Abstract
Background and aims
It is widely accepted that soil water repellency (SWR) is mainly caused by hydrophobic compounds from plants and microbes. The relation between these hydrophobic compounds, which are defined as SWR biomarkers, and SWR has been rarely known and the knowledge of their sources remains controversial. We aimed to select key SWR biomarkers predicting SWR and to trace their origin.
Methods
Topsoils under/around five dominant plant species (DPS) belonging to various plant functional types (PFTs) on the Chinese Loess Plateau were sampled, together with plant samples, i.e., plant leaves and roots. A sequential extraction procedure and hydrolysis approach were applied to obtain three organic fractions: dichloromethane (DCM)/ methanol (MeOH) soluble fraction (D), DCM/MeOH soluble fraction of isopropanol/ammonia solution (IPA/NH3) extract (AS), and DCM/MeOH insoluble fraction of IPA/NH3 extract (AI), which were analyzed by gas chromatography-mass spectrometry (GC–MS).
Results
The cutin concentrations in leaves and soils of evergreen trees with higher WDPT values were 10–60 times and 35.39–78.77% higher than those of other PFTs, respectively. However, the root-derived suberin in the soils under evergreen trees, deciduous trees, and shrubs were 19.55–62.69% lower than that around the legumes and grasses. Correlation analysis further revealed that cutin was positively correlated with SWR (r2 = 0.24, p < 0.001), whereas suberin was not (p > 0.05).
Conclusion
After considering the SWR behavior during extraction and the possible origin of the compounds, we concluded that leaf-derived cutin appears to have the greatest effect on SWR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil water repellency (SWR) is a widespread phenomenon that occurs in forest and agricultural soils under various plant cover and climatic conditions (Doerr et al. 2000; Jordán et al. 2013). It refers to the reduced ability of soil to absorb water, leading to the formation of water-repellent zones (Doerr et al. 2000; González-Pérez et al. 2008). The presence of SWR has significant negative impacts on soil and plant health, as well as on the hydrology and ecosystem functioning. One of the most significant impacts is soil waterlogging in lowland areas, as water is not absorbed by the soil but instead carries soil particles with it, increasing surface runoff and soil erosion in highlands (Coelho et al. 2005; Urbanek et al. 2015; DeBano 2000a; Doerr and Thomas 2000; Smettem et al. 2021; Zheng et al. 2017). Moreover, it hinders plant growth and lowers crop yields by reducing the water availability to plants (Doerr and Thomas 2000; Li et al. 2019; Müller et al. 2016). Soil water repellency also affects the cycling of nutrients, the storage of carbon, and microbial activity and function in soils, all of which have a cascading effect on ecosystem services (Doerr et al. 2000; Schonsky et al. 2014). For example, microbial activity can be inhibited in hydrophobic soils, leading to inhibited nutrient cycling and organic matter decomposition (Muñoz-Rojas et al. 2012; Kraemer et al. 2019). This may in turn affects plant growth and soil health. In addition, the increased runoff and erosion leveled up by SWR contributes to the loss of nutrients locally and the contamination elsewhere (Kodešová et al. 2008). This also affects the quality of surface and groundwater, as contaminants are carried away by runoff. In conclusion, understanding the mechanism and management of SWR is an important area of research with broad implications for agriculture, forestry, environmental science, and urban development. By developing effective management strategies to mitigate the negative impacts of SWR, we can promote sustainable land use and protect the health and productivity of our ecosystems.
Although SWR is common in soils and has many detrimental effects on ecosystems, its causes are still not completely understood. Several studies have focused on investigating the factors that contribute to SWR, including soil texture (Dekker and Ritsema 1996), soil particle-size distribution (Jiménez-Morillo et al. 2022), moisture content (Chau et al. 2014; Karunarathna et al. 2010), wildfires (DeBano 2000b; Arcenegui et al. 2007), microbial activity (White et al. 2017; Simpson et al. 2019), as well as soil organic carbon (SOC) (Mataix-Solera et al. 2007; Karunarathna et al. 2010; Hermansen et al. 2019; Balshaw et al. 2020; González-Pérez et al. 2004). Specifically, researches have shown that the presence of certain bacterial species can contribute to SWR (Chai et al. 2022; Seaton et al. 2019; Song et al. 2019). Therefore, the exact mechanism causing SWR remains a subject of debate, but an increasing number of studies consider hydrophobic organic compounds (i.e., SWR biomarkers), which mainly produced by plant exudates, as a critical factor (Jiménez-Pinilla et al. 2016; González-Peñaloza et al. 2013; Doerr et al. 2005). It has also been suggested that hydrophobic compounds produced by microbial activity or fire-induced changes in organic matter (OM) have a greater impact on SWR (Doerr et al. 2000; Jiménez-Morillo et al. 2022). Up till now, different extraction methods have been used to identify hydrophobic compounds, including n-alkanoic acids, n-alcohols, and n-alkanes (Franco et al. 2000; Horne and McIntosh 2000; Mao et al. 2014, 2015), alkenoic acids, α-alkenes, terpenes, sterols (Bull et al. 2000; Franco et al. 2000; Lozano et al. 2013), and some complex biopolymers, e.g., cutin and suberin (Mao et al. 2014, 2015). However, the mechanisms of how hydrophobic substances interact with soil particles and alter SWR are still uncertain. Several theories have been proposed to explain this phenomenon, but the most widely accepted one is the “coating” or “film” theory. This theory contends that soil particles are covered in hydrophobic materials, which form aggregates that repel water (DeBano 2000a; Doerr et al. 2000). According to this theory, hydrophobic substances bind to soil particles through van der Waals forces, hydrogen bonding, or chemical reactions, forming a hydrophobic film that repels water (DeBano 2000a; Doerr et al. 2000; Doerr and Thomas 2000; Bayry et al. 2012; Mao et al. 2019; Smettem et al. 2021; Leighton-Boyce et al. 2007). Overall, the understanding of the mechanisms underlying SWR formation is still evolving, and further research is needed to fully elucidate this complex phenomenon.
In spite of the fact that the occurrence of SWR is usually associated with wildfires in natural ecosystems, severe SWR is not always confined to burned sites, and the degree of repellency is often reported to vary with indigenous species-dominated plants. Most of soils under evergreen trees such as Pinus, Eucalyptus and Quercus always showed relatively high values of SWR (Rodríguez-Alleres and Benito 2011, 2012; Jordán et al. 2009, 2013; Lozano et al. 2013, 2014; Butzen et al. 2015; de Blas et al. 2010, 2013; Mao et al. 2014; Zavala et al. 2009; Walden et al. 2015), while soils under shrubs could be wettable to slight SWR (Alanís et al. 2016; Mao et al. 2014) and all bare soil samples were wettable (Lozano et al. 2013; Jordán et al. 2009, 2013). Probably, various dominant plant species (DPS) belonging to different plant functional types (PFTs) input different organic carbon (OC) to the soil, including plant residues, root exudates, and decaying vegetation, which differentially alter the extent of SWR (Keesstra et al. 2016; Seaton et al. 2019). For instance, evergreen trees tend to produce more recalcitrant OC (e.g., high-molecular-weight bound lipids), contributing to the development of strong SWR (DeBano 2000a; Mao et al. 2014). In contrast, grasslands may enhance microbial activities that mitigate repellency due to their more labile organic inputs (e.g., low-molecular-weight free lipids) (Doerr et al. 2000; Mao et al. 2014; Jordán et al. 2013). Nevertheless, de Blas et al. (2013) have studied the relationship between plant-derived extractable free lipids and bound lipids with the degree of SWR, achieving inconsistent results; therefore, the relationship between SWR and PFTs is intricate and multifaceted. As SWR occurs both in topsoil and subsoil (Dekker and Ritsema 1994), it is expected that both plant leaves and roots will contribute to SWR. In particular, the leaves of evergreen trees may have a strong influence on surface SWR, thereby affecting water infiltration (Mao et al. 2015). At this point, it is necessary to further investigate how the input and sequestration of source-specific SWR biomarkers modulate the effect of PFTs on SWR in artificial forest ecosystems.
