Abstract
Aims
Root system architecture (RSA) is important for nutrient and water acquisition efficiency. The adaptation of root growth and RSA to soil structure under conservative strip tillage (ST) system warrants further investigation.
Methods
A three-year field experimentation was conducted in Northeast China to investigate the RSA and dynamic root growth of rain-fed maize under ST system by comparison with the conventional tillage (CT).
Results
Grain yield in ST and CT was not significantly different, but their yield components differed. Compared to CT, grain number per ear was reduced by 4.4%, while 1000-grain weight was increased by 6.6% in ST. Root growth in ST plants was inhibited in the vegetative stage, as indicated by the reduced total root length (by 27.7–40.1%) compared to CT. During post-silking stage, the total root length was not different between ST and CT plants but the root xylem bleeding rate in ST plants was 70.7%-449.9% greater than that in CT. The uneven horizontal distribution of soil bulk density and soil temperature made the RSA of ST plants steeper compared to CT. Moreover, the D95 of root distribution in ST plant roots was greater.
Conclusions
In ST system, colder, more compacted soil in the inter-row soil likely caused the lower root growth and consequently lower shoot dry matter during the vegetative stage. However, root senescence was delayed which was beneficial for water and nitrogen acquisition during grain filling. Strategies to improve early root growth may increase maize productivity in ST systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant root system architecture (RSA), including root geometry and its spatial distribution characteristics, plays an important role in water and nutrient absorption (Lynch et al. 1995; Brady et al. 1995; Amato and Ritchie 2002; Doussan et al. 2006; Dorlodot et al. 2007; Hammer et al. 2009; Paez-Garcia et al. 2015; Maqbool et al. 2022). RSA is dynamic and is affected by external environmental conditions including soil temperature, moisture, nutrients, pH, and microbial communities (Bengough et al. 2006; Mi et al. 2010; Bao et al. 2014; Robbins and Dinneny 2015). Soil conditions in the field is locally different (Jackson and Caldwell 1993). Plants can sense the variation of soil environmental factors and make corresponding responses. For example, soil temperature can influence the direction of root growth (Mosher and Miller 1972). Low temperature induces smaller branching angles between primary and lateral roots, reduces root elongation, and reduces the volume that roots access (Nagel et al. 2009). In field conditions, roots are continuously exposed to mechanical resistance caused by soil compaction. In response to soil compaction, the total root length is reduced, root diameter and root hair number are increased, and root angle becomes steeper (Correa et al. 2019). Due to the soil heterogeneity, compensatory root growth may happen in the unimpeded soil and the total root length may not be altered (Unger and Kaspar 1994).
Tillage can change soil porosity, aggregate structure, bulk density and other characteristics, and affect soil respiration, temperature and humidity (Guan et al. 2014). These biological and non-biological processes can affect plant root growth (Lipiec and Stepniewski 1995; Mosaddeghi et al. 2009; Ioanna et al. 2019). No-till (NT) is a major form of conservation tillage system. Compared with conventional tillage (CT), NT reduces soil erosion and increases soil water content, but reduces soil temperature and increase soil bulk density (Cannell 1985; Dwyer et al. 1996; Munkholm et al. 2012; Muñoz-Romero et al. 2012; Soane et al. 2012), although long-term NT may reduce soil bulk density (Fiorini et al. 2018). To reduce the disadvantageous effect of NT on soil temperature, strip-till (ST) has been gradually developed as a major form of conservative tillage in maize production (Morrison 2002; Licht and Al-Kaisi 2005a; Trevini et al. 2013; Fernández et al. 2015; Sha et al. 2023). ST combines the benefits of CT and NT by cultivating the intra-row soil to form a planting strip and leaving the inter-row covered with crop residue through the whole maize growth period (Vyn and Raimbault 1992). By this way, soil temperature is increased and soil bulk density is reduced in the planting strip (Licht and Al-Kaisi 2005b). As a result, root growth is improved, and maize yield is guaranteed to the similar level of NT (Morrison 2002; Potratz et al. 2020). However, compared with CT, the low temperature and high soil compaction in the inter-row soil is still a disadvantage (Lipiec and Hatano 2003; Ren et al. 2019), which can cause maize yield fluctuation according to different soil types and climatic conditions. For example, in dry years or on sandy soils, the effect of ST on water and soil conservation is more obvious, and has yield advantages compared with CT (Temesgen et al. 2012). In rainy years or in clayey soils, maize yield can be lower in ST compared to CT (Vyn and Raimbault 1992; Licht and Al-Kaisi 2005a).
The adaptation of root growth and RSA to soil conditions greatly affect maize yield by affecting water and nutrient acquisition efficiency (Lynch 1995, 2013). In addition, root senescence, as indicated by root xylem bleeding sap (Fageria 2004; Doussan et al. 2006), also strongly affect nutrient and water absorption, especially during grain filling stage when grains become the main sink for assimilates (Wu et al. 2022a, b). A higher root xylem bleeding sap rate indicates greater water and nutrient absorption capacity (Guan et al. 2014; Wu et al. 2022a, b). Therefore, to understand the factors affecting maize yield formation and figure out the corresponding solutions to increase maize productivity under ST system, a full understanding of root growth and senescence, as well as RSA under ST system is crucial. Ren et al. (2019) has showed that the root distribution of maize in ST plants was restricted to the planting rows compared with intensive tillage. Nevertheless, the adaptation of root characteristics in relation to yield formation in ST is largely unclear. In this study, we made a systemic comparison of maize root growth dynamics and the 3-D spatial root distribution between ST and CT, and explored their relationship with yield formation.
