Abstract
Background and aims
Studies verify that intercropping effects soil nutrient content, enzyme activity, aggregate stability, arbuscular mycorrhizal fungi (AMF) community and glomalin-related soil protein (GRSP) content in Red Soil (Ultisol in the USDA Taxonomy, Acrisol in the WRB Soil Taxonomy) on sloping farmland. However, the comprehensive contribution of soil nutrients, enzyme activity, AMF community and GRSP to the characteristics of water-stable aggregates under different planting patterns of maize and soybean are not fully understood.
Methods
A long-term field experiment commenced in 2018. Three treatments of maize (Zea mays L.) monoculture, soybean (Glycine max L.) monoculture and maize-soybean intercropping were established in an experimental field. The planting patterns, crop varieties and fertilizer rates of each plot were consistent for each of the four years of experiments (2018–2021).
Results
Results showed that intercropping can improve the concentrations of alkali-hydrolysable nitrogen, available phosphorus and total extractable glomalin-related soil protein, the activities of enzyme (urease, invertase, acid phosphatase and catalase) and the mean weight diameter (MWD) in the rhizosphere soil of maize and soybean. Moreover, results proved that intercropping can potentially increase AMF diversity and macro-aggregates (> 2.0 mm) in the maize rhizosphere and macro-aggregates (0.5-2.0 mm) in the soybean rhizosphere.
Conclusion
Intercropping of maize and soybean can increase soil aggregate stability in the rhizosphere of the two crops. The easily extractable glomalin-related soil protein was the main factor affecting soil aggregate stability and the formation of > 2.0 mm aggregates in the maize rhizosphere. Soil organic matter was the main factor affecting soil aggregate stability and the formation of 0.5–2.0 mm aggregates in the soybean rhizosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil erosion is a common natural geological phenomenon in terrestrial ecosystems and has become a global problem in agroecosystems due to the loss of soil and nutrients from croplands, resulting in decreased cropland quality and productivity (Özşahin 2023). The key to solve this problem is to decrease soil erodibility. The stability of soil aggregates is an important index to determine soil erodibility, which is affected by the complex dynamics of physical, chemical and biological factors (Barthès and Roose 2002; Rillig et al. 2015). Among them, microorganisms are the most active biological factors, and their demand for nutrients can indirectly affect aggregate stability by regulating enzyme production (Rillig et al. 2015). Arbuscular mycorrhiza fungi (AMF) are a group of endophytic fungi belonging to Glomeromycota and can form mycorrhizal symbiosis with > 80% of terrestrial plants (Sharmah and Jha 2014). AMF can affect the composition and stability of soil aggregates due to the adhesion of AMF to the cell surface. Zhang et al. (2022) studied the effects of AMF on soil aggregates in maize-soybean intercropping and found that the AMF community was positively correlated with aggregate (> 5.0 mm) concentration. Furthermore, the entanglement of hyphae and the secretion of glomalin-related soil protein (GRSP) can also increase aggregate stability (Simpson et al. 2007; Ji et al. 2019). GRSP is a highly stable hydrophobic protein. According to the difficulty of extraction, it can be divided into easily extractable glomalin-related soil protein (EE-GRSP) and total glomalin-related soil protein (T-GRSP) (Sarapatka 2019). GRSP can adhere to sand, silt and clay particles and soil organic matter (SOM) to form aggregates (i.e., it is a pedological “super glue” in terms of its contribution to aggregate stability (Sarapatka 2019). Ji et al. (2019) demonstrated a significant positive correlation between GRSP concentration and water-stable aggregates.
Recent studies showed that intercropping can increase soil enzyme activity, GRSP concentration and aggregate stability, but different crops have different effects on soil nutrient concentrations and AMF community diversity (Zhao et al. 2020b; Wang et al. 2018). For example, Weng et al. (2021) studied the effect of maize/soybean intercropping on soil nutrients. They concluded that the concentration of available phosphorus (P), available potassium (K) and SOM in the rhizosphere decreased and the concentration of available nitrogen (N) increased in maize intercropping compared with maize monoculture. Fu et al. (2020) showed that compared with monoculture, maize/potato intercropping significantly increased SOM concentrations, and there was no significant difference in either available N or available K. Zhang et al. (2020) studied the effect of maize/soybean intercropping on the AMF community in the rhizosphere. The results showed that the planting pattern had no significant effect on the α diversity of AMF in the maize rhizosphere but could significantly increase α diversity of AMF in the soybean rhizosphere. However, Zhang et al. (2022) showed that maize/soybean intercropping increased α diversity of AMF in both maize and soybean soils. This may be due to differences between crops and soil types. Moreover, AMF community, enzyme activity, the concentration of soil nutrients and GRSP effects on the distribution characteristics of the water-stable aggregates on Red Soils on sloping farmland are poorly understood. Therefore, in this study, three treatments of maize monoculture, soybean monoculture and maize-soybean intercropping were established on sloping farmland Red Soils in Yunnan Province. Soil nutrient concentrations, GRSP concentrations, enzyme activity, water-stable aggregate distribution and AMF community changes under different planting patterns were analyzed. The mechanisms explaining the formation and stability of soil aggregate was clarified, which provided a theoretical basis for the selection of planting patterns on arable Red Soils.
Materials and methods
Description of the study site
The field experiment was conducted on a Red Soil in Dabai Community, Songhuaba, Panlong District, Kunming City, Yunnan Province (25°27’8’’N, 102°78’5’’E). The slope of the experimental site was 8°, altitude was 2210 m, mean annual temperature was 16 °C, and annual rainfall was 900–1000 mm. Thus, the site had a subtropical monsoon climate. The soil at 0–20 cm depth was collected by ‘S’ type five-point sampling method in each plot of the test site, and the soil collected at five points was mixed into one soil sample. The basic physical and chemical properties of each plot were measured, and the mean values calculated. The mean SOM concentration of the cultivated layer (Ap horizon) was 36.77 g·kg−1, soil pH was 4.96, alkali-hydrolysable N was 103.64 mg·kg−1, available P was 18.05 mg·kg−1 and available K was 332.33 mg·kg−1.
