Abstract
Background and aims
Phytotoxicity following addition of hydrothermal-carbonised waste amendments (hydrochar) to soils is primarily attributed to toxic-organic compounds formed in hydrochars during hydrothermal carbonisation (HTC). However, factors influencing toxin formation in hydrochar and subsequent phytotoxicity have not been elucidated. Here, we investigated the effects of hydrochar feedstock and HTC temperature on phytotoxicity.
Methods
Hydrochars from sawdust, rice straw, chicken manure, paunch-hair, pig manure, biosolids and digestate, produced at three HTC temperatures (170, 200 and 260 °C), were assessed for phytotoxicity using plant-bioassays, spectroscopy and wet-chemistry.
Results
Hydrochar had no effect on seed germination, but reduced (30 to 50%) or had no significant effect on wheat growth under limited nutrient supply. Importantly, under luxury-nutrient supply, hydrochars (170 and 200 °C) that reduced growth in limited-nutrient conditions had no significant effect, and only hydrochars produced at 260 °C consistently reduced (20 to 30%) growth. Elemental-analysis and fourier transform infrared spectra indicated an increase in potential toxic functional groups in hydrochars produced at high temperature (260 °C). This suggested that phytotoxicity was due to toxic organic compounds, and occurred at high temperature. Conversely, at low temperature (170 to 200 °C), apparent phytotoxicity in nutrient-limited conditions was not due to hydrochar toxins, but nutrient deficiency exacerbated by hydrochar-induced nutrient immobilisation. Feedstock-type had no significant effect on phytotoxicity.
Conclusion
Findings provide new understanding of hydrochar-induced phytotoxicity. Fundamentally, hydrochars (170 to 200 °C) are potential soil-amendments, but nutrition regimes to offset nutrient-drawdown need consideration. Research to mitigate toxicity in hydrochar-260 °C is warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exploitation of biowaste resources for use as soil amendments in agricultural and natural systems is one of the key strategies to achieve a circular economy (Barros et al. 2020). Biowaste incorporation in soil can improve soil health by recycling nutrients and carbon (C) (Sharma et al. 2019), and promoting favourable edaphic conditions for soil microorganisms (Luo et al. 2018). Biowaste recycling can also reduce the amount of greenhouse gas-producing wastes disposed in landfill (Lou and Nair 2009).
In agroecosystems, biowastes can be applied directly as soil amendments without treatment, or treated using biological (e.g., composting and anaerobic digestion) or thermochemical methods (e.g., pyrolysis and hydrothermal carbonisation (HTC)) (Lohri et al. 2017). Treated wastes are often preferred to untreated wastes because they contain less contaminants (e.g., pathogens, antibiotics or pharmaceuticals), which can have adverse effects on the environment (Ellis and McCalla 1978; Smith 2009). Moreover, treated wastes are usually concentrated and lower in volume, making transportation easier compared to untreated wastes (Lohri et al. 2017). Both biological and thermal treatments are widely used for waste valorisation, with thermal methods often favoured due to their short process duration (minutes to hours) (Fang et al. 2018; Tripathi et al. 2016), and their ability to transform wastes into carbonised material for soil C sequestration as a climate geo-engineering solution (Downie et al. 2012; Kambo and Dutta 2015). Hydrothermal carbonisation is gaining attention because it converts wet biomass into hydrochar (C-rich, coal-like material) with significantly lower energy inputs than pyrolysis (biochar), since prior biomass drying is not required (Kambo and Dutta 2015). The process is autocalytic, autogenic under subcritical water conditions, and proceeds at lower temperatures (170–270 °C) than pyrolysis (300–800 °C) (Gupta et al. 2020; Kambo and Dutta 2015). Furthermore, higher mass recovery of char is often realised with HTC than pyrolysis because of lower temperatures and the catalytic nature of the process (Fang et al. 2018).
Numerous studies have investigated hydrochar as a soil amendment; however, reported effects on seed germination and plant growth are inconsistent. Our recent meta-analysis study on hydrochar effects on plant growth revealed an overall reduction of both seed germination (38%) and shoot growth (10%), and further indicated that interactions among hydrochar dose, properties and edaphic variables were fundamental in understanding when and where benefits may be achieved (Luutu et al. 2021). Individually, studies have reported increased plant growth (Bargmann et al. 2014; Fornes and Belda 2018; Mau et al. 2020; Yu et al. 2019), decreased growth (Bargmann et al. 2013; Roehrdanz et al. 2019; Schimmelpfennig et al. 2014) or no effect on growth (de Jager and Giani 2021; George et al. 2012; Roehrdanz et al. 2019) following hydrochar addition. Generally, increased plant growth following hydrochar addition is attributed to nutrient addition or enhanced soil physical properties (Malghani et al. 2015; Schimmelpfennig et al. 2014), and reduced growth attributed to hydrochar toxicity (Busch et al. 2012; Kalderis et al. 2019) or nitrogen (N) immobilisation (Gajić and Koch 2012; Subedi et al. 2015). Toxicity may be caused by high pH, electrical conductivity (EC) or heavy metal content of hydrochar (George et al. 2012; Yue et al. 2017), but it is mainly ascribed to residual organic compounds on hydrochars, which can be absorbed by plants and subsequently inhibit growth (Becker et al. 2013; Poerschmann et al. 2014). These organic compounds include organic acids, phenolics and furans (Petrovic et al. 2016; Poerschmann et al. 2015; Stemann et al. 2013).
Although we found an overall plant growth reduction upon hydrochar addition in our recent meta-analysis (Luutu et al. 2021), elucidating which factors contribute to growth inhibition was difficult because most experiments simply quantified the net effect of hydrochar addition rather than attempting to isolate potential causal factors. This study was therefore designed to draw out potential feedstock and HTC temperature effects on hydrochar-induced phytotoxicity. Feedstock, and reaction temperature which is a key regulator of biomass degradation during HTC, are presumed to be the main factors controlling the production of toxins (Busch et al. 2013; Sun et al. 2014; Wang et al. 2018). We hypothesised that phytotoxic effects of hydrochar are more likely with lignocellulosic feedstocks, and effects would be reduced by increased HTC temperature. High HTC temperatures, and the use of animal manure feedstocks rather than lignocellulosic or biosolid feedstocks, are presumed to reduce the likelihood of phytotoxicity (Lang et al. 2019; Sun et al. 2014). High HTC temperatures may promote re-polymerisation of potentially phytotoxic organic fragments into secondary-hydrochars (Hitzl et al. 2018; Lang et al. 2019; Li et al. 2017), some of which are non-polar and may be insoluble in soil solution (Benavente et al. 2022; Lucian et al. 2018), thus reducing toxic effects. Animal manure feedstocks are assumed to consist of less precursors of toxins (complex organic compounds) (Song et al. 2020; Zhai et al. 2016) compared to lignocellulosic biomass or biosolids, and are also likely to have a positive nutrient effect that might outweigh any negative effect of toxins (Phillips et al. 2000).