Materials and methods
Experimental location
This research was conducted in a long-term positioning experimental field commenced in 2018, in San-Ke-Shu Village, Lishu County (Northeast China Plain), Jilin Province, China (43°2′ N, 123°5′ E). Plant samples were collected in 2019 and 2020, and in 2022. The typical cropping system in this region is rain-fed continuous spring maize. In the maize growing season from 1992 to 2022 (late April to early October), the average air temperature was 18.3 ℃, the rainfall was 513 mm, about 75% of which occurred during June—August (Table 1S). Rainfall was similar but unevenly distributed in 2019 and 2020, and relatively large in 2022, especially in June and July, but it had no destructive effect on maize growth (Fig. 1 and Table 1S). The spring of 2020 was cooler than 2019 and 2022. The soil texture was meadow black soil (Soil Survey Staff 1998). The soil (0—20 cm layer) was high in clay (45.6%) and silt (41.6%), and low in sand (12.8%). The initial soil properties before sowing in 2019 were pH 5.9 (1:2.5 g/v soil:water ratio), organic carbon 27.3 g kg−1, total N 1.4 g kg−1, Olsen-P 36.9 mg kg−1, available K 159 mg kg−1 in the 0—20 cm soil layer (measurement methods refer to Toth et al. 1948; Olsen 1954; Bremner and Tabatabai 1972; Nelson and Sommers 1983; Van Zwieten et al. 2010).
Experimental design and field management
The experiment was a single-factor completely random design with three replications. Two tillage treatments were set up: strip tillage (ST) and conventional tillage (CT). The size of each plot was 547 m2 (76 m long × 7.2 m wide), with 12 rows spaced 0.6 m apart. According to the local maize yield level and soil-test based fertilizer recommendation (Feng et al. 2017; Wang et al. 2018), the following fertilizers were applied: 180 kg N ha−1 (as urea), 75 kg P2O5 ha−1 (as superphosphate), 90 kg K2O ha−1 (as potassium chloride). All fertilizers were broadcasted before planting and no topdressing was applied during maize growth.
Maize was mechanically harvested and the residues remained in the field. The field was covered with the residues overwinter. For ST treatment (Table 2S), strip tillage was conducted using a strip-tiller (Yetter-2984, Illinois, USA) in 27 April 2019, 2 May 2020 and 8 May 2022, respectively. A clean soil strip about 12—15 cm in depth and 22—25 cm in width was created for planting and the residues were allocated to the inter-row area and were not disturbed through maize growth period. For CT treatment, the residues were removed from the field and then the soil was tilled using a rotary tiller to a depth around 15 cm. Maize (Zea mays L. cv. De-Mei-Ya 3) was sown with a row spacing of 0.6 m on 9 May 2019, 6 May 2020 and 10 May 2022, respectively. Plant density was controlled at 70,000 plant ha−1. Herbicides and pesticides were used to control weeds and pests, respectively. No irrigation was applied. Maize was harvested in 30 September 2019, 27 September 2020 and 29 September 2022, respectively.
Plant sampling and measurement
Shoot sampling, dry matter weight and grain yield
Plants were sampled in 2019 (2020) on 41 (41), 56 (53), 73 (76), and 87 (90) days after sowing (DAS), which were corresponding to seedling, jointing, silking and grain filling stage, respectively (Shen and Mao 2011), respectively. The GDD and the total rainfall for each growth stage were shown in Table 3S. For each sampling, three successive plants of each plot were cut on the soil surface to determine shoot biomass (g plant−1). All samples were oven-dried (DHG-9420A; Bilon Instruments Co. Ltd., Shanghai, China) at 80 ± 5 °C until constant weight (three or four days for seedling, four or five days for jointing, six or seven days for silking, more than seven days for grain filling stage) after heating at 105 °C for 30 min. Then the dry weight was measured. At maturity, the two center rows (10 m length and 1.2 m width) of each plot were harvested by hand. The number of ears was counted and was calculated into the ear number per ha (EN). From the harvested ears, 20 were chosen to measure the grain number per ear (GN). Grains were threshed by a grain thresher, and three 100-grain samples were weighted to determine the 1000-grain weight (TGW).
Root sampling, root distribution and root morphological traits
In 2019 and 2020, a modified monolith method was applied to figure out the spatial distribution characteristics of the root system (Böhm 1979). After the shoot was removed in each sampling, a soil volume centered on the plant root of 24 cm × 60 cm × 60 cm (distance between intra-row × distance between inter-row × depth) was excavated using a shovel. Three successive plant roots were sampled in each plot.
To analyze the vertical root distribution during maize seedling to grain filling stage, the soil volume was divided into 6 cm increments to the depth of 60 cm (Fig. 1S-A). To analyze the horizontal root distribution from the location of the plant to the middle of the inter-row, root growth at depth of 30 cm (5 layers with 6 cm deep in each layer) was also investigated at silking stage by reference to Shao et al. (2018) (Fig. 1S-B). In the inter-row direction, from the location of the plant, soil cubes were taken each 6 cm in the size of 24 cm × 6 cm × 6 cm cubes (distance between intra-row × distance between inter-row × depth). The soil cube size was 24 cm × 3 cm × 6 cm cube at the border, and totally 11 cubes were sampled between the neighboring rows in each layer. The soil cubes were crushed by hand and sieved through a 3 mm sieve. All visible selected living roots was temporarily stored in a self-sealing bag (numbered in advance), and then rinsed and frozen at—20 ℃ for further analysis.
Another three successive plant roots were sampled to investigate the root morphological traits in each plot. A soil volume centered the maize root of 24 cm × 60 cm × 30 cm (distance between intra-row × distance between inter-row × depth) was excavated using a shovel after cutting off the shoots. The soil volume was put into a pool and immersed in water for 12 h, then the roots were cleaned manually. Using a protractor, the expansion angle of the nodal roots from the upmost whorl to the 1st whorl was measured (Fig. 0.2S). Randomly three roots were measured in each whorl and the average value was used to represent the nodal roots angle (°). The nodal roots were cut off after measurement, and the number of nodal roots in each whorl was recorded.
In 2022, the same method was used to investigate root senescence from silking stage to the physiological maturity. After the collection of root xylem bleeding sap at 72, 82, 97,112, 127 DAS (see below), the successive soil cube (24 cm × 60 cm × 30 cm) per plot were excavated and the cleaned roots were cleaned for investigation of total root length using the same procedure as above.