Design of the experiment
In this study, the experiment was established in 2018 as a long-term experiment. A randomized block design was established with three treatments of maize monoculture, soybean monoculture and maize-soybean intercropping. Each treatment had three replicates and a total of 9 plots. The area of each plot was 40 m2 (4 × 10 m).
The maize monoculture was planted with wide and narrow row spacing (wide row spacing was 80 cm; narrow row spacing was 40 cm), and plant spacing was 25 cm. The soybean monoculture was planted with equal row spacing, with row spacing of 60 cm and plant spacing of 25 cm. The maize-soybean intercropping mode was that two rows of maize were intercropped with two rows of soybean. Row spacing between intraspecific crops was 40 cm, and row spacing between interspecific crops was 50 cm. Plant spacing was 30 cm. Hole application during sowing was one seedling per hole for maize and two seedlings per hole for soybean.
The fertilization method used the hole application mode. Monoculture and intercropped maize received fertilizers. These were urea (N 46%, 315 kg·ha−1); superphosphate (P2O5 16%, 120 kg·ha−1) and potassium sulfate (K2O 51%, 120 kg·ha−1). N fertilizer was applied twice (50% as base fertilizer, 50% as additional fertilizer at the large bell stage), while P and K were only applied as base fertilizer. Fertilization of monoculture and intercropped soybean consisted of urea (120 kg·ha−1), superphosphate (240 kg·ha−1) and potassium sulfate (180 kg·ha−1). These were all applied as base fertilizer. During this experiment, the crop varieties, planting patterns and fertilization amount of each plot planted in the field were the same each year (2018-21).
Sampling methods
Maize (Zea mays L., cultivar Yun rui 88) and soybeans (Glycine max L., cultivar Dian dou 7) were sown on April 24, 2021, and sampled on October 21, 2021. Soil samples were collected at the crop maturity stage. The ‘S’ type five-point sampling method was used to randomly select the target crop in each plot. The whole crop was dug out and rhizosphere soil was collected by the ‘shaking soil method’ (Qin et al. 2015). The rhizosphere soil samples of five plants of each species collected from each plot were combined to form a single soil sample and gently mixed. A subsample of the rhizosphere soil was put into a 10 ml sterile centrifuge tube and temporarily stored in an incubator with an ice bag. Samples were then brought to the laboratory and stored in a freezer (–80°C) prior to the analysis of the AMF community. The remaining soil was placed into a plastic box and allowed to air-dry. These subsamples were used to determine nutrient concentrations, GRSP, enzyme activity and the properties of soil aggregates.
Determination of soil properties
The concentration of soil available phosphorus (AP) and alkaline-hydrolysable nitrogen (AN) were determined by the molybdenum antimony colorimetric method and alkali solution diffusion method (Bao 2000). Total organic carbon (TOC) was determined using a carbon nitrogen analyzer (Multi N/C 3100 analyzer, Analytik Jena AG, Jena, Germany).
The activities of soil urease (URE), invertase (INV), acid phosphatase (ACP) and catalase (CAT) were determined by indophenol colorimetry, 3,5-dinitrosalicylic acid colorimetry, disodium phenyl phosphate colorimetry and potassium permanganate titration, respectively (Guan et al. 1986).
The extraction and determination of easily extractable glomalin-related soil protein (EE-GRSP) and total glomalin-related soil protein (T-GRSP) were conducted using the method of Wright and Upadhyaya (1996).
Soil aggregate screening was determined using the method of Elliott (1986). Soil aggregate stability was analyzed by the mean weight diameter (MWD) according to the method of Ali et al. (2022). The percentage concentration of > 0.25 mm water-stable aggregates (R0.25) were analyzed using the method of Barreto et al. (2009), using the equation:
where \({\overline{\mathrm{x}}}_{\mathrm{i}}\) is the mean diameter of water-stable aggregate of each particle size (mm), and Wi is the mass percentage of water-stable aggregate of each particle size (%).
where Mr > 0.25 is the cumulative mass of aggregates with particle size > 0.25 mm (g), and MT is the sum of the mass of water-stable aggregates (g).
Soil DNA extraction, PCR amplification and AMF community analysis
DNA was extracted from soil using a commercial kit (Soil/feces genomic DNA purification kit, MagaBio, Hangzhou, China) according to the manufacturer’s instructions. Primers AMV4.5 NF and AMDGR primers were used to PCR amplify fungal ITS regions. The forward primer sequence was 5’-AAGCTCGTAGTTGAATTTCG-3’, and the reverse primer sequence was 5’-CCCAACTATCCCTATTAATCAT-3’ (Van et al. 2014). The 300 bp amplified fragment was used to prepare a library (NEBNext ® Ultra TM II DNA Library Prep Kit for Illumina, New England Biolabs, USA) and the library was sequenced (Illumina Nova 6000 platform for PE250 sequencing, Guangdong Meige Gene Technology Co., Ltd., Guangdong, China) software was used for Cluster Analysis. The data mosaic was clustered with an identity of 97% to obtain representative sequences (Usearsh v. 10.0.240, using the UPARSE method) (Edgar 2013). Representative sequences of each OTU were compared with the 18s database to obtain species annotation information (usearch-sintax, v. 10.0.240, default confidence threshold of 0.8), and remove OTUs with Tags that were annotated as chloroplasts or mitochondria and could not be annotated to the boundary level. The effective Tags sequence number and OTU taxonomy comprehensive information table for each sample were obtained.
Data analysis
The independent sample T test comparison method of SPSS 25 software was used to analyze the variance of each index under different planting patterns (α = 0.05). The Bray Curtis distance algorithm was used to calculate the distance between any two samples to obtain the dissimilarity coefficient distance matrix, and then the matrix was hierarchically clustered. Based on the OTU information table, R software (V5.1.2) was used for common and endemic species statistics and community composition analysis. Usearch-alpha-div (V10.0.240) was used to calculate α Diversity Index (Chao1, Shannon, Simpson) and the dilution curve. Redundancy Analysis used Canoco 5 software.
