shi et al. 2019). These are main mechanisms for the inhibition of soil acidification and production of exchangeable Al by OC. The higher content of OC in the surface soil was also responsible for the greater soluble Al in this layer of the soil compared with subsurface soil. During soil acidification, mobilized Al formed organic-Al complexes with dissolved organic matter (DOM) (Zhang et al. 2018; Krettek and Rennert 2021). The higher contents of OC in surface Ultisol and Alfisol also increased the concentration of DOC and thus the content of soluble Al compared with subsurface soil (Table 1) (Jansen et al. 2003, 2004; Li et al. 2022a; Antonangelo et al. 2022). If soil exchangeable Al and soluble Al were put together as total mobilized Al, soluble Al only accounts for a small portion of total mobilized Al. Although higher OC in surface soil increased the content of soluble Al, still decreased total mobilized Al compared with subsurface soil.
However, the lower content of OC in the surface soil of Inceptisol led to smaller HCl consumption by surface soil and greater exchangeable Al content in surface soil compared with subsurface soil (Table 1, Fig. 5). The higher OC content in subsurface soil played a similar role in increasing soil acid buffering capacity and inhibited Al mobilization in the layer of the soil compared with surface soil of Inceptisol. For the same soil profile, there was little difference in the mineralogy of the clay fraction in surface and subsurface soils. Therefore, we only focused on the impact of SOC on Al mobilization. Much higher contents of OC in both surface and subsurface soils of Mollisol compared with other three soils (Table 1) led to similar HCl consumption and soluble Al contents from pH 4.3 to 4.9 in the two layers of the soil (Fig. 6). The higher content of OC in surface soil than subsurface soil was responsible for the lower content of exchangeable Al from pH 4.9 to 5.5 in surface soil compared with subsurface soil. Therefore, OC content was the main factor determining acid consumption and Al mobilization in surface and subsurface soils of four soils during their acidification.
Characteristics of Al mobilization for different soils
Although the CEC of Mollisol was greater than that of Inceptisol and Alfisol in surface and subsurface layers (Table 1), its higher content of OC inhibited the production of exchangeable Al and was responsible for the lower exchangeable Al of the soil in both soil layers. Comparatively, the OC content of Mollisol was higher than that of Ultisol (Table 1), nevertheless, the lower CEC and greater content of kaolinite (Table 2) led to a smaller exchangeable Al in Ultisol than that of Mollisol. This is probably because Al mobilization in 2:1 type phyllosilicate minerals of vermiculite, hydromica (illite), and chlorite is more readily mobilized than that in kaolinite (Li et al. 2023b). Therefore, higher contents of 2:1 type phyllosilicate minerals and greater CEC of Mollisol (Table 2) were responsible for its larger exchangeable Al than that of Ultisol. In addition, the higher contents of 2:1 type phyllosilicate minerals in Inceptisol and Alfisol were also responsible for the greater exchangeable Al in these two soils than that of Ultisol.
The effect of soil mineral composition on soil exchangeable Al was also related to the coordination nature of Al in these minerals. Previous research results showed that tetrahedral Al in minerals was more readily released (mobilized) than octahedral Al during mineral acidification (Li et al. 2023b). As can be seen from Fig. 2 and Table 3, Mollisol contained more tetrahedral Al than Ultisol, which indicated that the solid Al in Mollisol was more readily mobilized than that in Ultisol without the influence of other factors as octahedral Al is more stable than tetrahedral Al in the mineral lattice (Li et al. 2023b). As a result, more protons were absorbed and more exchangeable Al was mobilized in the acidification process of Mollisol. Also, since the OC content of surface soil in Alfisol was lower than that of Inceptisol (Table 1), the exchangeable Al content produced by Inceptisol was greater than that of Alfisol (Figs. 4B and 5B). This was mainly related to the difference in clay minerals between the two soils. There was more hydromica in Alfisol while Inceptisol contained more vermiculite and chlorite. The proportion of tetrahedral Al in clay minerals contained in Inceptisol was greater than that in Alfisol (Table 3), which also made the mobilization of exchangeable Al in Inceptisol greater than that in Alfisol.