Root xylem bleeding sap
Root xylem bleeding sap was collected from silking stage to physiological maturity in 2022 at 72, 82, 97,112, 127 DAS according to Yang et al. (2002). In brief, five evenly growing plants were selected from each plot, and their stems were cut at 10 cm above the soil surface at 18:00 on the first day. The residual stem was put into a plastic container containing pre-weighted degreasing cotton and the interface was tied tightly with a rubber band to prevent the sap from flowing out. The degreasing cotton was collected at 6:00 am the next day. The weight of the bleeding sap was calculated using the subtraction method.
Determination of root length and root biomass
All the root samples were scanned using a scanner at 600 dpi resolution (Epson, Perfection V800 Photo Scanner, Los Alamitos, USA) and analyzed using WinRhizo software (R´egent Instruments Inc., Qu´ebec, Canada) to determine the total root length (m plant−1). Root length density (cm cm−3) was calculated by dividing root length with the soil volume. After scanning, the roots were oven-dried at 80 ± 5 °C for a constant weight to obtain the root biomass (g plant−1). The rooting depth (D95), at which 95% of the coarse root length can be found within the 60 cm deep soil core, was estimated by linear interpolation (Schenk and Jackson 2002).
Soil sampling and measurements
After sowing (May 22, 2019 and May 24, 2020), soil samples were collected in 0—30 cm soil layer using a ring knife at two positions, the middle of neighboring plants in intra-row (0—9 cm from the center of the plants horizontally) and the middle of the inter-row (9—30 cm from the center of the plants horizontally), to measure the soil bulk density (g cm−3). The ring knife has a diameter of 5 cm and a volume of 100 cm3. Additional soil samples were collected to determine fresh weight as quickly as possible and oven-dried at 105 °C for 24 h to obtain dry soil weight (g). Volumetric soil water content (cm cm−3) was calculated by referring to Ren et al. 2019. At the same position of 0—20 cm soil layer, a portable soil thermometer (TP—101, XinTai wei, China) was used to measure soil temperature (℃) at 5 cm, 10 cm, 15 cm and 20 cm below soil surface.
At silking stage in 2020, centered on the plant that was used for root sampling, three soil cubes per plot was used to investigate the spatial distribution of soil bulk density and soil moisture. Soil samples were collected using a ring knife every 6 cm layer to a depth of 0–30 cm at 11 positions, including three positions in the intra-row direction: 0—3 cm (close to the plant), 3—9 cm at both sides from the plant, and eight positions in the inter-row direction: 9—15 cm, 15—21 cm, 21—27 cm, 27—30 cm, at both sides from the plant, respectively. The same soil samples for soil bulk density measurement were used for determining soil moisture.
Statistical analysis
All data were analyzed with analysis of variance using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) to examine the effects of tillage methods on grain yield and its components, shoot growth, root distribution, root morphological traits and soil physical properties. The statistical significance of differences among means was assessed by the Tukey HSD test (P ≤ 0.05). Linear regression model was used to analyze the relationship between soil bulk density and root length density. All the graphics were made using OriginPro 2022 (OriginLab., CA, USA).
Results
Grain yield, shoot and root biomass and root to shoot ratio
The interaction effect of tillage × year was not significant for grain yield and its components (Table 1). Across 2019, 2020 and 2022, there was not significant difference in grain yield between ST and CT. Grain number (GN) per ear of ST was 4.4% less compared to that of CT. In contrast, the thousand-grain weight (TGW) of ST was 6.6% higher than that of CT. Ear number (EN) per hectare was not affected by tillage systems.
Shoot and root biomass were reduced significantly in ST plants compared with CT in the vegetative stage. Shoot biomass in ST plants decreased by 12.3—15.1% at seedling, 8.3—16.8% at jointing, and root biomass in ST plants decreased by 8.7—31.3% at seedling, 16.0—17.7% at jointing (Table 2). The difference was gradually reduced during the late growth stage. During silking and post-silking stage, there was no significant difference in the shoot and root biomass between the ST and CT plants (Table 2). Root to shoot ratio was not different between ST and CT at any growth stage in two years (Table 2).
Soil properties
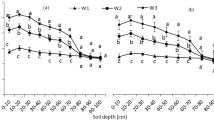
After sowing, soil bulk density (Fig. 2A), moisture (Fig. 2B) and temperature (Fig. 2C) were measured in the intra-row (0—9 cm from the center of the plants horizontally) and inter-row (9—30 cm from the center of the plants horizontally). Overall, the basic physical conditions of ST were similar to CT in the intra-row soil, but different from CT in inter-row soil, especially in the 0—15 cm soil layer. In 2019 and 2020, the soil bulk density and volumetric soil water content in the inter-row of ST were increased by 4.3%—17.9%, 20.8%—79.4%, respectively, in the 0—15 cm soil layer compared with CT. On the contrary, the soil temperature in the inter-row of ST was reduced by 6.9%—18.7% compared with CT. The effect of tillage on the soil temperature was highly significant in 2019.
At silking, compared with CT with uniform soil bulk density, the soil bulk density under ST had greater heterogeneity. The bulk density of ST was similar to that of CT in the intra-row soil, but significantly larger than CT in the inter-row soil (Fig. 3A). In 0—12 cm soil layer, the average soil bulk density of ST in the inter-row was 1.53 cm cm−3, which was 23.4% lager than that of CT (Table 4S). The bulk density in the two treatments did not differ significantly in deeper soil. ST practice increased the volumetric soil water content in inter-row soil (Fig. 3B). On average, the soil water content of ST in the inter-row was 59.5% lager than that of CT in 0—12 cm soil layer, but this significant difference did not extend to the 12–30 cm soil layer (Table 5S).
Spatial distribution of soil bulk density (g cm−3) (A) and volumetric soil water content (cm cm−3) (B) in the soil profile (0—30 cm depth) at silking stage as influenced by strip-till (ST) and conventional-till (CT) in 2020. The specific soil sampling and measurement methods are in Soil sampling and measurements
Root length and nodal root angle
Compared with CT, root growth in ST plants was significantly inhibited, especially in the vegetative stage. The total root length in ST plants decreased by 38.5—41.6% at seedling stage, 27.6—27.8% at jointing stage and 13.1—16.2% at silking stage. However, there was no significant difference between ST and CT at two weeks after silking (Fig. 4).