The Chinese Loess Plateau (6.4 × 105 km2) is a semi-arid region and is considered one of the most eroded areas (mean soil loss rate: 2860 t km−2 year−1); it is characterized by an extremely complex soil-eroding catena (Wang et al. 2017). Loess is the most widely distributed soil in the Chinese Loess Plateau. However, little is known about the behavior of SWR in loess soils, which are the initial soils in erosive environments with very low SOC content (Chai et al. 2022). In recent decades, with the accumulation of SOC after the world’s largest vegetation restoration project “Grain for Green”, the SWR of loess has emerged as an important topic (Liu and Zhan 2019). Investigating the effects of cover PFTs on SWR in loess and clarifying the possible relationship between SWR and SWR biomarkers could have significant implications for developing effective strategies to manage soil water availability, particularly in arid and semi-arid regions where water is scarce.
To investigate the main reasons for SWR differences under different DPS (i.e., Pinus tabulaeformis Carr., Robinia pseudoacacia L., Hippophae rhamnoides L., Coronilla varia L., Agropyron cristatum (L.) Gaertn.) belonging to various PFTs (i.e., evergreen trees, deciduous trees, shrubs, legumes, grasses). In this paper, we studied the SOM accrual and chemical composition of topsoils under/around these five DPS on the Chinese Loess Plateau. More importantly, we quantified the major leaf-derived (cutin), root-derived (suberin), and other sources biomarkers (short-chain fatty acids, long-chain fatty acids) to assess the relationship between the input and sequestration of source-specific group of SWR biomarkers with SWR, thus improving our mechanistic understanding of SWR occurrence at the molecular level. Based on the SWR performance during extraction, the possible origin of different chemical composition, and the distinct PFTs among the five DPS, we hypothesized that (1) evergreen trees would be more likely to cause more severe SWR than other PFTs; (2) all soils under/around different DPS would have a greater proportion of root-derived suberin than leaf-derived cutin, but the leaf-derived cutin would be the main cause of SWR; and (3) the abundance and composition of SWR biomarkers vary depending on PFTs; evergreen trees would provide more SWR-inducing cutin than deciduous trees, shrubs, legumes, and grasses.
Materials and methods
Sampling
This study was conducted in the Wangdonggou watershed of Changwu Country, Shaanxi Province, China (35°13′N, 107°40′E; 1220 m a.s.l.). The study watershed has an area of 6.3 km2, and the total length of the main gully was 4.97 km (Wang et al. 2021). Wangdonggou is located in the typical loess hilly and gully region of the Chinese Loess Plateau, and the topography is highly fragmented. The region features a semi-arid continental climate, with an annual mean temperature of 9.1 °C. The annual mean precipitation is 568 mm, and predominantly occurs from July to August as short heavy storms. The frost-free period is 194 days, lasting from early April to mid-October, and the potential evapotranspiration is 967 mm (Huang et al. 2003). There are four vegetation types in the watershed, including two typical artificial forests and two herbaceous vegetation. One artificial forest is dominated by Pinus tabulaeformis Carr. (PT), and the other one is Robinia pseudoacacia L.—Hippophae rhamnoides L. (RP-HR) forest; the two herbaceous vegetation are dominated by Coronilla varia L. (CV), and Agropyron cristatum (L.) Gaertn. (AC), respectively. The functional types of these five dominant species are as follows: evergreen trees, deciduous trees, shrubs, legumes, and grasses. All soils were classified as typical silt loam (USDA soil texture classes), developed from aeolian loess parent materials. More details on soil texture are given in Chai et al. (2022). Expect for the topsoil of PT and RP, most topsoil showed a light brownish or yellow–brown tint, of which the litter layer was scarcely existent and grass leaves decompose quickly, but debris of plant leaves and many fine roots were observed. The topsoil of PT and RP, however, had a dark grey or brownish hue, and a 1–3 cm litter layer was discovered, although only a few roots were visible.
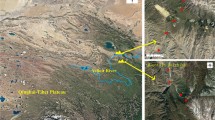
Locations of the four vegetation types were chosen for the collection of plant and soil samples in the watershed. The selected sampling locations do not have any human activity interfere with them, and have comparable soil type, vegetation restoration years, elevation, and management practices, to eliminate possible effects of non-experimental factors on SWR. The study region and sampling locations are summarized in Fig. 1 and Table 1. For all of the five dominant species, six stems for replicates were selected to take plant samples, and soil samples under/around them, as well. Although high variability of SWR was observed in our previous work (Chai et al. 2022), to avoid as far as possible distance-dependent sampling of SWR and other measurements, the six selected stems are not neighboring. Under/around each stem of each species, three micro-sampling positions with more than 50 cm spacing separation were placed to eliminate sampling variations. For trees and shrubs, the soil micro-sampling positions were located at an intermediate distance between the trunk and the edge of the canopy, where the microhabitat is most favorable for the degradation of plant litter by soil microorganisms (Moro et al. 1997). However, micro-sampling positions of herbaceous plants were as close to the stem as possible to ensure that the withered litter in the collected soil samples were formed by these plants themselves. Additionally, in the RP-HR forest, it was nevertheless possible to sample at micro-sampling places under each stem of each species without interfering with the others because of the relatively low density of vegetation. In May 2021, after the topsoil was almost air-dried, soil samples were taken from 0–3 cm depth of topsoil at micro-sampling positions under/around each of the five dominating plant species (n = 18 per species). The living plant leaf and root samples were taken separately from each plant species (n = 6 per species). Prior to further analysis, all collected soils were air-dried to constant weight at room temperature (20–25 ℃) and sieved (2 mm) to remove coarse soil particles. All the plant samples were freeze-dried and stored in a dry place.
Information for the study area (cited from Wang et al. 2016a) (a) and sampling locations (b). PT, Pinus tabulaeformis Carr.; RP-HR, Robinia pseudoacacia L.—Hippophae rhamnoides L.; CV, Coronilla varia L.; AC, Agropyron cristatum (L.) Gaertn
Soil characteristics and water repellency measurements
The soil organic matter (SOM) and total nitrogen (TN) were measured by the K2CrO7-H2SO4 oxidation method and the Kjeldahl method, respectively (Fu et al. 2011; Nelson and Sommers 1982; Bremner and Mulvaney 1982). The SOM value was converted to a total organic carbon (TOC) value using the established 1.724:1 SOM: TOC ratio.
For measuring SWR, approximately 15 g of soil was placed on a 50-mm diameter aluminum box and exposed to a controlled laboratory (20 °C, ~ 50% relative humidity) for one week to eliminate the potential impacts of preceding atmospheric humidity on SWR. The SWR was measured using the water drop penetration time (WDPT) test. This simple test measures the average time taken for three 100 μl water droplets to penetrate into the soil. The method is widely known and accepted to assess the persistence of SWR (Van’t Woudt 1959; Wessel 1988; Dekker and Ritsema 1994). The water-repellent classes were divided into five grades with different intervals according to Bisdom et al. (1993), with WDPT ≤ 5 s representing wettable and WDPT > 5 s water-repellent conditions. According to Lozano et al. (2013), to linearly rate the SWR, the logarithm of the WDPT value was used to determine a sample was water-repellent or not. Specially, if the value of log10 WDPT is > 0.7, it was considered as water-repellent (Table 2). After the determination of the WDPT value, a series of samples under/around the same DPS with different log10 WDPT intervals were obtained for subsequent SWR biomarkers extraction experiments. Finally, a total of 18, 12, 9, 9, and 6 soil samples were obtained from PT, RP, HR, CV, and AC. To compare the changes in SWR before and after extraction, the WDPT values were also measured on the air-dried soils after each extraction.
Soil and plant extraction procedures
To investigate SWR biomarkers, sequential extraction methods were applied to all the soils and the plant samples. Lipids were extracted by subsequently dichloromethane/methanol (DCM/MeOH) (9:1 v: v) and isopropanol/ammonia solution (IPA/NH3) (7:3 v: v, 25–28% ammonia solution) (Bull et al. 2000; Mao et al. 2014).
For extracting free lipids from samples, according to the method of Bull et al. (2000), each dried soil, leaf, and root sample was accurately weighed and extracted using a Soxhlet apparatus (AI-ZFCDY-6Z, Na ai Co., Ltd., Shanghai, China) containing DCM/MeOH (9:1 v: v) at 70 °C for 24 h to obtain the D fraction. The solvent was removed with a rotary evaporator (BUCHI Lab. AG, Flawil, Switzerland R-215). After redissolving the lipids in the solvent, extracts were passed through an SPE column filled with anhydrous Na2SO4 (2000 mg, 6 ml) to remove any water and were dried using a gentle stream of nitrogen, and then stored at -20 °C.