The complexation of DOM with Al was the main reason that affected the release of soluble Al from the soils (Jansen et al. 2003, 2004; Zhang et al. 2018; Krettek and Rennert 2021). The higher contents of OC in Mollisol and surface soil of Ultisol led to more DOC in soil solution and thus increased dissolution of Al through formation of soluble Al-DOC complexes, which was responsible for greater contents of soluble Al in Ultisol and Mollisol than these in Inceptisol and Alfisol (Table 1). In addition to SOC, the higher content of Al oxides in the Ultisol (Table 1) also contributed to greater soluble Al content in this soil compared with the other three soils (Zhang et al. 1991). The detailed mechanisms need to be further verified in the future studies. As discussed above, during soil acidification, acid consumption mainly depended on soil acid buffering performance which was influenced by soil CEC and OC content; the content of soil exchangeable Al depended on the CEC, the content of OC, and the composition of minerals as well as coordination nature of Al in soil Al-bearing minerals.
The study of Mládková et al. (2005) using traditional statistical analysis showed that soil exchangeable Al content was mainly affected by the altitudes of sampling sites. Boruvka et al. (2005) showed that the content of exchangeable Al in surface soils decreased with the increase of sampling site altitude. This was mainly related to the low temperature at high altitudes and the high content of OC in the surface soils, which inhibited the mobilization of soil Al. The content of exchangeable Al in deep soils was mainly affected by its parent materials (Boruvka et al. 2005). These observations were consistent with the results presented in this study. Mobilization of Al in surface soils was mainly affected by SOC contents, while the mobilization of Al in subsurface and deep soils was mainly influenced by the phyllosilicate minerals. The degree of soil weathering decreases with increasing latitude, which is reflected in the phyllosilicate minerals present in the soils from different climate zones. For example, Ultisol has the highest degree of weathering and contains mainly kaolinite. The proportion of 2:1 phyllosilicate minerals in soils gradually increases as the degree of weathering decreases. For example, the main phyllosilicate minerals in Alfisol, Inceptisol and Mollisol are hydromica and chlorite (Table 2). On the other hand, the decomposition of organic matter in Ultisol is faster, so the SOC content should be low. However, the clay content also affects SOC content as it is strongly bound to soil colloids. The SOC content of Ultisol is higher than that of Alfisol and Inceptisol due to its higher clay content. The degree of soil weathering in the Mollisol area is very low. This allows for easy accumulation of SOC, resulting in high SOC levels (Table 1).
Some other Al pools in the mollisol with partially removed OC also contributed to a higher production of mobilized Al. The rather high levels of oxalate-extractable Al and Fe in the mollisol (Table 1) may indicate the presence of: (i) short-range ordered (SRO) aluminosilicates, and (ii) Fe-oxyhydroxides with Al substitution. The co-existence of SRO aluminosilicates with 2:1 minerals has been reported for Alfisols (Lavkulich and Arocena 2011). Such ill-defined aluminosilicates can form due to continuous mineral weathering and the hydrolysis of Al3+ and Fe3+ cations (Basile-Doelsch et al. 2015). A previous study (Wang et al. 2020) showed that the Al3+ ions were readily released from the poorly defined SRO aluminosilicates during soil acidification. The Al mobilization characteristics and mechanisms of these soils in this study need to be validated in more soils in future investigations.
It is possible that iron oxides inhibited soil acidification and Al mobilization by coating the oxides on phyllosilicate minerals to reduce the reactions of H+ with minerals (Li et al. 2023a). The effect of iron oxides in the Ultisol on Al mobilization was greater than that in the other three soils due to the higher content of iron oxides in the former than in the latter. The effect of soil iron oxides on Al mobilization remains to be evaluated quantitatively in the future.