Results
Soil nutrient and GRSP concentrations under different planting patterns
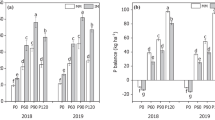
Compared with monoculture, the concentrations of AN, AP and GRSP in crop rhizosphere soil generally increased under the intercropping mode (Table 1). The concentrations of AN, AP, T-GRSP and EE-GRSP in maize intercropping were significantly (P < 0.05) higher than those in maize monoculture by 13.74, 39.78, 20.05 and 34.70%, respectively. The concentrations of SOM, AN, AP and T-GRSP in soybean intercropping were significantly higher (P < 0.05) than those in soybean monoculture by 13.83, 23.32, 36.38 and 21.82%, respectively.
Soil enzyme activities under different planting patterns
Intercropping can increase the activity of URE, INV, ACP and CAT in the crop rhizosphere (Table 2). The activities of URE, INV, ACP and CAT in maize-soybean intercropping were significantly (P < 0.05) higher than those in monoculture. The increases in maize were 31.05, 62.47, 15.43 and 18.53%, respectively. The added soybean values were significant (P < 0.05), at 77.65, 57.77, 23.55 and 33.68%, respectively.
The properties of water-stable aggregates under different planting patterns
Intercropping can increase aggregate stability in the crop rhizosphere (Table 3). Compared with maize monoculture, the concentration of > 2.0 mm aggregates and the MWD value in rhizosphere soil of maize intercropping significantly (P < 0.05) increased by 79.69 and 25.50%, respectively. Compared with soybean monoculture, the concentration of 0.5-2.0 mm aggregates, R0.25 and MWD values in rhizosphere soil of soybean intercropping were significantly (P < 0.05) increased by 21.38, 14.03 and 9.99%, respectively. The concentration of < 0.25 mm aggregates significantly (P < 0.05) decreased by 35.59%.
Operational taxonomic unit composition, ɑ Diversity Index and AMF community composition under different planting patterns
In this study, 450,445 valid sequences were obtained from the rhizosphere soil of maize and soybean, with a mean of 37,537 valid sequences in each sample. Rarefaction Analysis produced an asymptotic curve for each community (Fig. 1), indicating that the amount of sequence data from the soil samples was sufficiently large to accurately assess the arbuscular mycorrhizal fungi (AMF) community.
Based on the operational taxonomic units (OTU) classification level, the total number of OTUs of AMF communities in maize rhizosphere soil was 963. The OTU number of AMF communities in intercropped maize was 125% higher than that in maize monoculture (P > 0.05). The total number of OTUs of the AMF community in soybean rhizosphere soil was 1597, and the number of OTUs of AMF community in intercropped soybean was 25% of that in soybean monoculture (P > 0.05) (Fig. 2).
Analysis of Dissimilarity Clustering showed that the intercropped maize samples IM1 and IM2 were not clustered directly with IM3. Sample examples of monoculture and intercropping soybean treatments were also not directly sisters of each other in the hierarchy. Results showed that the similarity of AMF community in maize rhizosphere soil increased in maize-soybean intercropping compared with monoculture, but not to the extent it was in the soybean cropping system (Fig. 3).
Compared with maize monoculture, the Chao1 Index of AMF community in maize intercropping rhizosphere soil generally increased (P > 0.05), the Simpson Index generally decreased (P > 0.05), and the Shannon Index significantly (P < 0.05) increased by 9%. The Chao1, Shannon and Simpson Indexes of AMF community in rhizosphere soil of soybean intercropping were opposite to those of maize monoculture, and the differences were not significant (P > 0.05) (Table 4).
The flora not annotated in the database were classified as Unassigned, and the flora annotated but not isolated and cultured were classified as Uncultured. Except for Unassigned and Uncultured, Gigaspora, Acaulospora, Diversispora, Dentiscutata, Rhizophagus and Claroideoglomus were the six most dominant genera in maize monocropping and intercropping. The relative abundance of Acaulospora, Gigaspora, Diversispora and Claroideoglomus in intercropped maize was lower than that of monoculture maize, and the decrease was 0.40–68.32% (P > 0.05). The relative abundance of Dentiscutata and Rhizophagus increased, with values of 33.67 and 95.22% (P > 0.05), respectively. Acaulospora, Gigaspora and Diversispora were the three most dominant genera in soybean monoculture and intercropping treatments. Compared with soybean monoculture, the relative abundance of Acaulospora, Diversispora and Claroideoglomus in soybean intercropping increased. The increase was 7.70–233.40% (P > 0.05). However, the relative abundance of Gigaspora, Dentiscutata and Rhizophagus decreased by 0.05–79.05% (P > 0.05) (Table 5).
Associations between soil nutrients, GRSP, enzymes, α diversity and soil aggregates in the maize and soybean rhizosphere
A correlation matrix was constructed between nutrient, enzyme activity, GRSP, AMF community, α Diversity Index and water-stable aggregate distribution data of maize and soybean rhizosphere soil. For maize rhizosphere soil, the AN, AP, URE, INV, ACP, CAT, T-GRSP, EE-GRSP, Chao1 Index and Shannon Index were positively correlated with > 2.0 mm aggregates, MWD and R0.25 (Fig. 4A). For soybean rhizosphere soil, the AN, AP, SOM, URE, INV, ACP, CAT EE-GRSP and T-GRSP were positively correlated with 0.5–2.0 mm aggregates, 0.25–0.5 mm aggregates, MWD and R0.25, while they were all negatively correlated with < 0.25 mm (Fig. 4B).