The residual soils were air-dried and extracted by IPA/NH3 (7:3 v: v) using the Soxhlet apparatus at 95 °C for 48 h. After extraction, the solvent also needs to be concentrated using the rotary evaporator. The soluble lipids (AS fraction) and the insoluble residues (AI fractions) with ester bonds were separated from the dried IPA/NH3 extracts by DCM/MeOH (9:1 v: v). All solutions were combined and both this combined solution and the insoluble residue were dried under nitrogen, and then stored at -20 °C.
Prior to analysis, all the D and AS fractions of the soils and DCM/MeOH extracts of the plants were methylated using 100 µl of (trimethylsilyl)diazomethane (TMS-CH2N2) at room temperature. The AI fractions and the lipid-free dried leaves and roots were depolymerized through trans-methylation using BF3–MeOH at 70 °C for 24 h (Riederer et al. 1993). Then, all the extracts were eluted over a small silica gel (100–200 mesh) column with ethyl acetate and were silylated using N, O-bis (trimethylsilyl)trifluoroacetamide (BSTFA) at 70 °C for 30 min.
Derivatized extracts were analyzed using a triple quadrupole gas chromatography-mass spectrometry (GC–MS) GCMS-TQ8050NX instrument (Shimadzu Production Co., Kyoto, Japan) with a mass range of m/z 50–800. 1 μl of the derivatized extracts was injected onto an SH-Rxi-5Sil MS capillary column (Shimadzu 30 m × 0.25 mm inner diameter × 0.25 μm film thickness), using helium at a 1.0 ml min−1 constant flow rate as the carrier gas. The oven heating program started with an initial temperature of 70 °C, increased to 130 °C at 20 °C min−1, heated at 4 °C min−1 from 130 °C to 320 °C, and finally held at 320 °C for 20 min.
Identification of the individual compounds was carried out by their mass spectra using NIST libraries, interpreted spectra, retention indexes, or comparison to literature data (Feng et al. 2010; Otto and Simpson 2007; Tamura and Tharayil 2014). Based on GC–MS analysis, the relative correction factors of compound groups (alkanes, alcohols, fatty acids, ω-hydroxy fatty acids and α, ω-dicarboxylic acids) were rather similar and barely discriminated between various types of compounds. Therefore, 2 μl of squalane (40.5 μg/ml in ethyl acetate) as an internal standard was added to extracts, and the possible co-eluting compounds were quantified by peak area integration from GC–MS chromatograms and the following formula.
where \({c}_{ir}=\) the relative concentration of the identified compound; \({c}_{ia}=\) the absolute concentration of the identified compound; \({A}_{i}=\) the peak area of the identified compound; \({v}_{i}=\) the volume of the identified compound; \({v}_{is}=\) the volume of the internal standard; \({A}_{is}=\) the peak area of the internal standard; \({c}_{is}\) = the concentration of the internal standard; \({m}_{s}=\) the weight of the dried sample; \({f}_{{i}{\prime}}=\) the relative correction factor, 1; \(\alpha =\) the conversion coefficient, 1000.
Statistical data analysis
All data were tested for normality and homogeneity of variance using SPSS Statistics 26 (IBM crop., USA), and log-transformed when necessary. One-way ANOVA combined with LSD test was used to evaluate the significance of differences in soil properties and SWR biomarkers among different DPS. Simple linear regression was performed to quantify the relationship between soil characteristics, SWR biomarkers, and SWR (i.e., the measured WDPT value). In this study, the quality parameters of compound groups were defined as the ratio of the relative concentrations of the two different compound groups. In addition, functional compound groups were distinguished according to their compound classes (Table S1), i.e., long-chain fatty acids (LFA), short-chain fatty acids (SFA), cutin, or suberin.
Results
Soil chemical properties and SWR
One-way ANOVA analysis showed that TOC, TN, and C/N ratio differed significantly under/around different DPS. PT (48.01 g kg−1 soil) and RP (54.16 g kg−1 soil) had the highest TOC, followed by HR (38.53 g kg−1 soil) and CV (37.53 g kg−1 soil), and then AC (16.42 g kg−1 soil) (Fig. 2a). The highest TN was found in the soils under/around RP and CV with mean values of 4.54 g kg−1 soil and 4.57 g kg−1 soil, respectively, while CV had the lowest C/N ratio with a mean value of 8.22%, which were 1.4–1.9 times lower than those of the soils under/around other DPS (Fig. 2b–c). By the WDPT test, 78% of the measured samples were water repellency (Fig. S1). More importantly, only the soils under PT had strong to extreme SWR, while most soils under/around the other DPS were classified as slightly water-repellent (Fig. S1).
The soil TOC content, TN content, and C/N ratio under/around the different DPS (a–c). SWR (log 10 WDPT(s)) as a function of log10 TOC, log10 TN, and log10 C/N ratio before and after DCM/MeOH and IPA/NH3 extractions (d–f). TOC, total organic carbon; TN, total nitrogen; C/N, TOC: TN ratio. Error bars in (a–c) represent standard deviations of the mean values (n = 18, 12, 9, 9, 6) with lowercase letters above bars indicating significant differences among plant species treatments in specific fractions at p < 0.05
Before extraction, log10 TOC showed a strong positive linear correlation with SWR (r2 = 0.65, p < 0.001) (Fig. 2d). Moreover, Pearson's correlation was positive but weak between log10 TN (r2 = 0.31, p < 0.001) and log10 C/N ratio (r2 = 0.20, p < 0.001) with SWR (Fig. 2e–f). After DCM/MeOH extraction, SWR increased in most of the soils (p < 0.001) (Fig. S2), whereas the average TOC content (p < 0.001) and TN content (p < 0.01) of all soils decreased by 3.68% and 2.49%, respectively. (Fig. S2). The average C/N ratio also decreased, but the difference was not significant (p > 0.05) (Fig. S2). A strong positive correlation was found between log10 TOC and SWR for the soils (r2 = 0.66, p < 0.001) (Fig. 2d), while log10 TN (r2 = 0.40, p < 0.001) and log10 C/N ratio (r2 = 0.11, p < 0.05) also showed weak positive linear correlations with SWR (Fig. 2e–f). After IPA/NH3 extraction, again the average TOC content and TN content of all soils reduced by 4.62% and 4.50%, respectively (p < 0.001), and the average SWR class of all soils reduced to ‘wettable’ (p < 0.001) (Fig. S2). Meanwhile, log10 TOC (r2 = 0.16, p < 0.01) and log10 TN (r2 = 0.08, p < 0.05) showed positive but weak correlations with SWR (Fig. 2d–e), whereas log10 C/N ratio did not have significant correlation with SWR (p > 0.05) (Fig. 2f).
Biomarker groups and SWR
Soil biomarker groups
To get a more general view of the relationship between certain compounds and SWR, we have analyzed compound groups (i.e., the sum of all compounds of the same class in the three extraction fractions) (Table S1). As for extractable plant-derived biomarkers, the relative abundance of LFA were on average 52.17% higher in the soils under HR relative to the soils under PT (p < 0.01) (Fig. 3b). Cutin, primarily derived from leaf tissues, followed a trend contrary to that of LFA, in which the soils under PT had 35.39–78.77% higher cutin concentrations than that under/around other DPS (p < 0.05) (Fig. 3c). Additionally, the relative abundance of root-derived suberin under evergreen trees, deciduous trees, and shrubs (i.e., PT, RP and HR) were, on average, 56.77%, 19.55% and 62.69% lower than that around the legumes and grasses soils (i.e., CV and AC), respectively (p < 0.05) (Fig. 3d). To trace the microbial origin of TOC, we calculated the concentration of SFA. The lowest SFA concentrations (402.25 μg g−1 TOC) were found in the soils under PT as compared to RP, HR, CV and AC (p < 0.05) (Fig. 3a). Overall, the relative concentration of SFA in the soils under shrubs (HR) and legumes (AC), LFA in the soils under shrubs (HR), cutin in the soils under evergreen trees (PT), and suberin in the soils under legumes (AC) and grasses (CV) were highest, respectively (p < 0.05) (Fig. 3a–d).