Materials and methods
Hydrochar feedstock
Seven different biowastes categorised under three groups; plant based: rice straw (RS) and eucalyptus-saw dust (SD); animal wastes: chicken manure (CM), bovine paunch and hair mix (PH) and pig manure (PM); and sludges: biosolids (BS) and digestate (D) from an abattoir solid waste settling lagoon, were used as HTC feedstocks in this study. Feedstocks were oven-dried at 40 °C until no further change in weight was observed, then kept at 4 °C before being ground in a rotary mill (Gelder & Co., NSW, Australia) fitted with 2.4 mm sieve (ASTM). Milled materials were stored at room temperature in airtight plastic bags.
Hydrochar production
Hydrochar was produced by subjecting the seven feedstocks (oven-dried at 40 °C) to three temperatures (170, 200 and 260 °C) in a Parr® 4534 floor stand reactor, with a 2 L stirred vessel and a Parr® 4848 controller, for a reaction period of 1 h. A load of 15% w/w (dry feedstock: water, 225 g: 1275 g) for SD, BS, PM, PH, CM and D, and 13% (225 g: 1475 g) for RS were fed into the reactor-vessel, with the internal rotary mixer set at 110 rpm. Load capacity with RS was reduced by 2% compared to other feedstocks to reduce the mixture thickness hence allow better stirring. The reactor vessel was heated until it achieved the target temperature, and then temperature was held for a reaction period of 1 h by manually controlling the system with an allowance of ± 6 °C fluctuation. After 1 h of heating at a target temperature, the cooling system of the Parr heating-element was switched-on to allow rapid cooling with water (for about 15 min). When material reaction temperature dropped to below 100 °C (internal temperature), slurries were ready for collection. Reaction pressure was autogenerated, and at end of the processes, a total of 21 hydrochar-slurries were produced. The slurries were separated by vacuum filtration through a Filtech® Glass Microfibre 1.2 μm filter paper-Grade 333, and the recovered 21 hydrochars (Table 1) were oven dried at 60 °C without prior washing until no further change in weight was observed (at least 36 h). Dried hydrochars were then ground to < 1 mm and stored at 4 °C in airtight plastic bags before characterisation.
Hydrochar characterisation
Proximate analysis
Moisture content, volatile matter, fixed C and ash content of hydrochar were determined in accordance with the modified thermal analysis method (Zhang et al. 2017). Moisture content was determined as the weight loss after heating samples at 105 °C for 24 h, whilst volatile matter was determined as the weight loss after heating the samples at 450 °C for 1 h. Ash content was determined as the residue weight after heating the samples at 750 °C for 6 h whilst fixed C content was calculated by subtracting the summed-weight of moisture, volatile matter and ash content from the original sample weight.
Elemental analysis
The total C and N content of hydrochars was quantified as per Rayment and Lyons (2011) methods 6B2b and 7A5, respectively, using a LECO TruMac CNS Analyser (LECO Corporation, St Joseph, MI, USA). Phosphorus (P), calcium (Ca), magnesium (Mg), potassium (K), trace elements and heavy metals (Cd, As, Hg, Co and Pb) were determined using Inductively Coupled Plasma- Mass spectrometry (NexION 350D ICP-MS, Perkin Elmer, Waltham, MA, USA) according to method 17C1 of Rayment and Lyons (2011), after digesting samples in nitric acid and hydrochloric acid (HNO3: HCl, 1:3).
Hydrochar pH and electrical conductivity (EC)
Hydrochar pH (CaCl2) and EC were determined according to method 4A1 of Rayment and Lyons (2011), by mixing hydrochar/water (1:5), and extracting for 1 h on a tumbler. Suspensions were allowed to stand for 0.5 h, then pH and EC were determined using an automated pH/EC analyser (ManTech Incorporation, Guelph, Ontario, Canada).
Fourier transform infrared (FTIR) spectroscopy
Hydrocar samples for FTIR analysis were pulverised to < 1 mm, then FTIR spectra measured using a Thermo scientific iS50ABX FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Spectra were obtained from 500–4000 cm−1 at 4 cm−1 resolution, and data were processed using the Thermo scientific OMNIC spectra software (Thermo Fisher Scientific, Waltham, MA, USA).
Plant growth
Pot experiments were conducted to test the effect of hydrochar feedstock and HTC temperature combinations on the growth of wheat (Triticum aestivum L. cv. EGA-Wedgetail). Soil was collected from the 0–15 cm layer of an Arenosol (IUSC Working Group WRB, 2014) near Casino 28.988186° South; 153.008806° East, NSW, Australia, and was air-dried and sieved to 2 mm. This soil was selected because of the low pH (1:5, CaCl2) 5.25, EC (dS/m) 0.01, ECEC (cmol+/kg) 0.88, TC (%) 0.16, TN (%) 0.02 and clay content, properties thought to minimise the binding of potential hydrochar-toxic components to soil particles (see Table S1 for detailed soil properties). Experiments were conducted in a temperature-controlled glasshouse at NSW Department of Primary Industries, Wollongbar, Australia, with day/night temperatures maintained at 25 °C/15 °C.
Experiment 1
Air-dried soil (1 kg) was weighed into 1 L, free draining plastic pots. Basal nutrients were applied to pots as per Rose et al. (2007) in the following amounts; 0.58 g of N (NH4NO3), 0.38 g of P (KH2PO4), 1.48 g of K (K2SO4), 0.51 g of Ca (CaCl2.2H2O), 0.27 g of Mg (MgSO4 0.7H2O), 0.54 g of Mn (MnSO4.H2O), 0.57 g of Zn (ZnSO4.7H2O), 0.57 g of Cu ( CuSO4.5H2O), 0.23 g of B (H3BO3), 0.53 g of Co (CoSO4.7H2O) and 0.33 g of Mo (Na2MoO4.2H2O). The nutrients in each pot were then mixed by hand, and after 24 h, 10 g (1% w/w) of ground dry hydrochar with particle size < 1 mm was added to appropriate pots and thoroughly mixed by hand. Hydrochar application rate of 10 g (1% w/w) is equivalent to 13.3 t/ha which is within the optimum application rate of 10–40 t/ha for biochar (Gao et al. 2021a, b; Liu et al. 2018). Pots with raw feedstock, and a control ‘nil amendment’ treatment with no feedstock or hydrochar amendment (mineral fertiliser only), were also prepared. There were three replicate pots of each feedstock x temperature (raw, 170, 200 and 260 °C) combination, plus three nil-amendment control pots. All pots were then watered to 75% water holding capacity (WHC) (Dane and Topp 2020), and on 4 Dec 2020, six wheat seeds were sown at 10 mm depth in each pot. At 7 d after sowing (DAS), plants were thinned to three seedlings per pot. Soil moisture content was maintained at 75% WHC by watering to weight every day, and pots were randomised daily throughout the growth period of 38 d.