Correlation analysis of rhizosphere soil nutrients, enzymes, GRSP, AMF community alpha Diversity Index and Aggregate Index in maize (A) and soybean (B)1. 1* denotes P < 0.05; ** denotes P < 0.01; *** denotes P < 0.001. Red indicates positive correlation coefficient and blue indicates negative correlation coefficient
The indexes with significant correlation coefficients were analyzed by Redundancy Analysis based on the results of correlation analysis in the maize rhizosphere soil. These were the α Diversity Index, GRSP, URE, INV, ACP, and AP (Fig. 5A); and the AN, AP, GRSP, URE, CAT, MWD and > 2.0 mm aggregates (Fig. 5B). Results showed that the Shannon Index was the main factor affecting the variation of T-GRSP, EE-GRSP, URE, INV and ACP (P < 0.05). EE-GRSP was the main factor affecting the variation of MWD and > 2.0 mm aggregates (P < 0.05). In addition, the effects of α Diversity Index and dominant genera on the distribution characteristics of water-stable aggregates were analyzed (Fig. 5C and D). Results showed that the Shannon Index and dominant genus of Diversispora had the strongest effect on the variation of water-stable aggregate distribution characteristics, with contribution rates of 40.90 (P < 0.05) and 30.60% (P < 0.05), respectively.
Redundancy Analysis of α Diversity Index, GRSP, URE, INV, ACP and AP (A); Redundancy Analysis of AN, AP, GRSP, URE, CAT, MWD and > 2.0 mm aggregates (B); Redundancy Analysis of α Diversity Index and water-stable aggregate distribution (C); Redundancy Analysis of dominant genera and water-stable aggregate distribution (D), in maize rhizosphere soil
The indexes with significant correlation were analyzed by Redundancy Analysis based on the results of correlation analysis in the soybean rhizosphere soil, which were the AN, AP, SOM and enzymes (Fig. 6A); the AN, AP, SOM, T-GRSP, enzymes, MWD, R0.25, 0.5–2.0 mm and < 0.25 mm aggregates, (Fig. 6B). The results showed that AN was the main factor affecting the variation of URE, INV, CAT and ACP (P < 0.05). SOM was the main factor affecting the variation of MWD, R0.25, 0.5–2.0 mm and < 0.25 mm aggregates (P < 0.05). Analysis of the effect of dominant genera on the distribution characteristics of water-stable aggregates showed that Rhizophagus contributed most to data variability, with a contribution rate of 34.40% (P < 0.05) (Fig. 6C).
Discussion
Soil nutrients and GRSP concentrations under different planting patterns
Accumulation and composition of GRSP in soil are affected by many factors, including climate, AMF composition, vegetation species and soil types (Wang et al. 2016a). This study showed that compared with monoculture, intercropping could significantly increase the concentration of T-GRSP in rhizosphere soil of both maize and soybean, while the concentration of EE-GRSP tended to increase. This may be due to the maize and soybean intercropping crop roots secreting more C and energy substances conducive to the growth and reproduction of AMF, so that the mycelium density and length can increase (Shi et al. 2011). The results of this study accord with those of Zhao et al. (2020b) on the effect of maize and soybean intercropping on GRSP.
The reason why planting patterns change soil nutrient concentrations may be related to the secretion concentration of crop roots, soil enzyme activity and microbial metabolism (Lian et al. 2019). The results showed that the concentrations of AN and AP in maize rhizosphere soil and the concentrations of SOM, AN and AP in soybean rhizosphere soil were significantly increased by intercropping compared with monocropping. The reason for increased AP concentration in the rhizosphere soil of the two crops may be that intercropping increased the secretion of organic acids by roots, thus increasing the solubility of P compounds in the soil. In addition, insoluble P is released when root exudates chelate with metal elements (Dakora and Phillips 2002). The reason for increased AN concentration may be that the hyphal bridge formed between maize roots promoted the transfer of N fixed by soybean roots to the maize root zone, and the absorption of N in soybean soil by maize stimulated the growth of root nodules. In addition, maize and soybean can transform insoluble soil N by secreting extracellular enzymes (Zhao et al. 2020a). Increased SOM concentration in soybean soil may be related to the increase of root exudates and enzyme activities. Song et al. (2007) reported that wheat-faba bean and maize-faba bean intercropping systems could increase the availability of both soil N and P, which is consistent with the results of this study.
Soil enzyme activities under different planting patterns
Soil enzymes are secreted by plant roots or microorganisms and play important roles in material circulation and energy transformation in soil ecosystems. Their activity level is an important index of soil fertility (Aon and Colaneri 2001). This study showed that maize and soybean intercropping could increase the activities of URE, INV, ACP and CAT in the crop rhizosphere soil compared with monoculture. This may be attributed to intercropping increasing the metabolic activity of maize and soybean roots and their penetration into soil, which improved the microbial habitat and increased soil permeability, thus increasing soil enzyme activity (Muhammad et al. 2021). In addition, high concentration of sugars, amino acids and other substances secreted by roots may promote the growth and reproduction of microorganisms, thereby increasing enzyme activity (Aon and Colaneri 2001). The results of this study accord with those of Muhammad et al. (2021) on the effects of intercropping soybean on soil enzyme activity.