Previous studies have shown that organic matter interacts with soils to affect soil acidification and Al mobilization. For example, long-term application of organic manure effectively increased the content of OC in Ultisols, improved the pH buffer capacity (pHBC) of the soils, and thus reduced the contents of soluble and exchangeable Al in the soils (Shi et al. 2019). Similarly, incorporation of decayed crop straws into acidic Ultisols increased soil pH and pHBC, thereby inhibiting soil acidification and Al mobilization (Pan et al. 2020, 2021). Recent studies have shown that the interaction of soil bacteria (Nkoh et al. 2020) and their extracellular polymers (Nkoh et al. 2022) with an acidic soil can also improve the acid buffering performance and reduce the active Al content of the soil. These results, along with the observations presented in this study, indicated that SOC played an important role in inhibiting soil acidification and Al mobilization.
Al mobilization in soil colloids
Figure 7A shows that the order of Al mobilization of the four soil colloids at pH 4.3 was Alfisol > Inceptisol > Mollisol > Ultisol. This trend was consistent with the order of the 2:1 phyllosilicate minerals present. Therefore, the mobilization amount of Al during acidification of the four soil colloids in this study was mainly affected by the relative content of soil 2:1 clay minerals. Compared to the bulk soil, the amount of Al mobilization of the colloids was significantly increased, mainly due to the fact that almost all soil clay particles were present in the colloids. Thus, the content of phyllosilicate minerals in the soil was much higher than that in the bulk soil. The order of the increase of the Al mobilization amount in the four soil colloids was Alfisol > Inceptisol > Mollisol > Ultisol. This was also consistent with the order of the content of soil 2:1 phyllosilicate minerals in the four soils. On the other hand, the content of SOC in colloids also increased significantly compared to the bulk soil. Therefore, the Al mobilization during soil colloid acidification was also affected by the content of SOC. The effect of SOC on Al mobilization was further supported by the results of Fig. 7A, B.
The results obtained from this study also confirmed that the tetrahedral Al was more readily released from soil phyllosilicate minerals than octahedral Al during soil acidification, which was consistent with the observations in the systems of phyllosilicate minerals (Li et al. 2023b). Therefore, the Al coordination nature in soil minerals played an important role in the mobilization of soil solid Al during soil acidification. According to relevant literature and calculations, the theoretically maximum sphere radius accommodated by the central aperture of the Si-O tetrahedron is about 0.32 Å, and the central aperture of the Al-O octahedron is about 0.58 Å (Barnhisel and Bertsch 1989). Similarly, the ionic radius of Si4+ is 0.39 Å and that of Al3+ is 0.51 Å. As evident, the ionic radius of Si4+ is close to the central aperture of the Si-O tetrahedron, while that of Al3+ is consistent with the central pore size of the Al-O octahedron. Since the ionic radius of Al3+ is significantly larger than the central pore size of the Si-O tetrahedron, the isomorphic substitution of Si4+ by Al3+ makes the tetrahedral layer elements not closely packed. This means that the substitution of Si4+ by Al3+ makes the Al-O tetrahedron less stable than the closely packed Al-O octahedron. Thus, the tetrahedral Al is more readily mobilized than the octahedral Al during soil and phyllosilicate mineral acidification, which is consistent with the observations in this study.
The amount of Al mobilization in Ultisol was the lowest among the four soils when the influence of organic carbon was not considered during its acidification (Fig. 7B). This was due to the fact that the phyllosilicate minerals in the Ultisol with the highest degree of weathering were mainly kaolinite. The results of the study indicated that Al mobilization was more difficult in kaolinite than in other 2:1 minerals (Li et al. 2022a). We speculated that the easily mobilized form of Al was first mobilized in the weathering process and converted to other forms. Thus, the residual Al in phyllosilicate minerals was difficult to mobilize during soil acidification. In addition, the higher content of iron oxides in Ultisol inhibited soil acidification. There is also a possibility of selective dissolution of octahedral Al in the acidification process. Our unpublished data indicate that the Al in amorphous Al(OH)3 and gibbsite is octahedral Al. However, the Al mobilization amount and rate of amorphous Al(OH)3 were much higher than those of gibbsite in the acidification process.