Effects of tillage methods on total root length (m plant−1) in 2019 and 2020. The sampling volume for the roots is 24 cm × 60 cm × 60 cm (distance between intra-row × distance between inter-row × depth). ST: strip-till, CT: conventional-till. Each value is the mean of three replicates (± SE). The asterisks denote significant difference among different tillage treatments. *P ≤ 0.05, **P ≤ 0.01. ns denote no significant difference
The size of maize root system was determined by the growth of each nodal root in each whorl. The total number of the nodal roots of ST plants was similar as that of CT (Table 3). The growth angle of the nodal roots was changed by ST (Fig. 5A, 2S). That is, the nodal roots from node 5 and node 6 of the ST plants had a significantly smaller root extension angle than that of CT (decreased by 6.9—18.5% and 10.8—20.0% for the nodal roots from node 5 and node 6, respectively) (Fig. 5B). That is, the top nodal roots became steeper.
Effect of tillage methods on RSA. A, the representative RSA of ST and CT plant; B, nodal root angle (°) under ST and CT in 2019 and 2020. ST: strip-till, CT: conventional-till. Each value is the mean of three replicates (± SE). The asterisks denote significant difference among different tillage treatments. *P ≤ 0.05, **P ≤ 0.01. ns denote no significant difference
Root distribution
The root length density of ST plants in the topsoil (0—12 cm) was lower than that of CT (by 37.7—46.7% at seedling, by 26.0—38.2% at jointing, by 17.2—25.8% at silking, and by 5.7—22.8% at two weeks after silking) (Fig. 6A, B). For vertical root distribution, the average D95 of the vertical root distribution in ST plant were 3.3%, 6.0%, 7.0% and 3.5% higher than that of CT at seedling, jointing, silking and two weeks after silking, respectively (Table 4).
Effects of tillage methods on root length density (cm cm−3) at different maize growth stages in 2019 (A) and 2020 (B) in the soil profile (0—60 cm depth). The sampling volume for the roots is 24 cm × 60 cm × 60 cm (distance between intra-row × distance between inter-row × depth). ST: strip-till, CT: conventional-till. Each value is the mean of three replicates (± SE). The asterisks denote significant difference among different tillage treatments. *P ≤ 0.05, **P ≤ 0.01. ns denote no significant difference
Compared with CT, the horizontal root distribution in ST plants was severely inhibited (Fig. 7), especially in the topsoil (0—12 cm) which was the cultivated layer. The proportion of the intra-row roots of ST was increased by 7.0%—12.7% in the 0—12 cm soil layer compared with CT (Fig. 8). In contrast, in inter-row soil, at 9—15 cm, 15—21 cm, 21—27 cm, and 27—30 cm away from the plant, the average root length density of ST was reduced significantly by 23.2—32.0%, 24.3—35.6%, 28.6—55.7%, and 43.3—54.1% compared with CT, respectively (Table 6S). At different position in the inter-row soil, root length density was significantly negatively correlated with soil bulk density (Fig. 9).
Effect of tillage methods on the proportion of root length in intra-tow soil in 0—30 cm soil layer in 2019 and 2020. ST: strip-till, CT: conventional-till. Each value is the mean of three replicates (± SE). The asterisks denote significant difference among different tillage treatments. *P ≤ 0.05, **P ≤ 0.01. ns denote no significant difference
Root senescence
Root senescence during post-silking stage was studied in more detail in 2022 (Fig. 10). It was found that the total root length of ST plants was significantly less than CT until 97 days after sowing (early grain filling stage). This difference disappeared and the total root length of ST plants was significantly larger than that of CT at 112 days after sowing. This indicated that root senescence of ST plants was slower than CT. In accordance, at 112 and 127 days after sowing, the rate of root xylem bleeding sap of ST plants was 70.7% (P < 0.01) and 449.9% (P < 0.01) higher than that of CT plants, respectively.
Effect of tillage methods on total root length (m plant−1) and rate of root bleeding sap (g h−1 plant−1) during post-silking growth stage in 2022. The broken line in the figure shows the trend of total root length, and the bar chart shows the trend of root bleeding sap. 72 days after sowing is the sampling time of silking stage. The sampling volume for the roots is 24 cm × 60 cm × 30 cm (distance between intra-row × distance between inter-row × depth). ST: strip-till, CT: conventional-till. Each value is the mean of three replicates (± SE). The asterisks denote significant difference among different tillage treatments. *P ≤ 0.05, **P ≤ 0.01. ns denote no significant difference
Discussion
Root growth, root senescence and RSA in relation to soil environment under ST system
Maize shoot and root growth is often impeded by low root-zone temperature during early spring (Brouwer and Hoagland 1964; Knoll et al. 1964; Richner et al. 1996; Nagel et al. 2009). Mechanical resistance can also limit root growth and reduce total root length (Grzesiak et al. 2002; Bingham et al. 2010; Pfeifer et al. 2014). At seedlings stage, the inter-row soil in ST had a lower soil temperature and higher bulk density than CT, and this was associated with lower shoot and root biomass during the vegetative stage (Fig. 4 and Table 2). Chassot et al. (2001) showed that, in NT compared to CT, the poorer root and shoot growth of maize seedlings was presumably caused by the lower topsoil temperature rather than by mechanical impedance. Because low temperature can reduce the availability of soil nutrients, limit root absorption of nutrients, and thus have a negative impact on crop growth (Miedema 1982). The same explanation may be true in the present study. Although the difference in temperature between ST and CT was relatively small in 2020, the soil temperature in the inter-row of ST was about 3 ℃ lower than that of CT (Fig. 2). Licht and Al-Kaisi (2005b) showed that a temperature decrease of only 1.2—1.4 ℃ delayed the emergence of maize. With the increase of air temperature, the difference in root size (as shown in total root length) became smaller at jointing stage.