Experiment 2
In Experiment 1 at sub-optimal nutrient supply (limited-nutrient conditions), it was unclear whether reduced plant growth was due to phytotoxicity, or simply a result of nutrient immobilisation where applied raw or HTC-treated wastes had a high C:N ratio. A second experiment was therefore established to assess plant responses to hydrochar under high-nutrient supply (luxury-nutrient conditions), i.e., where the chance of nutrient deficiency due to nutrient immobilisation was minimised.
In this experiment, the same basal nutrients were applied at double the dose of Experiment 1 prior to sowing, with an additional single dose at 7 DAS. Pots were further replenished with 0.58 g of N (NH4NO3) and 0.38 g of P (KH2PO4) at 14, 21 and 28 DAS. This nutrient application regime was determined based on a preliminary experiment which indicated that any more than triple the basal nutrients mixed into the soil prior to sowing impacted wheat seedling emergence and growth (unpublished data). Hydrochar and raw feedstock amendments were added to appropriate pots as per Experiment 1 (1% w/w), with three replicate pots per treatment combination. Three nil-amendment (mineral fertiliser only) control pots were also prepared as per Experiment 1. Six wheat seeds were sown on 14 June 2021, and thinned to three seedlings 7 DAS.
Measurements
In both experiments, seedling emergence was recorded at 7 DAS. For shoot biomass, wheat shoots were cut at approximately 10 mm from the soil surface 38 DAS, oven dried at 40 °C for 5 d (until constant weight was reached), and final weight recorded. Dried plant samples were ground for nutrient analysis. Shoot N concentrations were determined with a LECO TruMac CNS Analyser (LECO Corporation, St Joseph, MI, USA) whilst P, K, trace element and heavy metal (Cd, As, Hg, Co and Pb) concentrations were determined using ICP-MS (NexION 350D ICP-MS, Perkin Elmer, Waltham, MA, USA), following acid digestion (nitric acid) of dry tissue according to method APHA 3125 of Rayment and Lyons (2011). Shoot nutrient content (uptake) was calculated by multiplying shoot biomass by the respective shoot nutrient concentration. Soil samples for pH and EC analysis were prepared by sieving to 2 mm to remove the roots, and drying at 40 °C overnight in the oven. Soil pH (1:5 CaCl2) and EC were then measured using an automated pH/EC analyser (ManTech Incorporation, Guelph, Ontario, Canada) according to method 4A1 of Rayment and Lyons (2011). All samples were analysed in triplicate.
Statistical analyses
All statistical analyses and visualisation were conducted in R version 2.4.1 (R Core Team, 2005). Principal components analysis (PCA) was initially conducted on the complete data set across both experiments to summarise the relationships across samples and measured variables. Variables were scaled (unit variance) and the PCA was visualised using the package ‘factoextra’ (Kassambara and Mundt 2021).
Separate ANOVA models were established for Experiments 1 and 2 to test the effects of hydrochar feedstock and HTC temperature on shoot biomass, soil pH and soil EC, since there were clear differences between experiments. The experimental design in this study was augmented, with a 7 × 4 factorial (feedstock × HTC temperature) and a control group (i.e., no amendment) (Piepho et al. 2006). The data were therefore clustered into two groups; control-treatment (C) and test-treatment (T), with a crossed two-way 7 × 4 structure (feedstock × HTC temperature) nested within T (McCullagh and Nelder 2019). With this approach of analysis, full ANOVAs were produced with all relevant sources of variation using single models, hence there was no need to specify contrasts. Data were log transformed prior to analysis to satisfy assumptions of normality, and back-transformed means are presented.
For seedling emergence, a similar model structure was fitted as above, except the model was specified as a binomial generalised linear with emergence success as a proportion. Inspection of the emergence data showed no effect of additional nutrients on germination in controls (100% germination in both experiments) and suggested similar trends in Experiment 1 and 2, so Experiment was also tested as a categorical factor in the initial model. This was found to be non-significant (P = 0.53), hence emergence data from experiments 1 and 2 were pooled to increase the statistical power to detect potential effects of feedstock/temperature (i.e., by increasing n = 3 to n = 6).
Post-hoc pairwise differences between means (for all response variables) were calculated using the ‘emmeans’ (Lenth et al. 2018) and ‘multcomp’ (Hothorn et al. 2008) packages. Figures were constructed using the package ‘ggplot2’ (Wickham 2016).
Results
Hydrochar physicochemical properties
Mass recovery of hydrochar ranged from 96% (D-170) to 49% (RS-260) of original feedstock, with notable reductions in yield at higher HTC temperatures (Table 1). Proximate analysis indicated that hydrochars produced at high temperature (> 200 °C) were more likely to have low volatile matter and high ash content. Fixed C (FC) content varied across feedstocks and HTC temperatures (Table 1). Elemental analysis indicated enrichment of C and P (except for saw dust, rice straw and paunch-hair), and depletion of K in hydrochar produced at high temperatures (Table 1). Hydrochar total N varied across feedstocks but was not affected by HTC temperatures (Table 1). Of the heavy metals, only Pb concentrations tended to increase consistently with increasing HTC temperature, but only in biosolids, pig manure, paunch-hair, chicken manure and digestate (Table S9). Hydrochar pH and EC also varied across feedstock, but only EC was affected by HTC temperatures (Table 1). Increasing HTC temperature typically reduced hydrochar EC compared to raw feedstock for all feedstocks except sawdust and biosolids. With sawdust, increasing HTC temperature increased hydrochar EC, whilst with biosolids, hydrochar EC was not affected by temperature.
The FTIR analysis of chemical bonding revealed that hydrochars produced from plant-based materials (saw dust and rice straw) featured a larger proportion of carboxyl C = O bonds, while hydrochars from animal wastes (pig manure, chicken manure and paunch-hair) were dominated by aliphatic C-H, and those from sludge wastes (biosolids and digestate) were dominated by aromatic C-H bonds (Fig. 1). High HTC temperature (260 °C) increased carboxyl C = O, aromatic C = C, aromatic C = O and phenolic O–H, especially in plant-based hydrochars (RS-260 and SD-260).