The total relative concentrations (sum of D, AS, and AI fractions) of SFA, LFA, cutin, and suberin under/around the different DPS. a Short-chain fatty acids (C16–C18 n-alkanoic and n-alkenoic acid). b Long-chain fatty acids (> C24 alkanes, > C22 n-alkanoic acids, and alkanols). c Cutin (C14–C18 ω-hydroxyalkanoic acids). d Suberin (α, ω-dicarboxylic acids [C16–C24; saturated and substituted] and ω-hydroxyalkanoic acids [C20–C30; saturated and substituted]). Error bars are ± SD (n = 18, 12, 9, 9, 6 correspond to the number of soil samples from PT, RP, HR, CV, and AC) of the mean with lowercase letters above bars indicating significant differences between DPS at p < 0.05
Plant biomarker groups
For group concentrations of SFA, LFA, and cutin of leaves (Fig. 4a–c), the SFA concentrations of AC were on average 41.15% higher than that of other DPS, and the LFA concentrations of RP were on average 61.67% higher than that of other DPS. More importantly, PT had the highest cutin concentrations (2.16 mg g−1 dried leaf), which was 10–60 times higher than other DPS. In contrast, the highest concentration of SFA and LFA was found in the roots of CV, followed by AC, and then HR, RP, and PT (Fig. 4d–e). Moreover, the suberin concentrations in the roots of HR were on average 36.13% higher than that of other DPS (Fig. 4f).
Relations between groups of soil–plant linked biomarkers and SWR
Linear regression analysis revealed that cutin was positively related to SWR (r2 = 0.24, p < 0.001), whereas SFA (r2 = 0.31, p < 0.001) and LFA (r2 = 0.17, p < 0.01) were negatively related to SWR (Fig. 5a–c). However, no significant correlation was found between suberin and SWR (p > 0.05) (Fig. 5d). In order to understand whether the quality parameters of biomarkers can describe the SWR, the correlation between the ratio of the two separate compound groups and SWR was analyzed. For all soils, cutin was essential for a significant combination, when cutin were acted as the numerator, the correlation between the ratio of two separate groups and SWR was positive, otherwise, no significant relationship was observed (Fig. 5e–j).
Discussion
High OC input induces water repellency in surface soils
By supplying additional OC inputs to the soil, the Grain for Green Program has long been acknowledged to play a significant role in preserving or boosting soil C sequestration (Wang et al. 2016b). The higher cumulative C inputs in all sampling locations may be primarily attributed to the Grain for Green Program's favorable effect on SOC accumulation. Due to the introduction of hydrophobic materials into the soil, one of the causes of the high WR in the examined soils may be the high SOC content (Zavala et al. 2009). According to multiple studies (Scott 2000; Harper et al. 2000; Mataix-Solera and Doerr 2004; Mataix-Solera et al. 2007; Doerr et al. 2005; Lozano et al. 2013; Hermansen et al. 2019; Mao et al. 2019; Wang et al. 2016a; Seaton et al. 2019), there is a positive relationship between SOC and SWR, particularly when samples were taken from the same soil type and under or near the same DPS. In the present study, we found that some soil characteristics were highly correlated with SWR, in particular, TOC. However, the very strong relation between TOC and SWR determined by WPDT found in our study is not supported by other studies (Horne and McIntosh 2000; de Blas et al. 2010; Dekker and Ritsema 1994; Doerr et al. 2005). This discrepancy may be primarily due to the soils used in others' work were from different soil types and geographical locations, having different effects on SWR, while the loess we used were all from the same area. The positive correlation between TOC and SWR demonstrated that SOC greatly contributes to SWR. Despite the soils under them having similar TOC content (Fig. 2a), we also found different water-repellency classes under PT and RP (Fig. S1). This inconsistency has been attributed to the fact that SWR can be controlled by the type and quality of SOM rather than by its amount (Wallis and Horne 1992; Dekker and Ritsema 1994; de Blas et al. 2010).
TN was positively but weakly correlated with SWR, as earlier found by Lachacz et al. (2009), which is probably caused by the ability of legumes to fix atmospheric N, high-C and high-N litter inputs may shift the stoichiometry of substrates, accelerating the mineralization rate of microorganisms and increasing the yield of polysaccharides, thereby improving SWR. The C/N ratios were also positively but weakly correlated with SWR, which is consistent with the findings of Lachacz et al. (2009) and Mao et al. (2014). A high C/N ratio implies a recalcitrant material that can only be decomposed by fungi (Holtkamp et al. 2011), which may contribute to more severe SWR than labile organic material (Schindler et al. 2007). Regarding the interrelation between soil parameters, the decomposition of TOC in soils likely cause the change in C/N ratio, which leads to the correlation between C/N ratio and SWR. Consequently, TOC will be considered an important soil parameter to predict the WR levels of loess after returning farmland to forest (grassland).
Leaf-derived cutin contributes to surface SWR
In the present work, sequential extraction methods have been used to identify SWR biomarkers, including n-alkanoic acids, n-alkanols, n-alkanes, alkenoic acids, and some monomers derived from cutin and suberin, which are all hydrophobic compounds (Franco et al. 2000; Horne and McIntosh 2000; Mao et al. 2014, 2015; Bull et al. 2000; Lozano et al. 2013); however, the severity of SWR is not related to all the constituents of SOC, or the effectiveness of individual organic compounds on SWR decreases with increasing concentration (Doerr et al. 2005), thus we analyzed the possible relations between compound groups and SWR. The identified compounds in the D fraction were mainly the extractable free lipids, while the AS fraction and AI fraction is a combination of free lipids and ester lipids hydrolyzed by microbes and BF3–MeOH, which agreed with some previous studies (Van Bergen et al. 1997; DeBano 2000b; Franco et al. 2000; Mao et al. 2014). In terms of the extent to which SWR was represented during the extraction sequence, the SWR of most soils increased after DCM/MeOH extractions (p < 0.001), while the SWR significantly reduced after IPA/NH3 extraction (p < 0.001) (Fig. S2). These results indicated that it is ester-bound lipids, not free lipids, that play a major role in causing SWR. Although no clear evidence for such molecular behavior has been given, this paradox can be explained by a certain degree of mobility of free lipids. Due to the longer chain and less mobility of ester-bound lipids, they may be more hydrophobic than free, low-molecular-weight lipids. DCM/MeOH solvent, by extracting more lipid material, leaves more hydrophobic adsorption sites on the surface. Once the free lipids were removed, the adsorbed extent of ester-bound lipids onto high-affinity hydrophobic sites increased and as a consequence of that SWR increased as well. Because ester-bound lipids exist as polyfunctional macromolecules, they may change their structural conformations due to interactions involving the carboxyl and alcoholic hydroxyl functional groups. These interactions are disturbed by the extraction of polar compounds by IPA/NH3 solvent, causing conformational disruption, hence desorption of polar compounds and exposure of surface polar adsorption sites, resulting in a wettable soil (Atanassova and Doerr 2010; Riederer et al. 1993; Mao et al. 2019). Thus, SWR increased in all the soils after DCM/MeOH solvent extraction and was eliminated by IPA/NH3. Although the IPA/NH3 solvent extracted less free lipids and more ester-bound lipids than the DCM/MeOH solvent, it was more suitable for extracting compounds of certain associated with SWR elimination.