When the soil environment is adverse to plant growth, plant roots tend to avoid the adverse environment and proliferate in the soil zones with favorable conditions, which is called a compensation adjustment (Bingham and Bengough 2003). RSA can adapt to the soil physical properties by changing root length density (Wu et al. 2022a, b), nodal roots angle (Jin et al. 2013), and root distribution (Ren et al. 2019). Ren et al. (2019) showed maize plants under ST had smaller root number density than CT in the top 40 cm layer at inter-row positions. In this research, when the soil temperature is no longer a limiting factor in ST system at silking stage, we found that the heterogeneous soil compactness caused by ST practice shaped the RSA. The inter-row soil bulk density in the 0—12 cm soil layer was significantly greater under ST than that of CT (Fig. 3 and Table 4S). The roots of ST plants tended to grow into the intra-row soil and avoided growing into the colder, more compacted inter-row soil, leading to a high proportion of root length in the intra-row in ST plants (Fig. 8). This was demonstrated by the negative correlation between soil bulk density and root length density (Fig. 9). Less root growth in the inter-row soil was associated with steeper root angles and, consequently, proportionately more roots at depth (Table 4). A deeper root system may help compensate for lack of soil exploration in the surface soil. Reducing root distribution in the top soil is believed to beneficial for drought tolerance (Wasson et al 2012).
The significance of roots in resource acquisition depends not only on root size but also root activity (Chen et al. 2015). Normally, maize root size begins to decrease since silking due to more root senescence (Yan et al. 2011; Chen et al. 2015). At grain filling stage, fast root senescence is a disadvantage for water and nutrient uptake and therefore grain development (Li et al. 2022). In the present study, although root size of ST plants was smaller than CT at silking stage, there was not significant difference in total root length between ST and CT at grain filling stage (Fig. 4), suggesting root senescence in ST plants was delayed in comparison to CT. In 2022, the total root length in ST plants was even significantly greater than that in CT plants at grain filling stage (Fig. 10). The greater rate of root xylem bleeding in ST plants in 2022 supports that root senescence may have been delayed. The smaller root size and slower shoot growth at vegetative growth stage of ST plants may save some soil water for later plant growth. It was found that during post-silking stage, there is normally more soil moisture storage in ST compared to CT (Licht and Al-Kaisi 2005b). Also, nutrients (especially nitrate) in the topsoil can gradually leach into deep soil (Thorup-Kristensen et al. 2009) and promote local later root proliferation there (Drew et al. 1973), so as to maintain the activity of the whole root system. More evidence is needed to validate and explain the delayed root senescence in ST practice.
Relationship between root characteristics and yield formation of maize in ST system
The smaller root system in ST plants can reduce nutrient uptake (Sha et al. 2023) and therefore restrict pre-silking dry matter accumulation. Pre-silking shoot growth has great effect on ear development (D’Andrea et al. 2008; Abendroth et al. 2011; Gonzalez et al. 2019; Mueller et al. 2019; Liu et al. 2021). Indeed, compared to CT, ST plants had few grains number per ear (Table 1), which is a major reason limiting grain yield under ST system. Therefore, improving early root development may be crucial to increase maize yield under ST system. Qin et al. (2005) suggested that developing new type starter fertilizer can be a promising way to enhance early root growth in the conservative tillage.
During grain-filling stage, at which 35 to 55% of the grain N was accumulated, maize root size and activity may greatly affect grain N accumulation, grain development and final grain yield (Hirel et al. 2007; Chen et al. 2015). At this stage, the sustained root activity may contribute to the efficient post-silking N uptake in ST plants (Sha et al. 2023). As a result, grain weight in ST plants was higher than that in CT plants (Table 1), which contributed to the stability of grain yield. It should be mentioned that root senescence and leaf senescence is interacted (Liu et al. 2018). Maize plants use a large amount of photosynthates to grow new roots and maintain the respiration of old roots during grain filling (Niu et al. 2010; Yan et al. 2011). Indeed, it is found that leaf senescence is also delayed in ST plants during grain filling stage (Sha et al. 2023).
It is well understood that plant root system architecture (RSA) is closely related to soil resource acquisition, and maize with steep, deeper RSA can increase water and nitrogen acquisition from the deeper soil (Mi et al. 2010, 2016; Lynch 2013; Trachsel et al. 2013). Feng et al. (2019) showed that more roots in deeper soil contribute to efficient N uptake and maize grain yield. In this study, although the growth of sallow roots in ST plants were restricted by the colder, more compacted inter-row soil environment, the deeper root growth was maintained (Fig. 7 and Table 4). This change of RSA in ST plants can help to improve water and nitrogen acquisition efficiency at grain filling stage (King et al 2003; Wasson et al. 2012).
Conclusion
In conclusion, compared with CT, the colder and more compacted inter-row soil in ST was unfavorable to early root growth, resulting in restricted shoot growth and few grain per ear. The heterogeneous soil environment in ST modified root distribution, resulting in proportionately more roots in the intra-row soil. While the shallow roots in ST plants was reduced, the deeper root growth was maintained. At grain filling stage, root senescence was delayed. The delayed root senescence and reshaped RSA may contribute to efficient resource acquisition at grain filling stage, contributing to greater grain weight and stabilized grain yield. It was suggested that improving early root growth may be crucial to increase maize productivity in ST system.
Data availability
Data will be made available on request.