FTIR spectra of hydrochars from rice straw, sawdust, chicken manure, paunch-hair, pig manure, biosolids and digestate, for raw-feedstock and at HTC temperature (170, 200 and 260 °C). Functional groups of the main FTIR bands: (1) O–H stretching of carboxylic acids, phenols, alcohols, 3336 cm−1; (2) Aliphatic C-H, 2906 cm−1; (3) Carboxyl C = O, 1702 cm−1; (4) Aromatic C = C, 1598 cm−1; (5) Aromatic C = O, 1514 cm−1; (6) Phenolic O–H, 1371 cm−1; and (7) Aromatic C-H, 609–895 cm.−1
Seedling emergence
There was a significant interaction effect of feedstock and HTC temperature on seedling emergence response (P < 0.001) (Table S2), with raw chicken manure reducing seedling emergence by 75% compared to the nil-amendment control (Table S3).
Plant growth and nutrient accumulation
Experiment 1
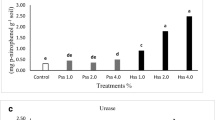
There was a significant interaction effect of feedstock and HTC temperature on shoot biomass response (P < 0.001) (Table S2; Fig. 2). Plant-based feedstocks including rice straw (RS-Raw, RS-170 and RS-200) and sawdust (SD-170 and SD-200), and biosolids (BS-170 and BS-200), reduced shoot biomass by 30–50% compared to the nil-amendment control (Table S4). Biosolid (BS-Raw) significantly increased shoot biomass whilst animal wastes including pig manure (PM-Raw, PM-170 and PM-200), chicken manure (CM-Raw, CM-170 and CM-200) and paunch-hair (PH-Raw, PH-170 and PH-200) had no significant effect on shoot biomass compared to the nil-amendment control (Table S4). Also, digestate (D-Raw, D-170, D-200 and D-260) had no significant effect on shoot biomass compared to the nil-amendment control (Table S4). Although not statistically significant, it was noted that raw amendments of high-nutrient feedstocks (pig manure, chicken manure, paunch-hair and digestate) increased shoot biomass compared to the nil- amendment control. All hydrochars produced at high temperature (260 °C) except for rice straw, sawdust and digestate, reduced plant growth by 30–50% compared to nil-amendment control (Table S4).
In reference to critical-threshold values below which wheat shoot growth is known to be compromised (Reuter and Robinson 1997), plant tissue N and P concentrations in Experiment 1 were below the critical values except for P in RS-260 and SD-Raw (Table S5). Potassium concentrations in plant tissue were also below the critical value except for PM-200 and PM-260 (Table S5). Other nutrients including S, Ca, Mg, Na, Cu, Zn, Mn, Fe, B and Mo were essentially within the adequate range as per critical threshold values (Table S6).
Experiment 2
Principal components analysis confirmed that the addition of nutrients in Experiment 2 led to substantial differences in overall plant nutrient uptake compared with Experiment 1 (Figure S1). However, similar to experiment 1, there was a significant interaction effect of feedstock and HTC temperature on shoot biomass response in experiment 2 (P < 0.001) (Table S2; Fig. 3). Plant-based feedstocks including rice straw (RS-raw, RS-170 and RS-200) and sawdust (SD-200), animal wastes including pig manure (PM-Raw, PM-170 and PM-200), chicken manure (CM-Raw, CM-170 and CM-200) and paunch-hair (PH-Raw, PH-170 and PH-200), and sludge wastes including biosolids (BS-Raw, BS-170 and BS-200) and digestate (D-Raw, D-170 and D-200) had no significant effect on shoot biomass (Table S4). Only hydrochar produced at 260 °C (except D-260) irrespective of feedstock and sawdust (SD-Raw and SD-170), significantly reduced (20–30%) shoot biomass (Table S4). Digestate (D-260) also reduced shoot biomass (10%) though the effect was not significant.
In reference to critical threshold values as per Reuter and Robinson (1997), tissue N and P concentrations were within the critical range (Table S5). Potassium concentration in plant tissue was above the critical range with SD-Raw and SD-170 having slightly less K compared to other treatments (Table S5). Other nutrients including S, Ca, Mg, Na, Cu, Zn, Mn, Fe, B and Mo were also within adequate ranges in all treatments (Table S6A, B). The concentrations of heavy metals in shoot tissue were generally not significantly higher in the 260 ºC chars than other chars, with the exception of Co concentrations in RS-260 and PM-260, and As levels in PM-260 (Table S6C).
Soil pH and EC
Experiment 1
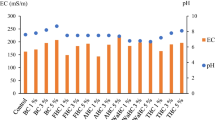
The effect of hydrochar addition on soil pH varied across feedstocks, and was not impacted by HTC temperature except for biosolids and paunch-hair (Table S7). Biosolid hydrochars (BS-170, BS-200 and BS-260) and paunch-hair (PH-260) significantly increased soil pH compared to the corresponding raw feedstock (Table S7). Soil pH following hydrochar addition ranged from 7.25 ± 0.07 (BS-170) to 4.75 ± 0.05 (SD-200) whilst soil pH of the nil-amendment control was 4.95 ± 0.05 (Table S7). Soil pH with raw feedstock addition ranged from 7.07 ± 0.07 (CM-Raw) to 4.49 ± 0.04 (SD-Raw) (Table S7).
Hydrochar addition resulted in varied soil EC across feedstocks, with a subtle influence of temperature (Table S7). Soil EC following hydrochar addition ranged from 0.13 ± 0.02 dS/m (RS-170) to 0.02 dS/m (SD-200) whilst soil EC for nil-amendment control was 0.02 dS/m (Table S7). Soil EC with addition of raw feedstock ranged from 0.13 ± 0.02 dS/m (RS-Raw) to 0.02 dS/m (D-Raw) (Table S7).
Experiment 2
The effect of hydrochar addition on soil pH in Experiment 2 followed a similar trend to Experiment 1 (Table S7). Soil pH following hydrochar addition ranged from 7.01 ± 0.19 (BS-200) to 4.13 ± 0.11 (SD-200) whilst soil pH for the nil-amendment control was 4.95 ± 0.13 (Table S7). Soil pH with raw feedstock addition ranged from 6.42 ± 0.17 (CM-Raw) to 4.54 ± 0.12 (SD-Raw) (Table S7).