Characteristics of water-stable aggregates under different planting patterns
The mean mass diameter of soil aggregates (MWD) is an important index of aggregate stability. With increased MWD, aggregate stability is also higher. The content of aggregates > 0.25 mm particle size (R0.25) is highly indicative of overall aggregate stability. With increased R0.25 value, the stability of aggregates also increases (Ji et al. 2021). MWD and R0.25 are negatively correlated with soil erosion and runoff intensity and thus can be used as measures of soil erodibility (Barthès and Roose 2002). This study showed that maize-soybean intercropping could increase the MWD value of aggregates in the rhizosphere soil of both crops. There may be multiple explanations of these results. Firstly, the cementing materials, crop roots, fungal mycelium length and density affected soil aggregate stability under intercropping conditions (Six et al. 2004; Hu et al. 2015; Zhao et al. 2020a). Secondly, the gravitational potential energy of raindrops can be effectively decreased when heavy raindrops collected on main leaves fall on soybean leaves, thus decreasing raindrop splash erosion and runoff surface erosion (Snelder and Bryan 1995). González Rosado et al. (2022) studied crop diversification effects on soil aggregation in short-term rainfed olive groves. They concluded that the olive orchards were diversified by Crocus sativus, which increased the mean weight diameter values and geometric mean values in the 0–10 cm soil depth. Seidel et al. (2017) evaluated aggregate distribution under maize–jack bean intercropping and demonstrated that the intercropping system increased soil aggregate stability and microporosity, which is consistent with this study. The high concentration of soil macro-aggregates is conducive to the formation of improved soil structure, especially the number and size of water-stable macro-aggregates strongly influence soil erodibility and water-holding capacity (Jha et al. 2012). This study showed that intercropping increased the concentration of > 2.0 mm aggregates in maize rhizosphere soil and the concentration of 0.5–2.0 mm aggregates and R0.25 values in soybean rhizosphere soil. This can be attributed to the main cementing material of macroaggregates being SOM, which increases in intercropping systems, thus promoting the formation of macroaggregates (Wu et al. 2021). Zhang et al. (2022) studied the effect of maize and soybean intercropping on soil aggregates and concluded that the concentration of large aggregates (> 5.0 mm) in maize intercropping mode was significantly higher than that in monoculture, which agrees with the results of this study. This study further indicated that intercropping of maize and soybean could decrease soil erosion.
Operational taxonomic unit composition, ɑ diversity and composition of AMF communities under different planting patterns
Studies show complementary relationships between the diversity and richness of AMF and plant diversity (Liu and Wang 2003). Marcel et al. (1998) identified that the Simpson Diversity Index of plant communities increased with an increased number of AMF species. However, some researchers have reported negative correlations between plant diversity and AMF community (Urcelay and Díaz 2010). The intercropping system of gramineae and leguminosae had different effects on soil fungi, depending on N fixation by Leguminosae. In this study, the number of OTUs in the unique AMF community of maize intercropping increased compared with that of maize monoculture, but the number of OTUs in the unique AMF community of soybean intercropping decreased compared with soybean monoculture. These results are consistent with the effects of intercropping patterns on the Diversity Index (both Shannon and Simpson) and Richness Index (Chao1) of AMF communities in maize and soybean rhizosphere soils. A plausible explanation is changes in soil properties caused by intercropping systems (Wang et al. 2016a, b). When maize was intercropped with soybean, maize showed a strong competitive advantage in soil nutrition compared with soybean, which promoted the growth of maize roots and provided more energy substances and larger area for AMF growth and infection (Stern 1993). The decrease of AMF diversity in rhizosphere soil of intercropping soybean may be caused by high AN and SOC concentrations in soil (Lian et al. 2018; Zhang et al. 2022). The results also showed that intercropping significantly increased the concentrations of both SOM and AN in the rhizosphere soil.
AMF diversity is an important factor affecting the productivity and stability of agroecosystems. Understanding the species composition of AMF is important for effective management of agroecosystems (Alguacil et al. 2010). Studies have shown that the difference of host plant types changes the community structure of AMF. For example, Zhang et al. (2022) studied the change of AMF composition in maize-soybean intercropping soil and concluded that Globus_f_Glomeraceae was the dominant genus in maize soil, unclassified_f_Gigasporaceae and Gigaspora were the dominant genera in soybean soil. This study found that the first several dominant genera of AMF in rhizosphere soil of maize and soybean under different planting patterns were the same, but the relative abundance of each genus in intercropping was different from that in monoculture. Compared with maize monoculture, the relative abundance of Dentiscutata and Rhizophagus in maize intercropping increased. The relative abundance of Acaulospora, Diversispora and Claroideoglomus increased in soybean intercropping compared with soybean monoculture. This may be due to differences in the habitat, distribution and competitiveness of maize and soybean roots (Oehl et al. 2010; Zhang et al. 2022). Because this study focused on the effect of AMF community on the formation and stability of soil aggregates, it is necessary to clarify whether intercropping can increase the relative abundance of beneficial genera. Previous studies have shown that the length and morphology of mycelium produced by different AMF vary considerably, which have different effects on aggregate formation and stability. For example, Piotrowski et al. (2004) showed that the hyphal length of Gigasporaceae was higher than that of Glomeraceae and Acaulosporaceae, but the WSA1–2 mm formation of soil aggregates by Glomeraceae and Acaulosporaceae were significantly higher than that of Gigasporaceae. In contrast, Barbosa et al. (2019) showed that mycelial length played the dominant important role in the formation of soil aggregates, and there was a positive correlation between WSA and mycelial length. Therefore, the results of this study indicate that maize-soybean intercropping can increase the diversity and richness of the AMF community in maize rhizosphere soil. Although the diversity and richness of the AMF community in soybean rhizosphere soil decreased, the relative abundance of AMF genera promoting aggregate formation increased. In addition, this study also concluded that Acaulospora, Gigaspora and Diversispora were widely present in the rhizosphere of both maize and soybean crops.
Associations between soil nutrients, GRSP, enzymes, α diversity and aggregates in the rhizosphere of maize and soybean crops
The physical, chemical and biological properties of soil are strongly influenced by network structures. When nutrients are bioavailable or converted into less efficient forms, plants and microorganisms secrete enzymes to catalyze biochemical processes in the soil to meet their own growth and development needs for nutrients and energy (Aon and Colaneri 2001). For AMF, when the energy material is sufficient, its growth and metabolic activities accelerate, resulting in more GRSP (Shi et al. 2011). GRSP can increase the concentration of SOC and the N pool after entering the soil (Shi et al. 2011). Studies have shown that GRSP in soil accounts for 27% of SOC concentration, and GRSP can be detected in cultivated land, forest land and grassland (Geeta et al. 2018; Wang et al. 2016b). In this study, correlation analysis of each index of maize and soybean rhizosphere soil was conducted. Significant positive correlations were found between AN, AP, URE, INV, ACP, CAT, T-GRSP, EE-GRSP, Chao1 Index, Shannon Index, > 2.0 mm, MWD and R0.25 in maize rhizosphere soil. In addition, AN, AP, SOM, URE, INV, ACP, CAT EE-GRSP and T-GRSP were positively correlated with 0.5–2.0 mm and 0.25–0.5 mm aggregates and were negatively correlated with < 0.25 mm in soybean rhizosphere soil. Results indicate that AN, AP, URE, INV, ACP, CAT, GRSP, MWD and R0.25 had positive effects on each other in maize and soybean rhizosphere soil.