Conclusions
In this study, four typical soils were selected for simulated acidification experiments to investigate the characteristics of acidification and Al mobilization in these soils. In surface and subsurface layers of the same soil profile, the Al mobilization was mainly affected by OC content during soil acidification. The mobilization amount of Al was mainly manifested as exchangeable Al, and the mobilization amount of soluble Al only accounted for a small part of the total amount of mobilized Al (sum of exchangeable and soluble Al). When different soils were compared, it was found that soil acidification and Al mobilization were mainly affected by CEC, OC content, and composition of clay minerals as well as the coordination nature of solid Al in phyllosilicate minerals. After OC was partially removed from soil colloids, the total amount of Al mobilization in soil colloids at pH 4.3 followed the order: Mollisol > Inceptisol > Alfisol > Ultisol. This was consistent with the order of relative content of tetrahedral Al in the soil colloids and confirmed that the Al in Al-O tetrahedrons of soil minerals was more readily mobilized than the Al in Al-O octahedrons during soil acidification. The results obtained in this study can provide useful references for soil acidification regulation and Al mobilization inhibition in acid soils. Protecting soil OC and slowing down the decomposition of soil OM is beneficial not only to mitigate soil acidification, but also to inhibit Al mobilization during soil acidification.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aitken RL (1992) Relationships between extractable Al, selected soil properties, pH buffer capacity and lime requirement in some acidic Queensland soils. Aust J Soil Res 30:119–130
Antonangelo JA, Neto JF, Crusciol CAC, Zhang HL, Alleoni LRF, Kinrade SD (2022) Comparative analysis of exchangeable aluminum in a tropical soil under longterm no-till cultivation. Soil Till Res 216:105242
Badreddine R, Vandormael D, Fransolet AM, Long GJ, Stone WEE, Grandjean F (2002) A comparative X-ray diffraction, Mössbauer and NMR spectroscopic study of the vermiculite s from Béni Bousera, Morocco and Palabora, Republic of South Africa. Clay Miner Miner 37:367–376
Barnhisel RI, Bertsch PM (1989) An introduction to soil mineralogy. In: Dixon JB, Weed SB (Eds.) Minerals in Soil Environments (Second Edition). SSSA Book Series, vol. 2, Madison, USA, pp 11–12
Basile-Doelsch I, Balesdent J, Rose J (2015) Are interactions between organic compounds and nanoscale weathering minerals the key drivers of carbon storage in soils? Environ Sci Technol 49:3997–3998
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interf Sci 140:114–131
Bian M, Zhou M, Sun D, Li C (2013) Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J 1:91–104
Blake L, Goulding KWT (2002) Effects of atmospheric deposition, soil pH and acidification on heavy metal contents in soils and vegetation of semi-natural ecosystems at Rothamsted Experimental Station, UK. Plant Soil 240:235–251
Bolan NS, Hedley MJ, White RE (1991) Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 134:53–63
Boruvka L, Mladkova L, Drabek O (2005) Factors controlling spatial distribution of soil acidification and Al forms in forest soils. J Inorg Biochem 99:1796–1806
Chien SH, Gearhart MM, Collamer DJ (2008) The effect of different ammonical nitrogen sources on soil acidification. Soil Sci 173:544–551
Deepthy R, Balakrishnan S (2005) Climatic control on clay mineral formation: evidence from weathering profiles developed on either side of the Western Ghats. J Earth Syst Sci 114:545–556
Di Pietro SA, Emerson HP, Katsenovich YP, Johnson TJ, Francis RM, Mason HE, Marple MA, Sawvel AM, Szecsody JE (2022) Solid phase characterization and transformation of illite mineral with gas-phase ammonia treatment. J Hazard Mater 424:127657