We separately analyzed the absolute and relative concentrations of compound groups in all extraction fractions. For all soils, the absolute concentrations of most compound groups associated with SWR (Fig. S4), which is supported by Mao et al. (2015) who also noted that the positive relationships between the absolute concentrations of all the compound groups and SWR follow the positive correlations between TOC and SWR. Obviously, the relationships between absolute concentrations of compound groups and SWR are determined by the TOC content, we cannot identify which compound group affects SWR by analyzing the absolute concentrations of the compound groups. Regarding the relative concentrations of SWR biomarker groups, PT with higher WDPT values had 59.70% higher cutin concentrations than that under/around other DPS (Fig. 3c), which was the only group to show positive relation with SWR (p < 0.001), while the groups of LFA, SFA showed negatively relations with SWR for all soils (p < 0.01) (Fig. 5a–c). However, no significant correlation was found between suberin and SWR (p > 0.05) (Fig. 5d). Similar results have been reported in many previous studies (Mao et al. 2014; Atanassova and Doerr 2010; Hansel et al. 2008), which showed that the differences in SWR studied cannot be attributed solely to a single factor or group, but also the interaction of multiple compound groups. In our study, we have tried to explain which SWR biomarker groups are the most relevant in the development of SWR in loess. Our correlation data of the ratio of the two separate compound groups and SWR revealed that when cutin were acted as the numerators, the ratio of the two separate compound groups was always positively correlated with SWR, and vice versa, with no significant relationship (Fig. 5e–j). The positive correlations between cutin / LFA (r2 = 0.27, p < 0.001) and cutin / SFA (r2 = 0.30, p < 0.001) with SWR were strongest (Fig. 5e and f). This finding might be mainly due to the increased adsorption of cutin onto high-affinity hydrophobic sites on the soil surface after more D fraction compounds were extracted, resulting in a larger SWR. According to the above results, SWR occurring on the loess surface seems to be the most influenced by cutin-derived C, and cutin / FA is considered an important soil quality parameter for predicting SWR levels on the loess surface (Fig. 6).
Cover PFTs-induced changes of the abundance and composition of hydrophobic compounds maintain SWR
Cover plant types may largely alter the content and composition of SOC by providing additional OC residues (Van Bergen et al. 1997; Nierop 2001; Kögel-Knabner 2002; Zhang et al. 2022). In the present work, the main groups of the extractable and ester-bound lipids present in the leaves and roots were all identified in the soils under/around the given plant species, which might be due to the cover plant are the main sources of the SWR biomarkers (Nierop et al. 2003; Naafs et al. 2004). In line with previous studies (Naafs et al. 2004; Nierop et al. 2006; Zhang et al. 2022), the predominance of even-over-odd numbered LFA (> C24 alkanes, > C22 n-alkanoic acids and alkanols) (Fig. S5) indicated that the primary sources are plants (Jansen et al. 2006; Zhang et al. 2022). By contrast, SFA (C16–C18 n-alkanoic and n-alkenoic acid), which were identified in all of our topsoil and plant samples, are mostly microbial-derived biomarkers. It was detected in plant samples, possibly due to the Endophytic microorganisms in plants (Jaffé et al. 1996; Jansen et al. 2006; Zhang et al. 2022). Cutin and suberin in the AS fractions may be from microbially hydrolyzed ester lipids, while those in the AI fractions are polymers hydrolyzed by BF3–MeOH from leaves/needles and roots of plants, respectively (Riederer et al. 1993; Mao et al. 2014). Therefore, the role of microorganisms in the hydrolysis of biopolymers cutin and suberin in the natural environment cannot be underestimated. In addition, the relationship between other microbial-derived C, such as ergosterol and glomalin-related soil protein, and SWR was not considered in our work. These carbon sources in soil may be produced by filamentous fungi (Feeney et al. 2004; Wessels 1996, 2000; White et al. 2017; Rillig et al. 2010; Bayry et al. 2012; Lozano et al. 2013), which directly affect SWR. However, our research only emphasized the influence of microbial-derived biomarkers SFA on SWR, the relationship between other microbial-derived biomarkers in loess and SWR may need further validation.
From the above analysis, the ester-bound lipids biomarkers after IPA/NH3 extraction represent the cutin and suberin-derived compounds in the plant leaves/needles and roots, respectively. The differences in suberin concentrations were observed, in which legumes, grasses soils, and shrubs soils were richer than evergreen trees and deciduous trees (Fig. 3d). This may be primarily due to plants with shallow and horizontal extended roots easily accumulating suberin than deep-rooted plants in topsoil. In our work, the small amounts of suberin under deep-rooted plants may derive from grasses providing suberin to the topsoil. Another source could be bark, which also contains suberin although they contribute less to the soil than roots (Preston et al. 1994). Evergreen trees with higher WDPT values had higher cutin concentrations in leaves and soils as compared to deciduous trees, legumes, grasses, and shrubs (Fig. 3c; Fig. 4c). These results are in line with Rodríguez-Alleres and Benito (2011) and Badía et al. (2013), who both manifested that the SWR persistence of grassland soil was much less than that under forest soil. Seaton et al. (2019) and Smettem et al. (2021) have successively reached the same conclusion that some deep-rooted plants can take advantage of SWR under drought stress, leading to the emergence of co-evolutionary behavior in natural ecosystems. This observation is also found in some previous studies, which showed SWR not only allows rainwater to form a preferential flow, penetrate into deep soil, and store in large quantities (De Boeck and Verbeeck 2011; Zeppenfeld et al. 2017; Alanís et al. 2016; Lozano et al. 2013), but also reduces soil water evapotranspiration loss through various mechanisms, making deep-rooted plants more resistant to drought than shallow-rooted plants (Doerr et al. 2006; Rye and Smettem 2018; Shahidzadeh-Bonn et al. 2007; Gupta et al. 2015). This can be inferred that deep-rooted evergreen trees tend to accumulate cutin rather than suberin on the loess surface, forming a hydrophobic layer and were hence more likely to cause SWR than other PFTs.
Conclusions
This study presents the first molecular investigation to explore the relationship between SOM composition and SWR output in the Chinese Loess Plateau, the extremely complex soil-eroding region. Since SWR increased after DCM/MeOH extraction and disappeared almost after IPA/NH3 extraction, we speculate that ester-bound lipids after IPA/NH3 extraction were stronger SWR-markers than free lipids. Our GC–MS data showed that the cutin concentrations in leaves and soils of evergreen trees with higher WDPT values were 10–60 times and 35.39–78.77% higher than those of other PFTs, respectively. However, the root-derived suberin in the soils under evergreen trees, deciduous trees, and shrubs were 19.55–62.69% lower than that around the legumes and grasses. Therefore, we demonstrate that leaf-derived cutin appears to have the greatest effect on SWR, which was also supported by the results of the correlation analysis. Besides, the correlation between the ratio of the two separate compound groups and SWR indicated that the combination cutin / FA was more strongly correlated with SWR, and thus it was considered as an important soil parameter for predicting the SWR. These results shed new light that the differentiation of cover PFTs alter the abundance and composition of hydrophobic compounds in soils, thereby affecting SWR levels in a semi-arid region of the Chinese Loess Plateau. Furthermore, this study highlights the important role of plant-soil interactions in mediating the high variability of SWR in typical loess soils, which could have significant implications for developing effective strategies to manage soil water availability at a later stage, particularly in arid and semi-arid regions where water is scarce.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Alanís N, Hernández-Madrigal VM, Cerdà A, Muñoz-Rojas M, Zavala LM, Jordán A (2016) Spatial gradients of intensity and persistence of soil water repellency under different forest types in Central Mexico. Land Degrad Dev 28(1):317–327. https://doi.org/10.1002/ldr.2544