To further clarify the main factors influencing the properties of rhizosphere soil of the two crops, the indexes with significant correlation were analyzed by Redundancy Analysis based on the results of correlation analysis. It was concluded that the Shannon Index was the main factor affecting the variation of T-GRSP, EE-GRSP, URE and ACP in maize rhizosphere soil, which may be due to the Shannon Index of AMF community under intercropping conditions being significantly higher than that of monoculture. Rillig (2004) proposed that GRSP may be the main factor affecting the influence of AMF on soil aggregate stability. This study also showed that EE-GRSP was the main factor affecting MWD and > 2.0 mm aggregates. This is because GRSP can increase the stability of soil water-stable aggregates by increasing the surface hydrophobicity of aggregates and decreasing the water infiltration rate (Rillig 2004). In addition, GRSP had a more direct effect on soil aggregate stability than host roots and mycelia (Wu et al. 2014). AN is the main factor affecting the variation of URE, INV, CAT and ACP in soybean rhizosphere soil, which is because the transformation process and transfer amount of N in maize and soybean intercropping systems are readily regulated and increased (Han et al. 2007). SOM is a cementing material contributing to the formation and stability of soil aggregates (Halder et al. 2023). This study showed that SOM was positively correlated with MWD, R0.25 and 0.5–2.0 mm aggregates in soybean rhizosphere soil and was the main influencing factor. The effects of AMF in the rhizosphere soil of maize and soybean on the distribution characteristics of water-stable aggregate were analyzed. It was concluded that AMF genus played decisive roles in the formation and stability of aggregates in the rhizosphere. In the case of maize, particularly Diversispora with longer mycelia. Rhizophagus promoted mycelial diffusion in the rhizosphere of soybean. This is due to the great variation in the branching patterns of the mycelia produced by different AMF species. Thus, the stability of soil aggregates may depend more on the hyphal dispersal of host roots than solely on hyphal length (Piotrowski et al. 2004). Schreiner et al. (1997) tested the effects of three AMFs on R0.25 formation in soybean soil. They showed that the influence of Glomus mosseae on 2.0–4.0 mm aggregates was greater than that of Glomus etunicatum and Gigaspora rosea, but there was no significant difference in the stability of either 1.0–2.0 mm or 0.25–1.0 mm aggregates.
Conclusions
This study indicated that maize and soybean intercropping increased aggregate stability and enzyme activity in the rhizosphere soil of both crops. In addition, intercropping significantly increased the diversity of the AMF community in the maize rhizosphere. Redundancy Analysis showed that EE-GRSP was the main factor affecting aggregate stability and the formation of > 2.0 mm aggregates in the maize rhizosphere. Diversispora contributed most to the formation and stability of aggregates in maize rhizosphere soil. SOM was the main factor affecting aggregate stability and the formation of 0.5-2.0 mm aggregates in the soybean rhizosphere. Rhizophagus contributed most to the formation and stability of soil aggregates in soybean rhizosphere soil.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further enquiries can be directed to the corresponding author.
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- MM:
-
Monoculture maize
- IM:
-
Intercropping maize
- MS:
-
Monoculture soybean
- IS:
-
Intercropping soybean
- SOM:
-
Soil organic matter
- AN:
-
Alkali-hydrolysable nitrogen
- AP:
-
Available phosphorus
- EE-GRSP:
-
Easily extractable glomalin-related soil protein
- T-GRSP:
-
Total glomalin-related soil protein
- URE:
-
Urease
- INV:
-
Invertase
- ACP:
-
Acid phosphatase
- CAT:
-
Catalase
- R 0.25 :
-
Percentage concentration of water-stable aggregates
- MWD:
-
Mean weight diameter
References
Alguacil MDM, Lozano Z, Campoy MJ, Roldán A (2010) Phosphorus fertilisation management modifies the biodiversity of AM fungi in a tropical savanna forage system. Soil Biol Biochem 42:1114–1122. https://doi.org/10.1016/j.soilbio.2010.03.012
Ali W, Yang MX, Long Q, Hussain S, Chen JZ, Clay D, He YB (2022) Different fall/winter cover crop root patterns induce contrasting red soil (Ultisols) mechanical resistance through aggregate properties. Plant Soil 477:461–474. https://doi.org/10.1007/s11104-022-05430-4
Aon MA, Colaneri AC (2001) II. Temporal and spatial evolution of enzymatic activities and physico-chemical properties in an agricultural soil. Appl Soil Ecol 18:255–270. https://doi.org/10.1016/S0929-1393(01)00161-5
Bao SD (2000) Soil agrochemical analysis. China Agricultural Press, 3rd edn. Beijing, China, pp 39–109
Barbosa MV, Pedroso DDF, Curi N, Carneiro MAC (2019) Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates. Ciência e Agrotecnologia 43:1–8. https://doi.org/10.1590/1413-7054201943003519
Barreto RC, Madari BE, Machado PLOA, Maddock JEL, Costa AR (2009) The impact of soil management on aggregation, carbon stabilization and carbon loss as CO2 in the surface layer of a rhodic ferralsol in Southern Brazil. Agric Ecosyst and Environ 132:243–251. https://doi.org/10.1016/j.agee.2009.04.008
