Abstract
Background and Aims
Polyamines (PAs) play important roles in drought tolerance, but the physiological significance of putrescine (Put) conversion into other forms of PAs is not clear in filling grain embryos of wheat (Triticum aestivum L.) plants under drought stress.
Methods
The changes in the levels of three main free PAs, Put, spermidine (Spd), and spermine (Spm), covalently conjugated PAs (perchloric acid-soluble), and covalently bound PAs (perchloric acid-insoluble), were investigated in embryos of filling grains, in two wheat cultivars, Longmai No. 079 (drought-tolerant) and Wanmai No. 52 (drought-sensitive). Furthermore, the activities of arginine decarboxylase, S-adenosylmethionine decarboxylase, and transglutaminase, which affect free Put biosynthesis, Spd and Spm biosynthesis from Put, and conversion of free PAs into bound PAs, respectively, were also determined. Exogenous PAs and PA biosynthesis inhibitors were also applied in the experiment.
Results
Under drought stress, the levels of conjugated PAs did not significantly differ between the two cultivars. However, the levels of free Spd, free Spm and bound Put increased more markedly (p < 0.05) in drought-tolerant Longmai No. 079 than in drought-sensitive Wanmai No. 52, suggesting that free Spd and Spm, and bound Put, which were converted from free Put, were possibly involved in drought tolerance. Exogenous Spd treatment enhanced the drought-induced increases in endogenous Spd and Spm contents in drought-sensitive Wanmai No. 52, and increased drought tolerance, as judged by the decrease in flag leaf relative plasma membrane permeability and increases in flag leaf relative water content, 1000-grain weight and grain number per spike. Methylglyoxal-bis guanylhydrazone and o-phenanthrolin inhibited drought-induced increases in free Spd, Spm, and bound-Put contents in drought-tolerant Longmai No. 079, and decreased in drought tolerance.
Conclusions
Collectively, the conversions of free Put into free Spd, Spm, and bound Put in filling grain embryos enhanced the tolerance of wheat plants to drought stress.
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental factors, such as drought, salt, high temperature, and heavy metal stress, greatly affect plant growth and development (Beshamgan et al. 2019; Ouyang et al. 2020; Ebmeyer et al. 2021; Zhong et al. 2020; Rakić et al. 2021) and thereby pose severe threats to agricultural productivity and sustainability (Lechowska et al. 2022).
Globally, arid and semi-arid areas are spreading rapidly and crop tolerance to drought stress is of particular importance. Therefore, it is increasingly interesting to analyse the mechanisms underlying crop drought tolerance. Wheat is one of the main food crops and often experiences drought and dry-hot wind stresses during grain filling and embryo maturation. Plant water deficits alter physiological metabolism, reduce photosynthetic performance, inhibit normal development of wheat, and ultimately decrease yield. Therefore, it is important to explore mechanisms of wheat drought tolerance at the grain filling stage.
Polyamines (PAs) are plant growth regulatory substances with positive charges and strong biological activity and are found in all living organisms (Martin-Tanguy 2001; Sobieszczuk-Nowicka et al. 2019). Three PAs, putrescine (Put), spermidine (Spd), and spermidine (Spm), are the most common members of the PA family. Diamine Put is mainly produced from arginine by the action of arginine decarboxylase (ADC; EC 4.1.1.19), which is one of the key enzymes in Put biosynthesis (Nam et al. 1997), characterized by absolute specificity for L-arginine, and can be inhibited by D-arginine (D-Arg) potently and exclusively (Lutts et al. 2013). Another key enzyme involved in Put synthesis is ornithine decarboxylase (ODC; EC 4.1.1.17), which catalyzes the transform from substrate ornithine to Put through decarboxylation (Martin-Tanguy 2001; Gardiner et al. 2010). Put can be converted to Spd and Spm by being linked with one and two aminopropyls by Spd synthase and Spm synthase, respectively. The aminopropyl group is from decarboxylated S-adenosylmethionine, which is converted from S-adenosylmethionine by S-adenosylmethionine decarboxylase (SAMDC; EC 4.1.1.50) (Martin-Tanguy 2001). Many processes and factors are involved in Put conversions into Spd and Spm. Among these, SAMDC is the most important enzyme and can be inhibited by methylglyoxal-bis guanylhydrazone (MGBG) potently and exclusively (Tiburcio et al. 1993; Lutts et al. 2013).