Ejeckam RB, Sherriff BL (2005) A 133Cs, 29Si, and 27Al MAS NMR spectroscopic study of Cs adsorption by clay minerals: implications for the disposal of nuclear wastes. Can Mineral 43:1131–1140
Flores CI, Clark RB, Gourley LM (1988) Growth and yield traits of sorghum grown on acid soil at varied aluminum saturations. Plant Soil 106:49–57
Fujii K, Toma T, Sukartiningsih (2021) Comparison of soil acidification rates under different land uses in Indonesia. Plant Soil 465:1–17
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Hajkowicz S, Young W (2005) Costing yield loss from acidity, sodicity and dryland salinity to Australian agriculture. Land Degrad Dev 16:417–433
Jansen B, Nierop KGJ, Verstraten JM (2003) Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: influence of solution pH and metal/organic carbon ratios. Geoderma 113:323–340
Jansen B, Nierop KGJ, Verstraten JM (2004) Mobilization of dissolved organic matter, aluminium and iron in podzol eluvial horizons as affected by formation ofmetal-organic complexes and interactions with solid soil material. Eur J Soil Sci 55:287–297
Jiang J, Xu RK, Zhao AZ (2011) Surface chemical properties and pedogenesis of tropical soils derived from basalts with different ages in Hainan, China. Catena 87:334–340
Kopittke PM, Menzies NW, Wang P, Blamey FPC (2016) Kinetics and nature of aluminium rhizotoxic effects: a review. J Exp Bot 67:4451–4467
Kozun YS, Kazeev KS, Kolesnikov SI (2022) Climatic gradients of biological properties of zonal soils of natural lands. Geoderma 425:116031
Krettek A, Rennert T (2021) Mobilisation of Al, Fe, and DOM from topsoil during simulated early Podzol development and subsequent DOM adsorption on model minerals. Sci Rep 11:19741
Krug EC, Frink CR (1983) Acid rain on acid soil: a new perspective. Science 221:520–525
Kunhikrishnan A, Thangarajan R, Bolan NS, Xu Y, Mandal S, Gleeson DB, Seshadri B, Zaman M, Barton L, Tang C, Luo J, Dalal R, Ding W, Kirkham MB, Naidu R (2016) Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv Agron 139:1–71
Larionova AA, Zolotareva BN, Kolyagin YG, Kvitkina AK, Kaganov VV, Kudeyarov VN (2015) Composition of structural fragments and the mineralization rate of organic matter in zonal soils. Eurasian Soil Sci 48:1110–1119
Lavkulich LM, Arocena JM (2011) Luvisolic soils of Canada: genesis distribution, and classification. Can J Soil Sci 91:781–806
Li Y, Sun J, Tian DS, Wang JS, Ha DL, Qu YX, Jing GW, Niu SL (2018) Soil acid cations induced reduction in soil respiration under nitrogen enrichment and soil acidification. Sci Total Environ 615:1535–1546
Li KW, Lu HL, Nkoh JN, Hong ZN, Xu RK (2022a) Aluminum mobilization as influenced by soil organic matter during soil and mineral acidification: a constant pH study. Geoderma 418:115853
Li S, Liu JJ, Yao Q, Yu ZH, Li YS, Jin J, Liu XB, Wang GH (2022b) Short-term lime application impacts microbial community composition and potential function in an acid black soil. Plant Soil 470:35–50
Li KW, Lu HL, Nkoh JN, Xu RK (2023a) The important role of surface hydroxyl groups in aluminum activation during phyllosilicate mineral acidification. Chemosphere 313:137570
Li KW, Shi YXX, Nkoh JN, Jiang J, Xu RK (2023b) Effect of coordination nature of aluminum on its activation from minerals and Oxisols during their acidification. Pedosphere (on line). https://doi.org/10.1016/j.pedsph.2023.07.003
Liu XB, Zhang XY, Wang YX, Sui YY, Zhang SL, Herbert SJ, Ding G (2010) Soil degradation: a problem threatening the sustainable development of agriculture in Northeast China. Plant Soil Environ 56:87–97
Locsey KL, Grigorescu M, Cox ME (2012) Water-rock interactions: an investigation of the relationships between mineralogy and groundwater composition and flow in a subtropical basalt aquifer. Aquat Geochem 18:45–75
Mládková L, Boruvka L, Drábek O (2005) Soil properties and selected aluminium forms in acid forest soils as influenced by the type of stand factors. Soil Sci Plant Nutr 51:741–744