Barthès B, Roose E (2002) Aggregate stability as an indicator of soil susceptibility to runoff and erosion; validation at several levels. CATENA 47:133–149. https://doi.org/10.1016/S0341-8162(01)00180-1
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:201–213. https://doi.org/10.1023/A:1020809400075
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Elliott ET (1986) Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50:627–633. https://doi.org/10.2136/sssaj1986.03615995005000030017x
Fu YZ, Ma K, Li Q, Li GW, Cui HZ (2020) Effects of potato intercropped with maize on soil bacterial diversity. Chin J Eco-Agriculture 28:1715–1725. https://doi.org/10.13930/j.cnki.cjea.200240
Geeta S, Ranjan B, Das TK, Sharma AR, Avijit G, Shrila D, Pramod J (2018) Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic Plains. Soil Tillage Res 184:291–300. https://doi.org/10.1016/j.still.2018.08.006
González Rosado M, Parras Alcántara L, Aguilera Huertas J, Lozano García B (2022) Crop diversification effects on soil aggregation and aggregate-associated carbon and nitrogen in short-term rainfed olive groves under semiarid Mediterranean conditions. Horticulturae 8:618. https://doi.org/10.3390/horticulturae8070618
Guan SY, Zhang D, Zhang Z (1986) Soil enzyme and its research methods. Chinese Agricultural Press, Beijing, pp 278–280
Halder M, Ahmad SJ, Rahman T, Joardar JC, Siddique Md, Abu B, Islam MS, Islam MU, Liu S, Rabbi S, Peng XH (2023) Effects of straw incorporation and straw-burning on aggregate stability and soil organic carbon in a clay soil of Bangladesh. Geoderma Reg 32:1–8. https://doi.org/10.1016/j.geodrs.2023.e00620
Han G, Zhou G, Xu Z, Yang Y, Liu J, Shi K (2007) Biotic and abiotic factors controlling the spatial and temporal variation of soil respiration in an agricultural ecosystem. Soil Biol Biochem 39:418–425. https://doi.org/10.1016/j.soilbio.2006.08.009
Hu FN, Xu CY, Li H, Li S, Yu ZH, Li Y, He XH (2015) Particles interaction forces and their effects on soil aggregates breakdown. Soil Tillage Res 147:1–9. https://doi.org/10.1016/j.still.2014.11.006
Jha P, Garg N, Lakaria BL, Biswas AK, Subba Rao A (2012) Soil and residue carbon mineralization as affected by soil aggregate size. Soil Tillage Res 121:57–62. https://doi.org/10.1016/j.still.2012.01.018
Ji B, Shi L, Xu JP, He JL, Wang ZJ, Wu XD, Jiang Q (2021) Distribution characteristics of soil aggregates and its organic carbon in typical natural grassland of Ningxia. Acta Ecol Sin 41:7669–7678. https://doi.org/10.5846/stxb202005061120
Ji LL, Tan WF, Chen XH (2019) Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res 185:1–8. https://doi.org/10.1016/j.still.2018.08.010
Lian TX, Mu YH, Jian J, Ma QB, Cheng YB, Cai ZD, Nian H (2019) Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. Peer J 1:14. https://doi.org/10.7717/peerj.6412
Lian TX, Mu YH, Ma QB, Cheng YB, Gao R, Cai ZD, Jiang B, Nian H (2018) Use of sugarcane-soybean intercropping in acid soil impacts the structure of the soil fungal community. Sci Rep 8:14488. https://doi.org/10.1038/s41598-018-32920-2
Liu RJ, Wang FY (2003) Selection of appropriate host plants used in trap culture of arbuscular mycorrhizal fungi. Mycorrhiza 13:123–127. https://doi.org/10.1007/s00572-002-0207-4
Marcel GA, van der Klironomos H, Ursic JN, Moutoglis M, Streitwolf-Engel P, Boller R, Wiemken T, Sanders A (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. https://doi.org/10.1038/23932
Muhammad I, Wang J, Sainju Upendra M, Zhang SH, Zhao FZ, Khan A (2021) Cover cropping enhances soil microbial biomass and affects microbial community structure: a meta-analysis. Geoderma 381:114696. https://doi.org/10.1016/j.geoderma.2020.114696
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, Heijden MVD, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738. https://doi.org/10.1016/j.soilbio.2010.01.006
Özşahin E (2023) Climate change effect on soil erosion using different erosion models: a case study in the Naip Dam basin. Türkiye. Comput Electron Agric 207:107711. https://doi.org/10.1016/j.compag.2023.107711
Piotrowski JS, Denich T, Klironomos JN (2004) Rillig JMGC. The effects of arbuscular mycorrhizas on soil aggregation depend on the interaction between plant and fungal species. New Phytol 164:365–373. https://doi.org/10.2307/1514778
Qin XM, Zheng Y, Tang L, Long GQ (2015) Effects of nitrogen application rates on rhizosphere soil enzyme activity and potential nitrification in maize and potato intercropping. J Yunnan Agricultural Univ (Natural Science) 30:886–894. https://doi.org/10.16211/j.issn.1004-390X(n).2015.06.010
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754. https://doi.org/10.1111/j.1461-0248.2004.00620.x
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2015) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388. https://doi.org/10.1111/nph.13045
Sarapatka P (2019) Can glomalin content be used as an indicator for erosion damage to soil and related changes in organic matter characteristics and nutrients. CATENA 181:1–8. https://doi.org/10.1016/j.catena.2019.104078
Schreiner RP, Mihara KL, McDaniel H, Bethlenfalvay GJ (1997) Mycorrhizal fungi influence plant and soil functions and interactions. Plant Soil 188:199–209. https://doi.org/10.1023/A:1004271525014
Seidel EP, Reis WD, Mottin MC, Fey E, Schneider APR, Sustakowski MC (2017) Evaluation of aggregate distribution and selected soil physical properties under maize jack bean intercropping and gypsum rates. Acad Journals 12:1209–1216. https://doi.org/10.5897/AJAR2016.11642