Arcenegui V, Mataix-Solera J, Guerrero C, Zornoza R, Mayoral AM, Morales J (2007) Factors controlling the water repellency induced by fire in calcareous mediterranean forest soils. Eur J Soil Sci 58:1254–1259. https://doi.org/10.1111/j.1365-2389.2007.00917.x
Atanassova I, Doerr S (2010) Organic compounds of different extractability in total solvent extracts from soils of contrasting water repellency. Eur J Soil Sci 61:298–313. https://doi.org/10.1111/j.1365-2389.2009.01224.x
Badía D, Aguirre JA, Martí C, Márquez MA (2013) Sieving effect on the intensity and persistence of water repellency at different soil depths and soil types from NE-Spain. Catena 108:44–49. https://doi.org/10.1016/j.catena.2012.02.003
Balshaw HM, Douglas P, Davies ML, Doerr SH (2020) Pyrene and nile red fluorescence probes for in-situ study of polarity and viscosity of soil organic coatings implicated in soil water repellency. Eur J Soil Sci 71:868–879. https://doi.org/10.1111/ejss.12925
Bayry J, Aimanianda V, Guijarro JI, Sunde M, Latgé J-P (2012) Hydrophobins–unique fungal proteins. PloS Pathog 8(5):e1002700. https://doi.org/10.1371/journal.ppat.1002700
Bisdom EBA, Dekker LW, Schoute JFTh (1993) Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 56:105–118. https://doi.org/10.1016/0016-7061(93)90103-R
Bremner J, Mulvaney C (1982) Nitrogen total content. In: Methods of soil analysis, 2nd edn. USA, pp 595–624
Bull ID, Bergen PF, Nott CJ, Poulton PR, Evershed RP (2000) Organic geochemical studies of soils from the Rothamsted classical experiments—V. The fate of lipids in different long-term experiments. Org Geochem 31(5):389–408. https://doi.org/10.1016/s0146-6380(00)00008-5
Butzen V, Seeger M, Marruedo A, Jonge LD, Wengel R, Ries JB, Casper MC (2015) Water repellency under coniferous and deciduous forest—experimental assessment and impact on overland flow. Catena 133:255–265. https://doi.org/10.1016/j.catena.2015.05.022
Chai X, Xu X, Li L, Wang W, Li S, Geming P, Qu Y, Zhang Q, Ren X, Xu Y, Li M (2022) Physicochemical and biological factors determining the patchy distribution of soil water repellency among species of dominant vegetation in loess hilly region of China. Front Plant Sci. https://doi.org/10.3389/fpls.2022.908035
Chau WH, Biswas A, Vujanovic V, Si BC (2014) Relationship between the severity, persistence of soil water repellency and the critical soil water content in water repellent soils. Geoderma 221–222:113–120. https://doi.org/10.1016/j.geoderma.2013.12.025
Coelho COA, Laouina A, Regaya K, Ferreira AJD, Carvalho TMM, Chaker M, Naafa R, Naciri R, Boulet AK, Keizer JJ (2005) The impact of soil water repellency on soil hydrological and erosional processes under eucalyptus and evergreen quercus forests in the Western Mediterranean. Aust J Soil Res 43:309–318. https://doi.org/10.1071/SR04083
DeBano LF (2000a) Water repellency in soils: a historical overview. J Hydrol 231–232:4–32. https://doi.org/10.1016/s0022-1694(00)00180-3
DeBano LF (2000b) The role of fire and soil heating on water repellency in wildland environments: a review. J Hydrol 231–232:195–206. https://doi.org/10.1016/S0022-1694(00)00194-3
de Blas E, Rodríguez-Alleres M, Almendros G (2010) Speciation of lipid and humic fractions in soils under pine and eucalyptus forest in Northwest Spain and its effect on water repellency. Geoderma 155:242–248. https://doi.org/10.1016/j.geoderma.2009.12.007
de Blas E, Almendros G, Sanz J (2013) Molecular characterization of lipid fractions from extremely water-repellent pine and eucalyptus forest soils. Geoderma 206:75–84. https://doi.org/10.1016/j.geoderma.2013.04.027
De Boeck HJ, Verbeeck H (2011) Drought-associated changes in climate and their relevance for ecosystem experiments and models. Biogeosciences 8(5):1121–1130. https://doi.org/10.5194/bg-8-1121-2011
Dekker LW, Ritsema CJ (1994) How water moves in a water repellent sandy soil: 1. Potential and actual water repellency. Water Resour Res 30(9):2507–2517. https://doi.org/10.1029/94WR00749
Dekker LW, Ritsema CJ (1996) Variation in water content and wetting patterns in dutch water repellent peaty clay and clayey peat soils. Catena 28:89–105. https://doi.org/10.1016/s0341-8162(96)00047-1
Doerr SH, Shakesby RA, Walsh RPD (2000) Soil water repellency: its causes, characteristics and hydro-geomorphological significance. Earth-Sci Rev 51:33–65. https://doi.org/10.1016/S0012-8252(00)00011-8
Doerr SH, Thomas A (2000) The role of soil moisture in controlling water repellency: new evidence from forest soils in Portugal. J Hydrol 231–232:134–147. https://doi.org/10.1016/s0022-1694(00)00190-6
Doerr SH, Llewellyn CT, Douglas P, Morley CP, Mainwaring KA, Haskins C, Johnsey L, Ritsema CJ, Stagnitti F, Allinson G, Ferreira AJD, Keizer JJ, Ziogas AK, Diamantis J (2005) Extraction of compounds associated with water repellency in sandy soils of different origin. Aust J Soil Res 43:225–237. https://doi.org/10.1071/SR04091
Doerr SH, Shakesby RA, Dekker LW, Ritsema CJ (2006) Occurrence, prediction and hydrological effects of water repellency amongst major soil and land-use types in a humid temperate climate. Eur J Soil Sci 57(5):741–754. https://doi.org/10.1111/j.1365-2389.2006.00818.x
Feeney DS, Paul D, Hallett TD, Karl Ritz JI, Young IM (2004) Does the presence of glomalin relate to reduced water infiltration through hydrophobicity? Can J Soil Sci 84(4):365–372. https://doi.org/10.4141/s03-095
Feng X, Simpson AJ, Schlesinger WH, Simpson MJ (2010) Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Global Change Biol 16:2104–2116. https://doi.org/10.1111/j.1365-2486.2009.02080.x
Franco CM, Clarke P, Tate M, Oades J (2000) Hydrophobic properties and chemical characterisation of natural water repellent materials in australian sands. J Hydrol 231–232:47–58. https://doi.org/10.1016/s0022-1694(00)00182-7
Fu B, Yu L, Lue Y, He C, Yuan Z, Wu B (2011) Assessing the soil erosion control service of ecosystems change in the loess plateau of China. Ecol Complex 8(4):284–293. https://doi.org/10.1016/j.ecocom.2011.07.003
González-Pérez JA, González-Vila FJ, Almendros G, Knicker H (2004) The effect of fire on soil organic matter—a review. Environ Int 30:855–870. https://doi.org/10.1016/j.envint.2004.02.003
González-Pérez JA, de Andrés JR, Clemente L, Martín JA, González-Vila FJ (2008) Organic carbon and environmental quality of riverine and off-shore sediments from the gulf of Cádiz, Spain. Environ Chem Lett 6(1):41–46. https://doi.org/10.1007/s10311-007-0107-0
González-Peñaloza FA, Cerdà A, Zaval LM, Jordán A, Giménez-Morera A, Arcenegui V (2013) Do conservative agriculture practices increase soil water repellency? A case study in citrus-cropped soils. Soil Till Res 124(2012):233–239. https://doi.org/10.1016/j.still.2012.12.001
Gupta B, Shah DO, Mishra B, Joshi PA, Gandhi VG, Fougat RS (2015) Effect of top soil wettability on water evaporation and plant growth. J Colloid Interf Sci 449:506–513. https://doi.org/10.1016/j.jcis.2015.02.018
Hansel FA, Aoki CT, Maia CMBF, Cunha JA, Dedecek RA (2008) Comparison of two alkaline treatments in the extraction of organic compounds associated with water repellency in soil under Pinus taeda. Geoderma 148(2):167–172. https://doi.org/10.1016/j.geoderma.2008.10.002