Free PAs can be converted into conjugated and bound forms. The former are perchloric acid-soluble and formed via covalent binding of PAs with small molecules, such as hydroxyl cinnamic acid and cinnamon, by the action of acyl-transferase. Conjugated PAs are regarded as temporary storage pools of free PAs (Bais et al. 2000). Most studies indicate that conjugated PAs are mainly involved in plant disease resistance with external exposure triggering hypersensitivity reactions (Rabiti et al. 1998; Musetti et al. 1999). However, conjugated PAs have been associated with the development of flowers (Scaramagli et al. 2000), fruits (Shinozaki et al. 2000) and seeds (Puga-Hermida et al. 2003) and PEG-osmotic stress tolerance (Kong et al. 1998). Bound PAs are perchloric acid-insoluble and formed via the linking of PAs to endo-glutamine residues of proteins by the action of transglutaminase (TGase; EC 2. 3. 2. 13), which can be inhibited by o-phenanthrolin (Zhong et al. 2020; Du et al. 2022). The conversion of PAs can play important roles in the process of post-translational modification of proteins (Del Duca et al. 1995, 2014). Our previous studies showed that this PA form in the plasma membrane could enhance the chilling tolerance of postharvest plum fruit (Du et al. 2021) and maize drought tolerance by maintaining the membrane stability of maturing grain embryos (Du et al. 2022).
In summary, PAs, as plant growth regulators, widely exist in plants and play important regulatory roles in plant growth, development, morphogenesis and response to environmental stress (Tomosugi et al. 2006; Kasukabe et al. 2004; Paschalidis and Roubelakis-Angelakis 2005; Alcázar et al. 2006; Chen et al. 2019; Gondor et al. 2021; Hashem et al. 2021; Pál et al. 2021). Therefore, the homeostatic balance of free, conjugated and bound PAs is vital in growth and development processes (Lutts et al. 2013). For example, Yang et al. (2007) reported that at a later stage of drought, the increases in free Spd/free Spm and bound Put in the flag leaves of rice plants were significantly positive correlated with rice grain yield. However, the mechanism underlying PA function in wheat (Triticum aestivum L.) drought tolerance during grain filling remains to be explored. Our recent research showed free Spd and Spm in filling grain embryos enhanced maize drought tolerance. Furthermore, non-covalently conjugated Spd and Spm, and conjugated covalently Put and Spd in embryo cell plasma membrane functioned in increasing H+-ATPase activity, mitigating cell plasmolysis and maintaining the plasma membrane conformation under drought stress (Du et al. 2022). However, due to sampling on the 7th day after treatment, dynamic changes in levels of free PAs were not elucidated. In particular, the significance of converting Put into other forms was not clear. Therefore, this research aimed to uncovering the significance of Put conversion by estimating the dynamic changes in levels of endogenous free, conjugated and bound PA accumulation in embryos during wheat grain filling under drought, with two wheat cultivars differing in drought tolerance. The hypothesis of the research was that the conversions of free Put into free Spd and Spm, and bound Put, rather than free Put itself, functioned in enhancing wheat drought tolerance. Furthermore, the finding could address the issue of whether free Put accumulation at an earlier stage of drought could bring about or alleviate injury.