Sharmah D, Jha DK (2014) Diversity of arbuscular mycorrhizal fungi in undisturbed forest, slash-and-burn field, and monoculture forest of Indo-Burma megadiverse region. Brazilian J Bot 37:339–351. https://doi.org/10.1007/s40415-014-0075-0
Shi S, Richardson AE, Maureen O, Angelis KM, Jones EE, Alison S, Firestone MK, Condron LM (2011) Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol Ecol 77:600–610. https://doi.org/10.1111/j.1574-6941.2011.01150.x
Simpson AJ, Simpson MJ, Smith E, Kelleher BP (2007) Microbially derived inputs to soil organic matter: are current estimates too low? Environ Sci Technol 41:8070–8076. https://doi.org/10.1021/es071217x
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res 79:7–31. https://doi.org/10.1016/j.still.2004.03.008
Snelder DJ, Bryan RB (1995) The use of rainfall simulation tests to assess the influence of vegetation density on soil loss on degraded rangelands in the Baringo District. Kenya 25:1–116. https://doi.org/10.1016/0341-8162(95)00003-b
Song YN, Zhang FS, Marschner P, Fan FL, Gao HM, Bao XG, Sun JH, Li L (2007) Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L). Biol Fertil Soils 43:565–574. https://doi.org/10.1007/s00374-006-0139-9
Stern WR (1993) Nitrogen fixation and transfer in intercrop systems. Field Crop Res 34:335–356. https://doi.org/10.1016/0378-4290(93)90121-3
Urcelay C, Díaz S (2010) The mycorrhizal dependence of subordinates determines the effect of arbuscular mycorrhizal fungi on plant diversity. Ecol Lett 6:388–391. https://doi.org/10.1046/j.1461-0248.2003.00444.x
Van GM, Busschaert P, Honnay O, Lievens B (2014) Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. J Microbiol Methods 106:93–100. https://doi.org/10.1016/j.mimet.2014.08.006
Wang J, Zhou ZY, Ling WT (2016a) Distribution and environmental function of glomalin-related soil protein: a review. Chin J Appl Ecol 27:634–642. https://doi.org/10.13287/j.1001-9332.201602.028
Wang XJ, Tang CX, Severi J, Butterly CR, Baldock JA (2016b) Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol 211:864–873. https://doi.org/10.1111/nph.13966
Wang T, Li YM, Wang ZL, Xiao JX, Bai LS, Fan MP (2018) Effects of intercropping on maize root exudates and soil aggregate stability. J Soil Water Conserv 32:185–190. https://doi.org/10.13870/j.cnki.stbcxb.2018.03.029
Weng QY, Huang XJ, Xu HL, Liu Y, Yuan XF, Ma HL, Yuan JC, Liu YH (2021) Effects of corn/soybean intercropping model on yield, quality, soil nutrition and rhizosphere microorganisms of silage corn. J Nuclear Agricultural Sci 35:462–470. https://doi.org/10.11869/j.issn.100-8551.2021.02.0462
Wright SF, Upadhyaya A (1996) Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci 161:575–586. https://doi.org/10.1097/00010694-199609000-00003
Wu QS, Cao MQ, Zou YN, He XH (2014) Direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stability in rhizosphere of trifoliate orange. Sci Rep 4:1–8. https://doi.org/10.1038/srep05823
Wu DM, Fan MP, Zhao JX, Li XM, Li YM (2021) Organic carbon mineralization characteristics of red soil aggregate fractions in sloping farmland with different planting patterns. J Agro-Environment Sci 40:1519–1528. https://doi.org/10.11654/jaes.2020-1446
Zhang S, Meng LB, Hou J, Liu XD, Ogundeji AO, Cheng ZY, Yin TJ, Clarke N, Hu BZ, Li SM (2022) Maize/soybean intercropping improves stability of soil aggregates driven by arbuscular mycorrhizal fungi in a black soil of northeast China. Plant Soil 481:63–82. https://doi.org/10.1007/s11104-022-05616-w
Zhang RZ, Mu Y, Li XR, Li SM, Sang P, Wang XR, Wu HL, Xu N (2020) Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates. Sci Total Environ 740:1–15. https://doi.org/10.1016/j.scitotenv.2020.139810
Zhao YJ, Liu XJ, Tong CC, Wu Y (2020a) Effect of root interaction on nodulation and nitrogen fixation ability of alfalfa in the simulated alfalfa/triticale intercropping in pots. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-61234-5
Zhao DQ, Yuan JC, Hou YT, Li T, Liao YC (2020b) Tempo-spatial dynamics of AMF under maize soybean intercropping. Chin J Eco-Agriculture 28:631–642. https://doi.org/10.13930/j.cnki.cjea.190720
Acknowledgements
We thank Mr. Chunpei Li, Mr. Hen Yang, Mr. Han Wu and Mr. Rongbiao Li (Yunnan Agricultural University) for helping in field planting. We thank Ms. Liqiong Zhao, Mr. Chengzhen Yu and Mr. Zitai Xia (Yunnan Agricultural University) for helping with field sampling. We thank Mrs. Zhimei Xu (Dabai Community, Panlong District, Kunming City) for helping to manage the field during our experiment.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 41661063) and the National Key Research and Development Program of China (Grant number 2022YFD1901500/2022YFD190150X).
Author information
Authors and Affiliations
Contributions
Maopan Fan, Yongmei Li, Jixia Zhao and Mei Lu designed the study and led the overall scientific questions. Mei Lu, Mingjiang Li, Zerang Lu and Jifen Yang performed the experiments. Mei Lu analyzed the data. Mei Lu, Mingjiang Li and Michael Fullen wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Hans Lambers.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, M., Zhao, J., Lu, Z. et al. Maize–soybean intercropping increases soil nutrient availability and aggregate stability. Plant Soil (2023). https://doi.org/10.1007/s11104-023-06282-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-06282-2