Harper RJ, McKissock I, Gilkes RJ, Carter DJ, Blackwell PS (2000) A multivariate framework for interpreting the effects of soil properties, soil management and landuse on water repellency. J Hydrol 231–232:371–383. https://doi.org/10.1016/s0022-1694(00)00209-2
Hermansen C, Moldrup P, Müller K, Jensen PW, Carlo VDD, Jeyakumar P, Jonge LWD (2019) Organic carbon content controls the severity of water repellency and the critical moisture level across New Zealand pasture soils. Geoderma 338:281–290. https://doi.org/10.1016/j.geoderma.2018.12.007
Holtkamp R, Van der Wal A, Kardol P, Van der Putten WH, De Ruiter PC, Dekker SC (2011) Modelling C and N mineralisation in soil food webs during secondary succession on ex-arable land. Soil Biol Biochem 43(2):251–260. https://doi.org/10.1016/S0022-1694(00)00182-7
Horne D, McIntosh J (2000) Hydrophobic compounds in sands in New Zealand–extraction, characterisation and proposed mechanisms for repellency expression. J Hydrol 231–232:35–46. https://doi.org/10.1016/s0022-1694(00)00181-5
Huang MB, Shao MG, Zhang L, Li YS (2003) Water use efficiency and sustainability of different long-term crop rotation systems in the loess plateau of China. Soil till Res 72:95–104. https://doi.org/10.1016/S0167-1987(03)00065-5
Jaffé R, Elismé T, Cabrera AC (1996) Organic geochemistry of seasonally flooded rain forest soils: molecular composition and early diagenesis of lipid components. Org Geochem 25:9–17. https://doi.org/10.1016/S0146-6380(96)00103-9
Jansen B, Nierop KGJ, Hageman JA, Cleef AM, Verstraten JM (2006) The straight-chain lipid biomarker composition of plant species responsible for the dominant biomass production along two altitudinal transects in the ecuadorian Andes. Org Geochem 37:1514–1536. https://doi.org/10.1016/j.orggeochem.2006.06.018
Jiménez-Morillo NT, Almendros G, Miller AZ, Hatcher PG, González-Pérez JA (2022) Hydrophobicity of soils affected by fires: an assessment using molecular markers from ultra-high resolution mass spectrometry. Sci Total Environ 817:152957. https://doi.org/10.1016/j.scitotenv.2022.152957
Jiménez-Pinilla P, Doerr SH, Ahn S, Lozano E, Mataix-Solera J, Jordán A, Zavala LM, Arcenegui V (2016) Effects of relative humidity on the water repellency of fire-affected soils. Catena 138:68–76. https://doi.org/10.1016/j.catena.2015.11.012
Jordán A, Zavala LM, Nava AL, Alanís N (2009) Occurrence and hydrological effects of water repellency in different soil and land use types in mexican volcanic highlands. Catena 79:60–71. https://doi.org/10.1016/j.catena.2009.05.013
Jordán A, Zavala LM, Mataix-Solera J, Doerr SH (2013) Soil water repellency: origin, assessment and geomorphological consequences. Catena 108:1–5. https://doi.org/10.1016/10.1016/s0167-1987(03)00065-5
Karunarathna AK, Kawamoto K, Moldrup P, Jonge LWD, Komatsu T (2010) A simple beta-function model for soil-water repellency as a function of water and organic carbon contents. Soil Sci 175:461–468. https://doi.org/10.1097/SS.0b013e3181f55ab6
Keesstra S, Pereira P, Novara A, Brevik EC, Azorin-Molina C, Parras-Alcántara L, Jordán A, Cerdà A (2016) Effects of soil management techniques on soil water erosion in apricot orchards. Sci Total Environ 551–552:357–366. https://doi.org/10.1016/j.scitotenv.2016.01.182
Kodešová R, Kočárek M, Kodeš V, Šimůnek J, Kozák J (2008) Impact of soil micromorphological features on water flow and herbicide transport in soils. Vadose Zone J 7:798–809. https://doi.org/10.2136/vzj2007.0079
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. https://doi.org/10.1016/s0038-0717(01)00158-4
Kraemer FB, Hallett PD, Morrás H, Garibaldi L, Cosentino D, Duval M, Galantini J (2019) Soil stabilisation by water repellency under no-till management for soils with contrasting mineralogy and carbon quality. Geoderma 355:113902. https://doi.org/10.1016/j.geoderma.2019.113902
Lachacz A, Nitkiewicz M, Kalisz B (2009) Water repellency of post-boggy soils with a various content of organic matter. Biologia 64:634–638. https://doi.org/10.2478/s11756-009-0096-5
Leighton-Boyce G, Doerr SH, Shakesby RA, Walsh RPD (2007) Quantifying the impact of soil water repellency on overland flow generation and erosion: a new approach using rainfall simulation and wetting agent onin situ soil. Hydrol Process 21:2337–2345. https://doi.org/10.1002/hyp.6744
Li Y, Yao N, Tang DX, Chau HW, Feng H (2019) Soil water repellency decreases summer maize growth. Agric Forest Meteorol 266–267:1–11. https://doi.org/10.1016/j.agrformet.2018.12.001
Liu F, Zhan Y (2019) Soil water repellency in China and Israel: synthesis of observations and experiments. Appl Ecol Environ Res 17(4):8599–8614. https://doi.org/10.15666/aeer/1704_85998614
Lozano E, Jiménez-Pinilla P, Mataix-Solera J, Arcenegui V, Bárcenas GM, González-Pérez JA, García-Orenes F, Torres MP, Mataix-Beneyto J (2013) Biological and chemical factors controlling the patchy distribution of soil water repellency among plant species in a Mediterranean semiarid forest. Geoderma 207–208:212–220. https://doi.org/10.1016/j.geoderma.2013.05.021
Lozano E, García-Orenes F, Bárcenas-Moreno G, Jiménez-Pinilla P, Mataix-Solera J, Arcenegui V, Morugán-Coronado A, Mataix-Beneyto J (2014) Relationships between soil water repellency and microbial community composition under different plant species in a mediterranean semiarid forest. J Hydrol Hydromech 62(2):101–107. https://doi.org/10.2478/johh-2014-0017
Mao J, Nierop KGJ, Sinninghe Damsté JS, Dekker SC (2014) Roots induce stronger soil water repellency than leaf waxes. Geoderma 232–234:328–340. https://doi.org/10.1016/j.geoderma2014.05.024
Mao J, Nierop KGJ, Rietkerk M, Dekker SC (2015) Predicting soil water repellency using hydrophobic organic compounds and their vegetation origin. Soil 1(1):411–425. https://doi.org/10.5194/soil-1-411-2015
Mao J, Nierop KGJ, Dekker SC, Dekker LW, Chen B (2019) Understanding the mechanisms of soil water repellency from nanoscale to ecosystem scale: a review. J Soil Sediment 19:171–185. https://doi.org/10.1007/s11368-018-2195-9
Mataix-Solera J, Doerr S (2004) Hydrophobicity and aggregate stability in calcareous topsoils from fire-affected pine forests in southeastern Spain. Geoderma 118(1–2):77–88. https://doi.org/10.1016/s0016-7061(03)00185-x
Mataix-Solera J, Arcenegui V, Guerrero C, Mayoral AM, Morales J, González J, Garcia-Orenes F, Gómez I (2007) Water repellency under different plant species in a calcareous forest soil in a semiarid Mediterranean environment. Hydrol Process 21:2300–2309. https://doi.org/10.1002/hyp.6750
Moro MJ, Pugnaire FI, Haase P, Puigdefabregas J (1997) Effect of the canopy of Retama sphaerocarpa on its understorey in a semiarid environment. Funct Ecol 11:425–431. https://doi.org/10.1046/j.1365-2435.1997.00106.x
Müller K, Carrick S, Meenken E, Clemens G, Hunter D, Rhodes P, Thomas S (2016) Is subcritical water repellency an issue for efficient irrigation in arable soils? Soil Till Res 161:53–62. https://doi.org/10.1016/j.still.2016.03.010
Muñoz-Rojas M, Jordán A, Zavala LM, Rosa DDL, Abd-Elmabod SK, Anaya-Romero M (2012) Organic carbon stocks in Mediterranean soil types under different land uses (southern Spain). Solid Earth Discuss 4:1095–1128. https://doi.org/10.5194/sed-4-1095-2012
Naafs DFW, Van Bergen PF, Boogert SJ, De Leeuw JW (2004) Solvent-extractable lipids in an acid andic forest soil; variations with depth and season. Soil Biol Biochem 36:297–308. https://doi.org/10.1016/j.soilbio.2003.10.005
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Methods of Soil Analysis, 3rd edn. USA, pp 961–1010
Nierop KGJ (2001) Temporal and vertical organic matter differentiation along a vegetation succession as revealed by pyrolysis and thermally assisted hydrolysis and methylation. J Anal Appl Pyrol 61:111–132. https://doi.org/10.1016/S0165-2370(01)00132-2
Nierop KGJ, Naafs DFW, Verstraten JM (2003) Occurrence and distribution of ester-bound lipids in dutch coastal dune soils along a pH gradient. Org Geochem 34:719–729. https://doi.org/10.1016/S0146-6380(03)00042-1