References
Abendroth LJ, Elmore RW, Boyer MJ, Marlay SK (2011) Corn growth and development. iowa state university extension. PMR 1009. Iowa State University: Ames
Amato M, Ritchie JT (2002) Spatial distribution of roots and water uptake of maize (Zea mays L.) as affected by soil structure. Crop Sci 42:773–780. https://doi.org/10.2135/cropsci2002.7730
Bao Y, Aggarwal P, Robbins NE, Sturrock CJ, Thompson MC, Tan HQ, Tham C, Duan L, Rodriguez PL, Vernoux T (2014) Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci USA 111:9319–9324. https://doi.org/10.1073/pnas.1400966111
Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot 57:437–447. https://doi.org/10.1093/jxb/erj003
Bingham IJ, Bengough AG (2003) Morphological plasticity of wheat and barley roots in response to spatial variation in soil strength. Plant Soil 250:273–282. https://doi.org/10.1023/:1022891519039
Bingham IJ, Bengough AG, Rees RM (2010) Soil compaction-N interactions in barley: root growth and tissue composition. Soil Tillage Res 106:241–246. https://doi.org/10.1016/j.still.2009.10.008
Brady DJ, Wenzel CL, Fillery I, Gregory PJ (1995) Root growth and nitrate uptake by wheat (Triticum aestivum L) following wetting of dry surface soil. J Exp Bot 46(5):557–564. https://doi.org/10.1093/jxb/46.5.557
Bremner JM, Tabatabai MA (1972) Use of an ammonia electrode for determination of ammonium in Kjeldahl analysis of soils. Commun Soil Sci Plant Anal 3:159–165
Brouwer R, Hoagland A (1964) Responses of bean plants to root temperatures. II. Anatomical aspects. Meded Inst Biol Scheik Onderz Landb Gewass 236:23–31. https://doi.org/10.1111/j.1438-8677.1969.tb00570.x
Böhm W (1979) Methods of studying root systems. Ecological studies, 33. Springer, Berlin, pp 20–25
Cannell RQ (1985) Reduced tillage in North-West Europe–A review. Soil Tillage Res 5:129–177. https://doi.org/10.1016/0167-1987(85)90028-5
Chassot A, Stamp P, Richner W (2001) Root distribution and morphology of maize seedlings as affected by tillage and fertilizer placement. Plant Soil 231(1):123–135. https://doi.org/10.1023/A:1010335229111
Chen YL, Zhang J, Li Q, He XL, Su XP, Chen FJ, Yuan LX, Mi GH (2015) Effects of nitrogen application on post-silking root senescence and yieldof maize. Agron J 107:835–842. https://doi.org/10.2134/agronj14.0509
Correa J, Postma JA, Watt M, Wojciechowski T (2019) Soil compaction and the architectural plasticity of root systems. J Exp Bot 70(21):6019–6034. https://doi.org/10.1093/jxb/erz383
D’Andrea K, Otegui ME, Cirilo AG (2008) Kernel number determination differs among maize hybrids in response to nitrogen. Field Crops Res 105:228–239. https://doi.org/10.1016/j.fcr.2007.10.007
Drew MC, Saker LR, Ashley TW (1973) Nutrient supply and the growth of the seminal root system in barley. J Exp Bot 24:1189–1202
Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X (2007) Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci 12(10):474–481. https://doi.org/10.1016/j.tplants.2007.08.012
Doussan C, Pierret A, Garrigues E, Pagès L (2006) Water uptake by plant roots: II-modelling of water transfer in the soil root-system with explicit account of flow within the root system-comparison with experiments. Plant Soil 283:99–117. https://doi.org/10.1007/s11104-004-7904-z
Dwyer LM, Ma BL, Stewart DW, Hayhoe HN, Balchin D, Culley JLB, McGovern M (1996) Root mass distribution under conventional and conservation tillage. Can J Soil Sci 76:23–28. https://doi.org/10.4141/cjss96-004
Fageria NK (2004) Influence of dry matter and length of roots on growth of five field crops at varying soil zinc and copper levels. J Plant Nutr 27:1517–1523
Feng GZ, He XL, Coulter JA, Chen YL, Gao Q, Mi GH (2019) Effect of limiting vertical root growth on maize yield and nitrate migration in clay and sandy soils in Northeast China. Soil till Res 195:104407. https://doi.org/10.1016/j.still.2019.104407
Feng GZ, Yan L, Wang Y, Wang SJ, Li JH, Chen XP, Cui ZL, Fan XL, Gao Q (2017) Establishment of Index System of Fertilizer Recommendation for Spring Maize in Jilin. J Maize Sci 25(6):142–147. https://doi.org/10.13597/j.cnki.maize.science.20170622
Fernández FG, Sorensen BA, Villamil MB (2015) A comparison of soil properties after five years of no-Till and strip-Till. Agron J 107:1339–1346. https://doi.org/10.2134/agronj14.0549
Fiorini A, Boselli R, Amaducci S, Tabaglio V (2018) Effects of no-till on root architecture and root-soil interactions in a three-year crop rotation. Eur J Agron 99:156–166. https://doi.org/10.1016/j.eja.2018.07.009
Gonzalez VH, Lee EA, Lewis LL, Swanton CJ (2019) The relationship between floret number and plant dry matter accumulation varies with early season stress in maize (Zea mays L.). Field Crops Res 238:129–138. https://doi.org/10.1016/j.fcr.2019.05.003
Grzesiak S, Grzesiak MT, Filek W, Hura T, Stabryła J (2002) The impact of different soil moisture and soil compaction on the growth of triticale root system. Acta Physiol Plant 24:331–342
Guan D, Al-Kaisi MM, Zhang Y, Duan L, Tan W (2014) Tillage practices affect biomass and grain yield through regulating root growth, root-bleeding sap and nutrients uptake in summer maize. Field Crops Res 157:89–97. https://doi.org/10.1016/j.fcr.2013.12.015
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the US corn belt? Crop Sci 49(1):299–312. https://doi.org/10.2135/cropsci2008.03.0152
Hirel B, Gouis JL, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58:2369–2387. https://doi.org/10.1093/jxb/erm097