Hydrochar addition in Experiment 2 had no significant effect on soil EC across all feedstocks and temperatures (Table S7). Soil EC following hydrochar addition ranged from 0.29 ± 0.03 dS/m (SD-260) to 0.19 ± 0.02 dS/m (SD-170) whilst soil EC for control treatment was 0.26 ± 0.03 dS/m (Table S7). Soil EC with addition of raw feedstock ranged from 0.37 ± 0.04 dS/m (CM-Raw) to 0.18 ± 0.02 dS/m (PM-Raw) (Table S7).
Discussion
Hydrothermal carbonisation may have a role in the circular economy by transforming waste products into soil amendments for sustainable soil performance, but concerns have been raised about potential phytotoxic effects of hydrochars on plants (Luutu et al. 2021). The aim of this study was to resolve the potential impact of feedstock and HTC temperature on hydrochar-induced phytotoxicity.
While addition of raw chicken manure reduced wheat emergence, no other raw feedstock, or hydrochar, had a significant effect on wheat emergence. The reduced emergence with chicken manure was likely due to ammonia or ammonium toxicity (El-Zeadani et al. 2018; Pan et al. 2016). In contrast to previous reports of reduced seed germination upon hydrochar addition to soil (Bargmann et al. 2013; Busch et al. 2013), hydrochar addition had no significant effect on emergence in either limited-nutrient (Experiment 1) or luxury-nutrient (Experiment 2) conditions.
Wheat shoot biomass increase following addition of raw biosolids, chicken manure, pig manure, paunch-hair and digestate compared to the nil-amendment control in limited-nutrient conditions is possibly due to improved N, P and K nutrition as indicated by feedstock nutrient composition (Table 1) and tissue nutrient content (Table S8). In contrast to the nutrient-rich feedstocks, raw rice straw and sawdust, which contained lower nutrients and had high C:N ratios (Table 1), significantly reduced shoot biomass compared to the nil-amendment control, as reported previously for amendments with high C:N ratios (Truong and Marschner 2019; van der Sloot et al. 2022).
Conversely, addition of biosolids, pig manure, paunch-hair, chicken manure and digestate hydrochars in limited-nutrient conditions reduced wheat shoot growth compared to the respective raw feedstock treatments, with a trend towards greater reduction at higher HTC temperatures (Fig. 2). This trend was not observed for rice straw or sawdust hydrochars where growth was instead promoted at the highest temperature (Fig. 2). These growth reductions are consistent with several reports from the literature describing plant growth reductions upon hydrochar addition to soil (Roehrdanz et al. 2019; Schimmelpfennig et al. 2014; Yin et al. 2022). However, attributing growth reductions to phytotoxicity can be difficult, because an apparent phytotoxic effect of hydrochar may be a result of nutrient deficiency following nutrient immobilisation when hydrochars have a high proportion of C compared to other key nutrients (N, P, S) (Luutu et al. 2021). Since hydrochar is a labile C source (Libra et al. 2011; Malghani et al. 2015), the potential for microbial nutrient immobilisation and subsequent deprivation of nutrients for plant growth is likely (Cao et al. 2021).
The higher shoot biomass production following amendment with 260 °C sawdust and rice straw hydrochars compared to the respective raw feedstock treatment in Experiment 1 (limited-nutrient) suggests that high HTC temperature of these feedstocks leads to plant growth stimulation. However, when adequate nutrients were supplied to the soil in Experiment 2, the results clearly indicated that 260 °C sawdust and rice straw hydrochars were phytotoxic, and that the nutrient deficiencies in Experiment 1 were masking the toxicity. The results from Experiment 2 (luxury-nutrient) support the notion that where soils are nutrient-responsive, any toxicity effects may be masked by growth stimulation from a fertiliser effect of nutrient-rich hydrochars. It should also be noted that the significantly lower biomass production following addition of sawdust amendments (SD-Raw and SD-170) compared to the nil-amendment control in Experiment 2 (luxury-nutrient) is likely due to insufficient K as suggested by tissue-nutrient concentrations (Table S5). This is likely because the sawdust amendment was low in K (Table 1) and K was not supplemented on a weekly basis like N and P. Ultimately, feedstock had little effect on the phytotoxicity of hydrochars once nutrient differences between chars were nullified by application of fertiliser to soils.
The poor growth of wheat in soils amended with all hydrochars produced at 260 °C irrespective of feedstock type or nutrient-condition confirms hydrochar-induced phytotoxicity at high HTC temperature. This is contrary to our hypothesis that higher temperatures would mitigate phytotoxic effects of hydrochars. Phytotoxicity following hydrochar addition may be a result of reduced soil pH (George et al. 2012) or increased soil EC (Macdonald et al. 2014), heavy metals or toxic residual compounds in hydrochar (Poerschmann et al. 2014). In this study, soil pH and EC following addition of hydrochar produced at 260 °C were in a similar range to corresponding hydrochars produced at 170 °C and 200 °C that did not reduce shoot biomass in Experiments 1 and 2 (Table S7). Moreover, soil pH (4.29 -7.08) and EC (0.03–0.3 dS/m) with hydrochar produced at 260 °C were within a range suitable for the wheat variety (EGA-Wedgetail) used in this study (Zhang et al. 2006). The concentrations of heavy metals in wheat shoots in the hydrochar 260 °C treatments were not substantially higher than those from raw or hydrochar 170 °C treatments, with the exception of Co levels in RS-260 and PM-260, and As levels in PM-260, suggesting heavy metals were not the cause of phytotoxicity. This implies that toxic residual compounds in hydrochar are the most likely underlying cause of phytotoxicity at high HTC temperature (260 °C).