Nelson PN, Su N (2010) Soil pH buffering capacity: a descriptive function and its application to some acidic tropical soils. Aust J Soil Res 48:201–207
Nkoh JN, Yan J, Xu RK, Shi RY, Hong ZN (2020) The mechanism for inhibiting acidification of variable charge soils by adhered Pseudomonas fluorescens. Environ Pollut 260:114049
Nkoh JN, Hong ZN, Xu RK (2022) Laboratory studies on the effect of adsorbed microbial extracellular polymeric substances on the acidity of selected variablecharge soils. Soil Sci Soc Am J 86:162–180
Pan XY, Shi RY, Hong ZN, Jiang J, He X, Xu RK, Qian W (2020) Characteristics of crop straw-decayed products and their ameliorating effects on an acidic Ultisol. Arch Agron. Soil Sci 67:1708–1721
Pan XY, Xu RK, Nkoh JN, Lu HL, Hua H, Guan P (2021) Effects of straw decayed products of four crops on the amelioration of soil acidity and maize growth in two acidic Ultisols. Environ Sci Pollut Res 28:5092–5100
Pansu M, Gautheyrou J (2006) Handbook of soil analysis: mineralogical, organic and inorganic methods. Springer, Berlin
Peng J, Wu XL, Ni SM, Wang JG, Song YT, Cai CF (2022) Investigating intra-aggregate microstructure characteristics and influencing factors of six soil types along a climatic gradient. Catena 210:105867
Raza S, Miao N, Wang PZ, Ju XT, Chen ZJ, Zhou JB, Kuzyakov Y (2020) Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob Change Biol 26:3738–3751
Reddy KJ, Wang L, Gloss SP (1995) Solubility and mobility of copper, zinc and lead in acidic environments. Plant Soil 171:53–58
Reis S, Grennfelt P, Klimont Z, Amann M, ApSimon H, Hettelingh JP, Holland M, LeGall AC, Maas R, Posch M (2012) From acid rain to climate change. Science 338:1153–1154
Riggs CE, Hobbie SE (2016) Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol Biochem 99:54–65
Rozhkov VA (2009) Soils and the soil cover as witnesses and indicators of global climate change. Eurasian Soil Sci 42:118–128
Sanderman J (2012) Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agric Ecosyst Environ 155:70–77
Sanz J, Serratosa JM, Stone WEE (1986) Distribution of isomorphous substitutions in silicates by NMR-spectroscopy. J Mol Struct 141:269–277
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Shi RY, Liu ZD, Li Y, Jiang T, Xu M, Li JY, Xu RK (2019) Mechanisms for increasing soil resistance to acidification by long-term manure application. Soil Till Res 185:77–84
Slessarev EW, Lin Y, Bingham NL, Johnson JE, Dai Y, Schimel JP, Chadwick OA (2016) Water balance creates a threshold in soil pH at the global scale. Nature 540:567–569
Soil Survey Staff Keys to Soil Taxonomy (2022) 13th ed. U.S. Department of Agriculture Natural Resources Conservation Service. Washington, DC, USA
Stefaan D, Stephan M, Seppe D (2022) Factors of soil formation: Climate. In: Michael JG, Margaret O (eds) Encyclopedia of Soils in the Environment, 2nd edn. Academic Press, Oxford, pp 14–24
Ulrich B (1986) Natural and anthropogenic components of soil acidification. Z Pflanzenernahr Bodenk 149:702–717
Van Breemen N, Buurman P (2002) Soil formation. Kluwer Academic, Dordrecht
Vonuexkull HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Wang S, Du PX, Yuan P, Liu YQ, Song HZ, Zhou JM, Deng LL, Liu D (2020) Structural alterations of synthetic allophane under acidic conditions: implications for understanding the acidification of allophanic andosols. Geoderma 376:114561
Winkler A, Wolf-Welling TCW, Stattegger K, Thiede J (2002) Clay mineral sedimentation in high northern latitude deep-sea basins since the middle Miocene (ODP leg 151, NAAG). Int J Earth Sci 91:133–148
Woessner DE (1989) Characterization of clay minerals by 27Al nuclear magnetic resonance spectroscopy. Am Mineral 74:203–215
Wu X, Cai C, Wang J, Wei Y, Wang S (2016) Spatial variations of aggregate stability in relation to sesquioxides for zonal soils, south-Central China. Soil Till Res 157:11–22