Ioanna P, Kakabouki IR, Dimitra H, Panayiota P, Antigolena F, Dimitrios B (2019) Root growth dynamics and productivity of quinoa (Chenopodium quinoa Willd.) in response to fertilization and soil tillage. Folia Hort 31(2):285–299. https://doi.org/10.2478/fhort-2019-0023
Jackson RB, Caldwell MM (1993) The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 74:612–614. https://doi.org/10.2307/1939320
Jin KM, Shen JB, Ashton RW, Dodd IC, Parry MAJ, Whalley WR (2013) How do roots elongate in a structured soil? J Exp Bot 64(15):4761–4777. https://doi.org/10.1093/jxb/ert286
King J, Gay A, Sylvester-Bradley R, Binham I, Foulkes J, Gregory P, Robinson R (2003) Modelling Cereal Root Systems for Water and Nitrogen Capture: Towards an Economic Optimum. Ann Bot 91:383–390
Knoll MA, Lathwell DJ, Brady NC (1964) Effect of root zone temperature at various stages of the growing period on the growth of corn. Agron J 56:143–145
Li GH, Fu PX, Cheng GG, Lu WP, Lu DL (2022) Delaying application time of slow-release fertilizer increases soil rhizosphere nitrogen content, root activity, and grain yield of spring maize. Crop J 10(3):853–863. https://doi.org/10.1016/j.cj.2022.04.014
Licht MA, Al-Kaisi M (2005a) Corn response, nitrogen uptake, and water use in strip tillage compared with no tillage and chisel plow. Agron J 97:705–710. https://doi.org/10.2134/agronj2004.0102
Licht MA, Al-Kaisi M (2005b) Strip-tillage effect on seedbed soil temperature and other soil physical properties. Soil Tillage Res 80:233–249. https://doi.org/10.1016/j.still.2004.03.017
Lipiec J, Stepniewski W (1995) Effects of soil compaction and tillage systems on uptake and losses of nutrients. Soil Tillage Res 35:37–52. https://doi.org/10.1016/0167-1987(95)00474-7
Lipiec J, Hatano R (2003) Quantification of compaction effects on soil physical properties and crop growth. Geoderma 116(1–2):107–136. https://doi.org/10.1016/s0016-7061(03)00097-1
Liu HY, Wang WQ, He AB, Nie LX (2018) Correlation of leaf and root senescence during ripening in dry seeded and transplanted rice. Rice Sci 25:279–285. https://doi.org/10.1016/j.rsci.2018.04.005
Liu Z, Hu CH, Wang YN, Sha Y, Hao ZH, Chen FJ, Yuan LX, Mi GH (2021) Nitrogen allocation and remobilization contributing to low-nitrogen tolerance in stay-green maize. Field Crops Res 263:108078. https://doi.org/10.1016/j.fcr.2021.108078
Lynch JP (1995) Root architecture and plant productivity. Plant Physiol 109:7–13. https://doi.org/10.1104/pp.109.1.7
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112(2):347–357. https://doi.org/10.1093/aob/mcs293
Maqbool S, Hassan MA, Xia XC, York ML, Rasheed A, He ZH (2022) Root system architecture in cereals: progress, challenges and perspective. Plant J 110:23–42. https://doi.org/10.1111/tpj.1566
Mi GH, Chen FJ, Wu QP, Lai NW, Yuan LX, Zhang FS (2010) Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Sci China Life Sci 53:1369–1373. https://doi.org/10.1007/s11427-010-4097-y
Mi GH, Chen FJ, Yuan LX, Zhang FS (2016) Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Adv Agron 139:73–97. https://doi.org/10.1016/bs.agron.2016.05.002
Miedema P (1982) The effects of low temperature on zea mays. Adv Agron 35(s 1–2):93–128
Morrison JE (2002) Strip tillage for “no-till” row crop production. Appl Eng Agric 18:277–284
Mosaddeghi MR, Mahboubi AA, Safadoust A (2009) Short-term effects of tillage and manure on some soil physical properties and maize root growth in a sandy loam soil in western Iran. Soil Tillage Res 104:173–179. https://doi.org/10.1016/j.still.2008.10.011
Mosher PN, Miller MH (1972) Influence of soil temperature on the geotropic response of corn roots (Zea mays L). Agron J 64(4):459. https://doi.org/10.2134/agronj1972.00021962006400040015x
Mueller SM, Messina CD, Vyn TJ (2019) The role of the exponential and linear phases of maize (Zea mays L.). Ear Growth for Determination of Kernel Number and Kernel Weight. Eur J Agron 111:125939. https://doi.org/10.1016/j.eja.2019.125939
Munkholm LJ, Heck RJ, Deen B (2012) Long-term rotation and tillage effects on soil structure and crop yield. Soil Tillage Res 127:85–91. https://doi.org/10.1016/j.still.2012.02.007
Muñoz-Romero V, López-Bellido L, López-Bellido RJ (2012) The effects of the tillage system on chickpea root growth. Field Crops Res 128:76–81. https://doi.org/10.1016/j.fcr.2011.12.015
Nagel KA, Kastenholz B, Jahnke S, Van Dusschoten D, Aach T, Mühlich M, Truhn D, Scharr H, Terjung S, Walter A, Schurr U (2009) Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Funct Plant Biol 36(11):947–959. https://doi.org/10.1071/FP09184
Nelson DA, Sommers L (1983) Total carbon, organic carbon, and organic matter. Methods Soil Anal.: Part 2 Chem. Microbiol Prop 9:539–579
Niu JF, Peng YF, Li CJ, Zhang FS (2010) Changes in root length at the reproductive stage of maize plants grown in the field and quartz sand. J Plant Nutr Soil Sci 173:306–314. https://doi.org/10.1002/jpln.200800316
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium carbonate. United States Department of Agriculture. Circular No. 939, p 19
Paez-Garcia A, Motes C, Scheible WR, Chen R, Blancafor E, Monteros M (2015) Root traits and phenotyping strategies for plant improvement. Plants 4:334–355. https://doi.org/10.3390/plants4020334
Pfeifer J, Faget M, Walter A, Blossfeld S, Fiorani F, Schurr U, Nagel KA (2014) Spring barley shows dynamic compensatory root and shoot growth responses when exposed to localised soil compaction and fertilisation. Funct Plant Biol 41:581–597. https://doi.org/10.1071/FP13224
Potratz DJ, Mourtzinis S, Gaska J, Lauer J, Arriaga FJ, Conley SP (2020) Strip-till, other management strategies, and their interactive effects on corn grain and soybean seed yield. Agron J 112:72–80. https://doi.org/10.1002/agj2.20067