Since biomass degradation and release of breakdown compounds including potential phytotoxins during HTC is thought to occur between 200 °C to 240 °C (Funke and Ziegler 2010; Kambo and Dutta 2015), further increase in temperature to 260 °C may promote adsorption or absorption of these compounds from the liquid fraction back into the formed hydrochar (Reza et al. 2013), hence conferring toxicity. Residual organic compounds on hydrochar may include O-functionalised breakdown products (phenols, acids, lactones, furans/ alcohols/ketones, furfurals, etc.), fatty acids (α-linolenic, nonanoic, palmitic, etc.), short-chain carboxylic acids (levulinic, acetic, formic acid, etc.), N-containing organic compounds (3-pyridinol, 5-oxoproline carboxylate, pyrrolidine, etc.) and polycyclic aromatic hydrocarbons (PAH) (benzene, toluene, naphthalene, etc.), some of which have been associated with phytotoxicity (Liu et al. 2021; Poerschmann et al. 2014, 2015). This is evident in the present study where carboxyl C = O, aromatic C = C, aromatic C = O, and phenolic O–H increased with increasing HTC temperature, particularly with plant-based hydrochars (RS-260 and SD-260) (Fig. 1). Therefore, it is likely that when hydrochar produced at 260 °C was added to the soil, sorbed toxins dissolved into soil pore water, assimilated into the plant system and compromised plant metabolism (Luutu et al. 2021). It should also be noted that some studies assume that increasing HTC temperature would reduce hydrochar toxicity owing to possible re-polymerisation of toxic compounds into secondary-hydrochars (Benavente et al. 2022; Lucian et al. 2018), or increasing decomposition rate more than the synthesis rate of toxic compounds (Gao et al. 2021a, b; Lang et al. 2019; Li et al. 2017). Unfortunately, with these studies, no plant growth experiments were conducted to confirm the assumptions. In this study FTIR results indicated an increase in secondary hydrochars at higher temperature. However, this was associated with a decrease in plant biomass, which contradicts the notion that secondary char formation may reduce toxicity of hydrochar.
Conclusion
This study generally revealed that phytotoxicity following hydrochar addition to soils is influenced by HTC temperature irrespective of feedstock type. Hydrochar produced at high HTC temperature (260 °C) is phytotoxic and can reduce shoot growth up to 30% but has no effect on seedling emergence. Conversely, hydrochar produced at low HTC temperature (170–200 °C) is not phytotoxic per se. However, a seeming phytotoxic effect with hydrochar produced at low HTC temperature may occur as a result of nutrient deficiency, likely induced by nutrient immobilisation when hydrochars have a high proportion of C compared to other key nutrients (N, P, S). Results also indicated that phytotoxicity effect of hydrochar can be masked by nutrient deficiency in soils.
These findings highlight the prospects of application of hydrochar produced at low HTC temperature in agroecosystems. Moreover, results highlight the need for further research to mitigate the phytotoxic organic compounds in hydrochars produced at high HTC temperature (260 °C). Of importance, the use of HTC at low temperature (170–200 °C) for wet feedstocks, and the use of pyrolysis where positive results regarding crop production have been shown (Jeffery et al. 2011) for low moisture feedstocks, could be a suitable strategy. In this case, care needs to be taken to ensure adequate nutrition where nutrient drawdown caused by hydrochar addition could occur.
References
Bargmann I, Rillig MC, Buss W, Kruse A, Kuecke M (2013) Hydrochar and biochar effects on germination of spring barley. J Agron Crop Sci 199:360–373. https://doi.org/10.1111/jac.12024
Bargmann I, Rillig MC, Kruse A, Greef JM, Kücke M (2014) Effects of hydrochar application on the dynamics of soluble nitrogen in soils and on plant availability. J Plant Nutr Soil Sci 177:48–58. https://doi.org/10.1002/jpln.201300069
Barros MV, Salvador R, de Francisco AC, Piekarski CM (2020) Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew Sustain Energy Rev 131:109958. https://doi.org/10.1016/j.rser.2020.109958
Becker R, Dorgerloh U, Helmis M, Mumme J, Diakité M, Nehls I (2013) Hydrothermally carbonized plant materials: patterns of volatile organic compounds detected by gas chromatography. Biores Technol 130:621–628. https://doi.org/10.1016/j.biortech.2012.12.102
Benavente V, Lage S, Gentili FG, Jansson S (2022) Influence of lipid extraction and processing conditions on hydrothermal conversion of microalgae feedstocks–Effect on hydrochar composition, secondary char formation and phytotoxicity. Chem Eng J 428:129559
Busch D, Kammann C, Grünhage L, Müller C (2012) Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J Environ Qual 41:1023–1032. https://doi.org/10.2134/jeq2011.0122
Busch D, Stark A, Kammann CI, Glaser B (2013) Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol Environ Saf 97:59–66. https://doi.org/10.1016/j.ecoenv.2013.07.003
Cao Y, He Z, Zhu T, Zhao F (2021) Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 383:114784. https://doi.org/10.1016/j.geoderma.2020.114784
Dane JH, Topp CG (2020) Methods of soil analysis, Part 4: Physical methods (vol. 20), Wiley, New Jersey, pp 417–545
de Jager M, Giani L (2021) An investigation of the effects of hydrochar application rate on soil amelioration and plant growth in three diverse soils. Biochar 1–17. https://doi.org/10.1007/s42773-021-00089-z
Downie A, Munroe P, Cowie A, Van Zwieten L, Lau DM (2012) Biochar as a geoengineering climate solution: hazard identification and risk management. Crit Rev Environ Sci Technol 42:225–250. https://doi.org/10.1080/10643389.2010.507980
El-Zeadani H, Abubaker J, Essalem M, Alghali A (2018) Germination of several wheat cultivars in desert soil after amendment with raw and digested poultry manure with and without combination with mineral fertilizer. Int J Recycl Org Waste Agric 7:335–343. https://doi.org/10.1007/s40093-018-0219-5
Ellis J, McCalla T (1978) Fate of pathogens in soils receiving animal wastes—a review. Transactions of the ASAE 21:309–0313. https://doi.org/10.13031/2013.35294
Fang J, Zhan L, Ok YS, Gao B (2018) Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J Ind Eng Chem 57:15–21. https://doi.org/10.1016/j.jiec.2017.08.026
Fornes F, Belda RM (2018) Biochar versus hydrochar as growth media constituents for ornamental plant cultivation. Sci Agric 75:304–312. https://doi.org/10.1590/1678-992X-2017-0062