Xiong Y, Li QK (1987) Soils in China, second edn. Science Press, Beijing (in Chinese)
Xu RK, Ji GL (1998) Chemical species of aluminum ions in acid soils. Pedosphere 2:127–133
Xu RK, Zhao AZ, Yuan JH, Jiang J (2012) pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments 12:494–502
Yang SX, Liao B, Li JT, Guo T, Shu WS (2010) Acidification, heavy metal mobility and nutrient accumulation in the soil-plant system of a revegetated acid mine wasteland. Chemosphere 80:852–859
Yin N, Geng N, Wang TT, Wang H, Pan H, Yang QA, Lou YH, Zhuge YP (2023) Effect of acidification on clay minerals and surface properties of brown soil. Sustainability 15:179
Yu TR (1997) Chemistry of variable charge soils. Oxford University Press, New York
Yu HL, He NP, Wang QF, Zhu JX, Xu L, Zhu ZL, Yu GR (2016) Wet acid deposition in Chinese natural and agricultural ecosystems: evidence from national-scale monitoring. J Geophys Res Atmos 121:10995–11005
Zhang FS, Zhang XN, Yu TR (1991) Reactions of hydrogen-ions with variable charge soils .1. Mechanisms of reaction. Soil Sci 151:436–443
Zhang JB, Song CC, Wang SM (2007) Dynamics of soil organic carbon and its fractions after abandonment of cultivated wetlands in Northeast China. Soil Till Res 96:350–360
Zhang XM, Liu W, Zhang GM, Jiang L, Han XG (2015) Mechanisms of soil acidification reducing bacterial diversity. Soil Biol Biochem 81:275–281
Zhang YT, He XH, Liang H, Zhao J, Zhang YQ, Xu C, Shi XJ (2016) Long-term tobacco plantation induces soil acidification and soil base cation loss. Environ Sci Pollut Res 23:5442–5450
Zhang JC, Hu A, Wang BR, Ran W (2018) Al(III)-binding properties at the molecular level of soil DOM subjected to long-term manuring. J Soils Sediments 19:1099–1108
Zhang ZY, Huang L, Liu F, Wang MK, Ndzana GM, Liu ZJ (2019) Transformation of clay minerals in nanoparticles of several zonal soils in China. J Soils Sediments 19:211–220
Zhang XM, Guo JH, Vogt RD, Mulder J, Wang YJ, Qian C, Wang JG, Zhang XS (2020) Soil acidification as an additional driver to organic carbon accumulation in major Chinese croplands. Geoderma 366:114234
Zhao MX, Sun B, Wu LW, Gao Q, Wang F, Wen CQ, Wang MM, Liang YT, Hale L, Zhou JZ (2016) Zonal soil type determines soil microbial responses to maize cropping and fertilization. Msystems 1:e00075–e00016
Zhao WR, Li JY, Jiang J, Lu HL, Hong ZN, Qian W, Xu RK, Deng KY, Guan P (2020a) The mechanisms underlying the reduction in aluminum toxicity and improvements in the yield of sweet potato (Ipomoea batatas L.) after organic and inorganic amendment of an acidic Ultisol. Agric Ecosyst Environ 288:106716
Zhao YD, Hu X, Li XY (2020b) Analysis of the intra-aggregate pore structures in three soil types using X-ray computed tomography. Catena 193:104622
Zhou J, Xia F, Liu XM, He Y, Xu JM, Brookes PC (2014) Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J Soils Sediments 14:415–422
Zhu QC, Liu XJ, Hao TX, Zeng MF, Shen JB, Zhang FS, de Vries W (2020) Cropland acidification increases risk of yield losses and food insecurity in China. Environ Pollut 256:113145
Acknowledgements
We thank Dr. Jacson Nkoh Nkoh from Senzhen University, China for polishing the English of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (U19A2046).
Author information
Authors and Affiliations
Contributions
Ke-wei Li and Ren-kou Xu designed the experiments. Ke-wei Li conducted the experiments and analyzed the data. Ze-jiang Cai assisted in experiments and discussed the results. Ke-wei Li drafted the manuscript. Ren-kou Xu revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Chao-Feng Huang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Kw., Xu, Rk. & Cai, Z. Aluminum mobilization characteristics in four typical soils from different climate zones during their acidification. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06476-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06476-2