Qin RJ, Stamp P, Richner W (2005) Impact of Tillage and Banded Starter Fertilizer on Maize Root Growth in the Top 25 Centimeters of the Soil. Agron J 97:674–683. https://doi.org/10.2134/agronj2004.0059
Ren LD, Nest TV, Ruysschaert G, D’Hose T, Cornelis WM (2019) Short-term effects of cover crops and tillage methods on soil physical properties and maize growth in a sandy loam soil. Soil Tillage Res 192:76–86. https://doi.org/10.1016/j.still.2019.04.026
Richner W, Soldati A, Stamp P (1996) Shoot-to-root relations in field-grown maize seedlings. Agron J 88:56–61. https://doi.org/10.2134/agronj1996.00021962008800010012x
Robbins NE, Dinneny JR (2015) The divining root: Moisture-driven responses of roots at the micro-and macro-scale. J Exp Bot. https://doi.org/10.1093/jxb/eru496
Schenk HJ, Jackson RB (2002) The global biogeography of roots. Ecol Monogr 72(3):311–328. https://doi.org/10.2307/3100092
Sha Y, Hao ZH, Liu Z, Huang YW, Feng GZ, Chen FJ, Mi GH (2023) Regulation of maize growth, nutrient accumulation and remobilization in relation to yield formation under strip-till system. Arch Agron Soil Sci. https://doi.org/10.1080/03650340.2023.2169281. Published online
Shao H, Xia TT, Wu DL, Chen FJ, Mi GH (2018) Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 430:395–411. https://doi.org/10.1007/s11104-018-3720-8
Shen JB, Mao DR (2011) Methodology of Plant Nutrition. China Agricultural University Press, Beijing
Soane BD, Ball BC, Arvidsson J, Basch G, Moreno F, Roger-Estrade J (2012) No-till in Northern, Western and South-western Europe: a review of problems and opportunities for crop production and the environment. Soil Tillage Res 118:66–87. https://doi.org/10.1016/j.still.2011.10.015
Soil Survey Staff (1998) Keys to Soil Taxonomy. United States Department of Agriculture, Natural Resources Conservation Service, Washington, DC, USA, p 211
Temesgen M, Savenije HHG, Rockström J, Hoogmoed WB (2012) Assessment of strip tillage systems for maize production in semi-arid Ethiopia: Effects on grain yield, water balance and water productivity. Phys Chem Earth 47–48:156–165. https://doi.org/10.1016/j.pce.2011.07.046
Thorup-Kristensen K, Cortasa MS, Loges R (2009) Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 322:101–114. https://doi.org/10.1007/s11104-009-9898-z
Toth SJ, Prince AL, Wallace A, Mikkelsen DS (1948) Rapid quantitative determination of eight mineral elements in plant tissue by a systematic procedure involving use of a flame photometer. Soil Sci 66:459–466
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2013) Maize root growth angles become steeper under low N conditions. Field Crop Res 140:18–31. https://doi.org/10.1016/j.fcr.2012.09.010
Trevini M, Pa B, Guiducci M (2013) Strip tillage effect on seedbed tilth and maize production in Northern Italy as case-study for the Southern Europe environment. Europ J Agronomy 48:50–56. https://doi.org/10.1016/j.eja.2013.02.007
Unger PW, Kaspar TC (1994) Soil compaction and root growth: a review. Agron J 86:759–766. https://doi.org/10.2134/agronj1994.00021962008600050004x
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Vyn T, Raimbault B (1992) Evaluation of strip tillage systems for corn production in Ontario. Soil Tillage Res 23(1–2):163–176. https://doi.org/10.1016/0167-1987(92)90012-Z
Wang Y, Gao Q, Feng GZ, Yan L, Li CL, Song LX, Liu ZG, Fang J (2018) N, P and K requirement and fertilizer use efficiencies of spring maize in Jilin Province. Plant Nutr Fert Sci 24(2):306–315. https://doi.org/10.11674/zwyf.17199
Wasson AP, Richards RA, Chatrath R, Misra SC, Sai Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63:3485–3498
Wu P, Liu F, Wang JY, Liu YH, Gao Y, Zhang XQ, Chen GZ, Huang FY, Ahmad S, Zhang P, Cai T, Jia ZK (2022) Suitable fertilization depth can improve the water productivity and maize yield by regulating development of the root system. Agric Water Manage 271. https://doi.org/10.1016/j.agwat.2022.107784
Wu XB, Li HB, Rengel Z, Whalley WR, Li HG, Zhang FS, Shen JB, Jin KM (2022b) Localized nutrient supply can facilitate root proliferation and increase nitrogen-use efficiency in compacted soil. Soil Tillage Res 215:105198. https://doi.org/10.1016/j.still.2021.105198
Yan HF, Shang AX, Peng YF, Yu P, Li CJ (2011) Covering middle leaves and ears reveals differential regulatory roles of vegetative and reproductive organs in root growth and nitrogen uptake in maize. Crop Sci 51:265–272. https://doi.org/10.2135/cropsci2010.03.0180
Yang JC, Zhang JH, Wang ZQ, Zhu QS, Liu JJ (2002) Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta 215:645–652
Acknowledgements
We gratefully acknowledge the National Science Foundation of China (No. U19A2035) for financial support.
Author information
Authors and Affiliations
Contributions
Ye Sha: Methodology, Investigation, Data curation, Writing-original draft. Guohua Mi: Writing-review & editing, Supervision, Project administration, Funding acquisition. All authors contributed to the conceptual development and writing of this article. All authors reviewed the manuscript and contributed to the interpretation and manuscript revisions.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Hans Lambers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sha, Y., Liu, Z., Hao, Z. et al. Root growth, root senescence and root system architecture in maize under conservative strip tillage system. Plant Soil 495, 253–269 (2024). https://doi.org/10.1007/s11104-023-06322-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06322-x