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioprod Biorefin 4:160–177. https://doi.org/10.1002/bbb.198
Gajić A, Koch HJ (2012) Sugar beet (Beta vulgaris L.) growth reduction caused by hydrochar is related to nitrogen supply. J Environ Qual 41:1067–1075. https://doi.org/10.2134/jeq2011.0237
Gao Y, Remón J, Matharu AS (2021a) Microwave-assisted hydrothermal treatments for biomass valorisation: a critical review. Green Chem 23:3502–3525. https://doi.org/10.1039/D1GC00623A
Gao Y, Shao G, Yang Z, Zhang K, Lu J, Wang Z, ... Xu D (2021b) Influences of soil and biochar properties and amount of biochar and fertilizer on the performance of biochar in improving plant photosynthetic rate: A meta-analysis. Eur J Agron 130:126345. https://doi.org/10.1016/j.eja.2021.126345
George C, Wagner M, Kücke M, Rillig MC (2012) Divergent consequences of hydrochar in the plant–soil system: Arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl Soil Ecol 59:68–72. https://doi.org/10.1016/j.apsoil.2012.02.021
Gupta D, Mahajani SM, Garg A (2020) Opportunities for resource recovery after hydrothermal pretreatment of biodegradable municipal solid waste: A mini-review. In: Ghosh S (eds) Waste management as economic industry towards circular economy. Springer, Singapore. https://doi.org/10.1007/978-981-15-1620-7_16
Hitzl M, Mendez A, Owsianiak M, Renz M (2018) Making hydrochar suitable for agricultural soil: a thermal treatment to remove organic phytotoxic compounds. J Environ Chem Eng 6:7029–7034. https://doi.org/10.1016/j.jece.2018.10.064
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J: J Math Methods Biosci 50:346–363. https://doi.org/10.1002/bimj.200810425
Jeffery S, Verheijen FG, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agr Ecosyst Environ 144:175–187. https://doi.org/10.1016/j.agee.2011.08.015
Kalderis D, Papameletiou G, Kayan B (2019) Assessment of orange peel hydrochar as a soil amendment: impact on clay soil physical properties and potential phytotoxicity. Waste Biomass Valorization 10:3471–3484. https://doi.org/10.1007/s12649-018-0364-0
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renewable and Sustainable Energy Reviews 45:359–378. https://doi.org/10.1016/j.rser.2015.01.050
Kassambara A, Mundt F (2021) Factoextra: Extract and visualize the results of multivariate data analyses, R Package Version 1.0. 7. 2020. https://cir.nii.ac.jp/crid/1370004235968325765
Lang Q, Zhang B, Li Y, Liu Z, Jiao W (2019) Formation and toxicity of polycyclic aromatic hydrocarbons during CaO assisted hydrothermal carbonization of swine manure. Waste Manage 100:84–90. https://doi.org/10.1016/j.wasman.2019.09.010
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2018) Emmeans: Estimated marginal means, aka least-squares means. R Package Version 1(1):3
Libra J A, Ro K S, Kammann C, Funke A, Berge N D, Neubauer Y, ... Kern J (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2:71–106. https://doi.org/10.4155/bfs.10.81
Li Y, Zhai Y, Zhu Y, Peng C, Wang T, Zeng G, ... Zhao X (2017) Distribution and conversion of polycyclic aromatic hydrocarbons during the hydrothermal treatment of sewage sludge. Energy Fuels 31:9542–9549. https://doi.org/10.1021/acs.energyfuels.7b01523
Liu T, Tian L, Liu Z, He J, Fu H, Huang Q, ... Huang Z (2021) Distribution and toxicity of polycyclic aromatic hydrocarbons during CaO-assisted hydrothermal carbonization of sewage sludge. Waste Manag 120:616–625. https://doi.org/10.1016/j.wasman.2020.10.025
Liu Q, Zhang Y, Liu B, Amonette J E, Lin Z, Liu G, ... Xie Z (2018) How does biochar influence soil N cycle? A meta-analysis. Plant Soil 426:211–225. https://doi.org/10.1007/s11104-018-3619-4
Lucian M, Volpe M, Gao L, Piro G, Goldfarb JL, Fiori L (2018) Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 233:257–268
Lohri C, Diener S, Zabaleta I, Mertenat A, Zurbrügg C (2017) Treatment technologies for urban solid biowaste to create value products: a review with focus on low- and middle-income settings. Rev Environ Sci Biotechnol 16:81–130. https://doi.org/10.1007/s11157-017-9422-5
Lou X, Nair J (2009) The impact of landfilling and composting on greenhouse gas emissions–a review. Biores Technol 100:3792–3798. https://doi.org/10.1016/j.biortech.2008.12.006
Luo G, Li L, Friman V-P, Guo J, Guo S, Shen Q, Ling N (2018) Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol Biochem 124:105–115. https://doi.org/10.1016/j.soilbio.2018.06.002
Luutu H, Rose M T, McIntosh S, Van Zwieten L, Rose T (2021) Plant growth responses to soil-applied hydrothermally-carbonised waste amendments: a meta-analysis. Plant Soil 1–15. https://doi.org/10.1007/s11104-021-05185-4
Macdonald LM, Farrell M, Van Zwieten L, Krull ES (2014) Plant growth responses to biochar addition: an Australian soils perspective. Biol Fertil Soils 50:1035–1045. https://doi.org/10.1007/s00374-014-0921-z
Malghani S, Jüschke E, Baumert J, Thuille A, Antonietti M, Trumbore S, Gleixner G (2015) Carbon sequestration potential of hydrothermal carbonization char (hydrochar) in two contrasting soils; results of a 1-year field study. Biol Fertil Soils 51:123–134. https://doi.org/10.1007/s00374-014-0980-1
Mau V, Arye G, Gross A (2020) Poultry litter hydrochar as an amendment for sandy soils. J Environ Manag 271:110959. https://doi.org/10.1016/j.jenvman.2020.110959
McCullagh P, Nelder JA (2019) Generalized linear models. Routledge
Pan WL, Madsen IJ, Bolton RP, Graves L, Sistrunk T (2016) Ammonia/ammonium toxicity root symptoms induced by inorganic and organic fertilizers and placement. Agron J 108:2485–2492. https://doi.org/10.2134/agronj2016.02.0122
Petrovic J, PerišiĿ N, MaksimoviĿ J D, MaksimoviĿ V, KragoviĿ M, StojanoviĿ M, ... MihajloviĿ M (2016) Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase. J Anal Appl Pyrolysis 118:267–277. https://doi.org/10.1016/j.jaap.2016.02.010
Phillips T, Liu D, Seech A, Lee H, Trevors J (2000) Monitoring bioremediation in creosote-contaminated soils using chemical analysis and toxicity tests. J Ind Microbiol Biotechnol 24:132–139. https://doi.org/10.1038/sj.jim.2900789
Piepho H, Williams E, Fleck M (2006) A note on the analysis of designed experiments with complex treatment structure. HortScience 41:446–452. https://doi.org/10.21273/HORTSCI.41.2.446
Poerschmann J, Weiner B, Wedwitschka H, Baskyr I, Koehler R, Kopinke F-D (2014) Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Biores Technol 164:162–169. https://doi.org/10.1016/j.biortech.2014.04.052