Nierop KGJ, Jansen B, Hageman JA, Verstraten JM (2006) The complementarity of extractable and ester-bound lipids in a soil profile under pine. Plant Soil 286:269–285. https://doi.org/10.1007/s11104-006-9043-1
Otto A, Simpson MJ (2007) Analysis of soil organic matter biomarkers by sequential chemical degradation and gas chromatography—Mass spectrometry. J Sep Sci 30:272–282. https://doi.org/10.1002/jssc.200600243
Preston CM, Hempfling R, Schulten HR, Schnitzer M, Trofymow JA, Axelson DE (1994) Characterization of organic matter in a forest soil of coastal British Columbia by NMR and pyrolysis-field ionization mass spectrometry. Plant Soil 158:69–82. https://doi.org/10.1007/BF00007919
Riederer M, Matzke K, Ziegler F, Kögel-Knabner I (1993) Occurrence, distribution and fate of the lipid plant biopolymers cutin and suberin in temperate forest soils. Org Geochem 20:1063–1076. https://doi.org/10.1016/0146-6380(93)90114-Q
Rillig MC, Mardatin NF, Leifheit EF, Antunes PM (2010) Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol Biochem 42(7):1189–1191. https://doi.org/10.1016/j.soilbio.2010.03.027
Rodríguez-Alleres M, Benito E (2011) Spatial and temporal variability of surface water repellency in sandy loam soils of NW Spain under Pinus pinaster and Eucalyptus globulus plantations. Hydrol Process 25:3649–3658. https://doi.org/10.1002/hyp.8091
Rodríguez-Alleres M, Benito E (2012) Temporal fluctuations of water repellency in forest soils of Galicia, NW Spain. do soil samples dried at laboratory reflect the potential soil water repellency? Hydrol Process 26:1179–1187. https://doi.org/10.1002/hyp.8209
Rye CF, Smettem KRJ (2018) Seasonal variation of subsurface flow pathway spread under a water repellent surface layer. Geoderma 327:1–12. https://doi.org/10.1016/j.geoderma.2018.04.008
Schonsky H, Peters A, Wessolek G (2014) Effect of soil water repellency on energy partitioning between soil and atmosphere: a conceptual approach. Pedosphere 24(4):498–507. https://doi.org/10.1016/S1002-0160(14)60036-9
Schindler F, Mercer E, Rice J (2007) Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil Biol Biochem 39:320–329. https://doi.org/10.1016/j.soilbio.2006.08.017
Scott DF (2000) Soil wettability in forested catchments in South Africa as measured by different methods and as affected by vegetation cover and soil characteristics. J Hydrol 231–232:87–104. https://doi.org/10.1016/S0022-1694(00)00186-4
Seaton FM, Jones DL, Creer S, George PBL, Smart SM, Lebron I, Barrett G, Emmett BA, Robinson DA (2019) Plant and soil communities are associated with the response of soil water repellency to environmental stress. Sci Total Environ 687:929–938. https://doi.org/10.1016/j.scitotenv.2019.06.052
Shahidzadeh-Bonn N, Azouni A, Coussot P (2007) Effect of wetting properties on the kinetics of drying of porous media. J Phys-Condens Mat 19(11):112101. https://doi.org/10.1088/0953-8984/19/11/112101
Simpson RM, Mason K, Robertson K, Müller K (2019) Relationship between soil properties and enzyme activities with soil water repellency. Soil Res 57(6):689–702. https://doi.org/10.1071/SR18199
Smettem K, Rye C, Henry DJ, Sochacki SJ, Harper RJ (2021) Soil water repellency and the five spheres of influence: a review of mechanisms, measurement and ecological implications. Sci Total Environ 787:147429. https://doi.org/10.1016/j.scitotenv.2021.147429
Song E, Pan X, Kremer RJ, Goyne KW, Anderson SH, Xiong X (2019) Influence of repeated application of wetting agents on soil water repellency and microbial community. Sustainability 11:4505. https://doi.org/10.3390/su11164505
Tamura M, Tharayil N (2014) Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol 203:110–124
Urbanek E, Walsh RPD, Shakesby RA (2015) Patterns of soil water repellency change with wetting and drying: the influence of cracks, roots and drainage conditions. Hydrol Process 29(12):2799–2813. https://doi.org/10.1002/hyp.10404
Van Bergen PF, Bull ID, Poulton PR, Evershed RP (1997) Organic geochemical studies of soils from the rothamsted classical experiments—I. Total lipid extracts, solvent insoluble residues and humic acids from broadbalk wilderness. Org Geochem 26:117–135. https://doi.org/10.1016/S0146-6380(96)00134-9
Van’t Woudt BD (1959) Particle coatings affecting the wettability of soils. J Geophys Res 64:263–267.https://doi.org/10.1029/JZ064i002p00263
Walden LL, Harper RJ, Mendham DS, Henry DJ, Fontaine JB (2015) Eucalyptus reforestation induces soil water repellency. Soil Res 53(2):168–177. https://doi.org/10.1071/sr13339
Wallis MG, Horne DJ (1992) Soil water repellency. Adv Soil Sci 20:91–146. https://doi.org/10.1007/978-1-4612-2930-8_2
Wang J, Zheng Y, Hu H, Li J, Zhang L, Chen B, Chen W, He J (2016a) Coupling of soil prokaryotic diversity and plant diversity across latitudinal forest ecosystems. Sci Rep 6:19561. https://doi.org/10.1038/srep19561
Wang S, Fu BJ, Piao SR, Lü YH, Ciais P, Feng XM, Wang YF (2016b) Reduced sediment transport in the Yellow River due to anthropogenic changes. Nat Geosci 9(1):38–41. https://doi.org/10.1038/ngeo2602
Wang Z, Hu Y, Wang R, Guo S, Du L, Zhao M, Yao Z (2017) Soil organic carbon on the fragmented chinese loess plateau: combining effects of vegetation types and topographic positions. Soil till Res 174:1–5. https://doi.org/10.1016/j.still.2017.05.005
Wang R, Hu Y, Khan A, Du L, Wang Y, Hou F, Guo S (2021) Soil prokaryotic community structure and co-occurrence patterns on the fragmented chinese loess plateau: effects of topographic units of a soil eroding catena. Catena 198:105035. https://doi.org/10.1016/j.catena.2020.105035
Wessel AT (1988) On using the effective contact angle and the water drop penetration time for classification for water repellency in dune soils. Earth Surf Proc Land 13(6):555–561. https://doi.org/10.1002/esp.3290130609
Wessels JGH (1996) Hydrophobins: proteins that change the nature of the fungal surface. Adv Microb Physiol 38:1–45. https://doi.org/10.1016/s0065-2911(08)60154-x
Wessels JGH (2000) Hydrophobins, unique fungal proteins. Mycologist 14(4):153–159. https://doi.org/10.1016/s0269-915x(00)80030-0
White AM, Lockington DA, Gibbes B (2017) Does fire alter soil water repellency in subtropical coastal sandy environments? Hydrol Process 31:341–348. https://doi.org/10.1002/hyp.11000
Zavala LM, González FA, Jordán A (2009) Intensity and persistence of water repellency in relation to vegetation types and soil parameters in Mediterranean SW Spain. Geoderma 152:361–374. https://doi.org/10.1016/j.geoderma.2009.07.011
Zeppenfeld T, Balkenhol N, Kóvacs K, Carminati A (2017) Rhizosphere hydrophobicity: a positive trait in the competition for water. PLoS One 12(7):e0182188. https://doi.org/10.1371/journal.pone.0182188
Zhang Z, Kaye JP, Bradley BA, Amsili JP, Suseela V (2022) Cover crop functional types differentially alter the content and composition of soil organic carbon in particulate and mineral-associated fractions. Global Change Biol 28:5831–5848. https://doi.org/10.1111/gcb.16296
Zheng S, Lourenço SDN, Cleall PJ, Chui TFM, Ng AKY, Millis SW (2017) Hydrologic behavior of model slopes with synthetic water repellent soils. J Hydrol 554:582–599. https://doi.org/10.1016/j.jhydrol.2017.09.013
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number 41977426). We would like to thank the staff of Changwu Agro-Ecological Experiment Station for their assistance in field investigations and sample collections.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Raúl Ochoa-Hueso.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chai, X., Wang, W., Ren, X. et al. Cover plant functional types alter the abundance and composition of hydrophobic compounds: The relationship with soil water repellency on the Chinese Loess Plateau. Plant Soil 497, 687–704 (2024). https://doi.org/10.1007/s11104-023-06424-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06424-6