Materials and methods
Plant materials and growth conditions
The typical winter wheat (Triticum aestivum L.) cultivars (Longmai No. 079 and Wanmai No. 52) were applied in the research. Longmai No. 079 (F1 generation of Loumai No. 1 and TW98-829–1) and Wanmai No. 52 (F1 generation of Zhengmai No. 8329 and Wanmai 19) are all hybrids and bred by Pingliang Academy of Agricultural Sciences, Gansu Province, China, and Suzhou Seed Company, Anhui Province, respectively. The former was approved by Gansu Provincial Variety Certification Committee in 2016 (No. 2016007) and the latter was examined by the second session of the National Crop Variety Approval Committee of China in 2007 (No. 2007009). The wheat seeds were obtained from Prof. Guozhang Kang of Henan Agricultural University and were permitted to be used in this experiment according to the legislation and guidelines of China. Longmai No. 079 is distributed in Northwest China, a drought ecotope, while Wanmai No. 52 is mainly planted in Central China, a rainy ecotope. From the geographical distribution of the two cultivars, it could be prospectively inferred that Longmai No. 079 is drought-tolerant, and Wanmai No. 52 is drought-sensitive. In our preliminary experiment, under the normal water supplying condition, the 1000-grain weight of Longmai No. 079 and Wanmai No. 52 were about 44 and 41 g, respectively, while under D.S., the parameter of both cultivars were about 40 and 30 g, respectively. Furthermore, the tolerance difference in D.S. between the two cultivars was verified in this research. This experiment was carried out at Zhoukou Normal University in Henan Province, North China, from 2017 to 2020 by the scheme (Fig. 1).
On October 25, 2017, 2018 and 2019, wheat seeds were planted in plastic basins (33 seeds/basin) after the seed surface was sterilized for 5 min with 0.1% HgCl2 (w/v) and rinsed with distilled water three times. Each basin (height: 50 cm, bottom diameter: 35 cm, rim diameter: 40 cm) was filled with 15 kg of screened soil, which was taken from the surface in the wheat experimental field and contained 13.5 g organic matter kg−1, 120 mg available nitrogen kg−1, 30 mg available phosphorus kg−1, 105 mg available potassium kg−1 and the other macroelements and microelements, meeting wheat’s demand for nutrients during the growth period. The basins with seeds were placed in the field and the seedlings were vernalized under natural conditions in winter. Then they were thinned to 10 seedlings/basin and cultivated under natural conditions. After pollination, wheat plants were transferred to a greenhouse with a 25 °C/15 °C (day/night) temperature, 75% relative air humidity, 16 h/8 h light/dark photoperiod, and 600 μmol m−2 s−1 photosynthetic quantum flux density and grown for 10 d.
Material treatments and sampling
As soon as the basins with wheat plants had been placed in the greenhouse, the soil water potentials of all basins were detected with a potential instrument (Zhejiang Top Yunong Technology Co., LTD, Hangzhou, China, Model: TRS-II), of which the probe sensor was buried at a depth of approximately 15 cm in the soil of the basin. The soil water potentials of the control and treated groups were monitored every 3 h. When the water potential readings of the control and treated groups dropped to -0.15 and -1.0 MPa, respectively, the basins were replenished manually in a timely manner with tap water to ensure that the water potential of the control and treated groups were -0.15 and -1.0 MPa, respectively. On the 10th day after pollination, the wheat plants were treated as follows. The greenhouse was under the aforementioned conditions except for the 35% relative air humidity.
Control
Flag leaves and spikes were sprinkled with deionized water, and the roots were in a soil water potential of -0.15 MPa.
Drought treatment
Flag leaves and spikes were sprinkled with water, and the roots were in a soil water potential of -1.0 MPa.
Drought + D-Arg treatment
Flag leaves and spikes were sprinkled with 1 mM D-Arg, and the roots were in a soil water potential of -1.0 MPa.
Drought + D-Arg + Put treatment
Flag leaves and spikes were sprinkled with 1 mM D-Arg + 1 mM Put, and the roots were in a soil water potential of -1.0 MPa.
Drought + Spd treatment
Flag leaves and spikes were sprinkled with 1 mM Spd, and roots were in a soil water potential of -1.0 MPa.
Drought + MGBG treatment
Flag leaves and spikes were sprinkled with 0.5 mM MGBG, and the roots were in a soil water potential of -1.0 MPa.