Poerschmann J, Weiner B, Wedwitschka H, Zehnsdorf A, Koehler R, Kopinke F-D (2015) Characterization of biochars and dissolved organic matter phases obtained upon hydrothermal carbonization of Elodea nuttallii. Biores Technol 189:145–153. https://doi.org/10.1016/j.biortech.2015.03.146
Rayment GE, Lyons DJ (2011) Soil chemical methods: Australasia, vol 3. CSIRO publishing
R Core Team R (2005) R: A language and environment for statistical computing.The R Foundation for Statistical Computing, Vienna, Austria. http://www. R-project. org
Reuter DJ, Robinson JB (1997) Plant analysis : an interpretation manual, 2nd edn. CSIRO Publishing, Collingwood, Vic
Reza MT, Lynam JG, Uddin MH, Coronella CJ (2013) Hydrothermal carbonization: Fate of inorganics. Biomass Bioenerg 49:86–94. https://doi.org/10.1016/j.biombioe.2012.12.004
Roehrdanz M, Greve T, de Jager M, Buchwald R, Wark M (2019) Co-composted hydrochar substrates as growing media for horticultural crops. Sci Hortic 252:96–103. https://doi.org/10.1016/j.scienta.2019.03.055
Rose TJ, Rengel Z, Ma Q, Bowden JW (2007) Differential accumulation patterns of phosphorus and potassium by canola cultivars compared to wheat. J Plant Nutr Soil Sci 170:404–411. https://doi.org/10.1002/jpln.200625163
Schimmelpfennig S, Müller C, Grünhage L, Koch C, Kammann C (2014) Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agr Ecosyst Environ 191:39–52. https://doi.org/10.1016/j.agee.2014.03.027
Sharma B, Vaish B, Singh UK, Singh P, Singh RP (2019) Recycling of organic wastes in agriculture: an environmental perspective. Int J Environ Res 13:409–429. https://doi.org/10.1007/s41742-019-00175-y
Smith SR (2009) A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ Int 35:142–156. https://doi.org/10.1016/j.envint.2008.06.009
Song C, Yuan W, Shan S, Ma Q, Zhang H, Wang X, ... Wang H (2020) Changes of nutrients and potentially toxic elements during hydrothermal carbonization of pig manure. Chemosphere 243:125331.https://doi.org/10.1016/j.chemosphere.2019.125331
Stemann J, Putschew A, Ziegler F (2013) Hydrothermal carbonization: process water characterization and effects of water recirculation. Biores Technol 143:139–146. https://doi.org/10.1016/j.biortech.2013.05.098
Subedi R, Kammann C, Pelissetti S, Taupe N, Bertora C, Monaco S, Grignani C (2015) Does soil amended with biochar and hydrochar reduce ammonia emissions following the application of pig slurry? Eur J Soil Sci 66:1044–1053. https://doi.org/10.1111/ejss.12302
Sun Y, Gao B, Yao Y, Fang J, Zhang M, Zhou Y, ... Yang L (2014) Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem Eng J 240:574–578. https://doi.org/10.1016/j.cej.2013.10.081
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew Sustain Energy Rev 55:467–481. https://doi.org/10.1016/j.rser.2015.10.122
Truong THH, Marschner P (2019) Plant growth and nutrient uptake in soil amended with mixes of organic materials differing in C/N ratio and decomposition stage. J Soil Sci Plant Nutr 19:512–523. https://doi.org/10.1007/s42729-019-00049-4
van der Sloot M, Kleijn D, De Deyn GB, Limpens J (2022) Carbon (C) to nitrogen (N) ratio and quantity of organic amendment interactively affect crop growth and soil mineral N retention. Crop Environ. https://doi.org/10.1016/j.crope.2022.08.001
Wang T, Zhai Y, Zhu Y, Li C, Zeng G (2018) A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew Sustain Energy Rev 90:223–247. https://doi.org/10.1016/j.rser.2018.03.071
Wickham H (2016) Data Analysis. In: ggplot2. Use R!. Springer, Cham. https://doi.org/10.1007/978-3-319-24277-4_9
Yin S, Zhang X, Suo F, You X, Yuan Y, Cheng Y, ... Li Y (2022) Effect of biochar and hydrochar from cow manure and reed straw on lettuce growth in an acidified soil. Chemosphere 298:134191. https://doi.org/10.1016/j.chemosphere.2022.134191
Yu S, Feng Y, Xue L, Sun H, Han L, Yang L, ... Chu Q (2019) Biowaste to treasure: Application of microbial-aged hydrochar in rice paddy could improve nitrogen use efficiency and rice grain free amino acids. J Clean Prod 240:118180. https://doi.org/10.1016/j.jclepro.2019.118180
Yue Y, Yao Y, Lin Q, Li G, Zhao X (2017) The change of heavy metals fractions during hydrochar decomposition in soils amended with different municipal sewage sludge hydrochars. J Soils Sediments 17:763–770. https://doi.org/10.1007/s11368-015-1312-2
Zhai Y, Liu X, Zhu Y, Peng C, Wang T, Zhu L, ... Zeng G (2016) Hydrothermal carbonization of sewage sludge: the effect of feed-water pH on fate and risk of heavy metals in hydrochars. Bioresour Technol 218:183–188. https://doi.org/10.1016/j.biortech.2016.06.085
Zhang H, Chen C, Gray EM, Boyd SE (2017) Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenerg 105:136–146. https://doi.org/10.1016/j.biombioe.2017.06.024
Zhang H, Turner N, Poole M, Simpson N (2006) Crop production in the high rainfall zones of southern Australia—potential, constraints and opportunities. Aust J Exp Agric 46:1035–1049. https://doi.org/10.1071/EA05150
Funding
The work has been supported by the Cooperative Research Centre for High Performance Soils whose activities are funded by the Australian Government's Cooperative Research Centre Program. Thanks to Lee Kearney and Craig Hunt for their technical support.
Author information
Authors and Affiliations
Contributions
All authors listed have made a significant contribution to this work, and have approved this article for publication. Henry Luutu: Conceptualisation, methodology, investigation, formal analysis, writing-original draft; Michael T. Rose: Conceptualisation, methodology, formal analysis, writing-review and editing, validation, supervision; Shane McIntosh: Conceptualisation, methodology, investigation, resources, writing-review and editing, supervision; Lukas Van Zwieten: Conceptualisation, methodology, writing-review and editing, validation, supervision; Han H. Weng: Formal analysis, writing-review and editing; Matt Pocock: Methodology, formal analysis; Terry J. Rose: Conceptualisation, methodology, writing-review and editing, validation, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Ana Catarina Bastos.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luutu, H., Rose, M.T., McIntosh, S. et al. Phytotoxicity induced by soil-applied hydrothermally-carbonised waste amendments: effect of reaction temperature, feedstock and soil nutrition. Plant Soil 493, 647–661 (2023). https://doi.org/10.1007/s11104-023-06265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06265-3