Drought + o-phenanthrolin treatment
Flag leaves and spikes were sprinkled with 0.2 mM o-phenanthrolin, and the roots were in a soil water potential of -1.0 MPa.
The PAs and inhibitors mentioned-above were from Sigma Chemical Co. (USA). The dose determination of the abovementioned reagents was based on our preliminary experiments (Supplementary Data, Fig. 1). Wheat flag leaves and spikes of treated groups were sprayed with 25 mL/basin of a solution containing the abovementioned reagents, 0.1% ethanol and 0.01% (V/V) Tween 20 at 6:00 and 18:00 every day. The control group was sprayed with water containing 0.1% ethanol and 0.01% (V/V) Tween-20. The flag leaves and seeds in the middle of the spikes were collected at 6:00 on the 0, 2nd, 4th, 6th, 8th, 10th and 12th days after treatment. The embryos were carefully separated from the seeds with a scalpel under a dissection microscope and washed with deionized water.
Assessment of flag leaf relative water content (FLRWC)
After the fresh flag leaves were weighed for fresh weight (LFW), they were immediately immersed in deionized water for approximately 5 h to fully absorb water until the weight of the flag leaves was constant, which was regarded as the saturation weight (LSW). Then, the sample was put in an oven and dried at 75 °C for approximately 10 h until its weight was constant. The dried constant flag leaf weight was named the dry weight (LDW). FLRWC was assessed by the formula:
Assessment of flag leaf relative plasma membrane permeability (FLRPMP)
Wheat FLRPMP was assessed according to the method described by Jahan et al. (2019) with minor adjustments. Wheat flag leaves (1 g) were immersed in 10 mL of deionized water. Then, under dark conditions, the samples were incubated in a water bath at 25 °C for 2 h. The original electrical conductivity (OEC) of the water medium containing flag leaves was determined with a portable conductivity metre (Guangzhou Ruibin Technology Co., Ltd, Guangzhou, China, Model: DDB-11A). Afterwards, the flag leaves were boiled for 20 min at 100 °C, cooled to 25 °C, and left to stand for 30 min. The terminal electrical conductivity (TEC) was determined. DEC represents the electrical conductivity of deionized water. FLRPMP was assessed by the formula:
Determination of 1000-grain weight and grain number per spike
The wheat plants, from which leaves and grains were not sampled, were left growing in a greenhouse under normal growing conditions with a soil water potential of -0.15 MPa, 25 ℃/15 ℃ (day/night) temperature, 75% relative air humidity, 16 h/8 h light/dark photoperiod, and 600 μmol m−2 s−1 photosynthetic quantum flux density until the grains were fully matured. Then, 1000 grains and spikes, which were randomly selected from the control and treatment groups, were sampled for the determination of 1000-grain weight and grain number per spike, respectively. At the tillering stage, the tillering number was controlled to ensure 20 wheat plants per basin, so the spike number per basin was 20, and the grain yield per basin was approximately 20 to 50 g.
Assay of arginine decarboxylase (ADC) in filling grain embryos
ADC was extracted and assayed by the method of Nam et al. (1997) with modifications. Wheat embryos (0.2 g) were homogenized with 4 mL of precooled extract solution, which was composed of 50 mM phosphate buffer (pH 6.3), 5 mM ethylene diamine tetraacetic acid (EDTA), 0.1 μM phenyl methyl sulfonyl fluoride (PMSF), 40 μM phosphopyridoxal, 5 mM dithiothreitol (DTT), and 20 mM vitamin E, in an ice bath and filtered with 4 layers of gauze. The solution was precipitated by 20%-50% (NH4)2SO4. The precipitate was redissolved in 10 mM phosphate buffer (pH 6.3), dialyzed overnight at 4 °C, and then added to a double volume of -15 °C acetone. After centrifugation at 5,000 × g for 5 min at 4 °C, the precipitate was redissolved in 10 mM phosphate buffer, dialyzed overnight at 4 °C and centrifuged at 15,000 × g for 15 min. The supernatant was used to measure enzyme activity. The ADC reaction system, including Tris HCl buffer (100 mM, pH 7.5), 5 mM EDTA, 40 μM phosphopyridoxal and 5 mM DTT and ADC extraction above, was placed in a water bath at 37 °C for 2 min. The reaction lasted for 60 min at 37 °C and was terminated with perchloric acid (PCA, 5% final concentration). The sample was centrifuged at 3,000 × g for 10 min. Then, 0.5 mL of supernatant was added to 1 mL of 2 M NaOH and 10 μL of benzoyl chloride, vortexed vigorously for 20 s, left to stand for 60 min at 25 °C and added to 2 mL of saturated NaCl. After mixing, 2 mL of diethyl ether was added, vortexed vigorously for 20 s, and centrifuged at 1,500 × g for 5 min. One millilitre of diethyl ether was collected and evaporated at 50 °C. The precipitate was redissolved in 3 mL of methanol and measured by an ultraviolet-spectrophotometer at 254 nm. A total of 1 nmol agmatine g−1 fresh weight (FW) h−1 was regarded as one enzyme activity unit.
Assay of S-adenosylmethionine decarboxylase (SAMDC) and transglutaminase (TGase) activities in filling grain embryos
SAMDC activity was assayed by examining the release of 14CO2 using substrates labelled with the isotope 14C according to the method of Kaur-Sawhney and Shin (1982) with a few modifications. Wheat embryos (0.2 g) were homogenized with 0.5 mL of phosphate buffer (100 mM, pH 7.6) and then centrifuged at 25,000 × g for 20 min at 4 ºC. The supernatant was used to assess SAMDC activity. The assay solution consisted of the aforementioned supernatant, EDTA (0.2 mM), Tris–HCl buffer (0.1 M, pH 8.3) and 2-mercaptoethanol (2.0 mM). S-adenosyl-L-[carboxyl-14C] methionine (0.5 nM) was added to the assay solution, and at 30 ºC, the reaction went on for 30 min. Then, 0.4 ml of KH2PO4 (1 M) was used to quench the reaction, and 14CO2 was collected in a glass vial. One microlitre of 14CO2 min−1 was defined as one unit.
TGase activity was determined by detecting the rate of incorporation of Put labelled with 3[H] into proteins according to the method of Icekson and Apelbaum (1987) with proper adjustments. Embryo samples of wheat grains (0.2 g) were homogenized with 1 mL of 100 mM Tris–HCl (pH 8.8) and centrifuged for 30 min at 1200 × g at 4 °C. The supernatant was used to assess TGase activity. Put labelled with 3[H] was added to the enzyme solution, and the determination of TGase activity was performed by assessing the conjugation rate of 3[H] Put into proteins. One TGase activity unit was expressed with 1 nmol of 3[H] Put mg−1 protein h−1. Protein content was detected by the Bradford method of protein–dye binding (1976), with bovine serum albumin used as a standard.
Extraction and quantification of free PAs
The Extraction and quantification of free PAs were conducted by the method of Quinet et al. (2010) with minor adjustments. Wheat embryos (0.2 g) were homogenized in 3 mL of perchloric acid (5%, v/v), and the homogenate was left to stand for 1 h in a refrigerator at 4 °C. Then it was centrifuged at 21,500 × g for 30 min. The supernatant was collected for the free PA assay. By the method of Di Tomaso et al. (1989), benzyol chloride was used for the derivatization of the free PAs in the supernatant. Then, 0.5 mL of supernatant was added to 1 mL of 2 M NaOH and 7 μL of benzoyl chloride, vortexed vigorously for 5 s three times, left to stand for 60 min at 25 °C and added to 2 mL of saturated NaCl. Then the mix was added to 2 mL of diethyl ether, vortexed vigorously for 5 s three times, and centrifuged at 1,500 × g for 5 min. One millilitre of diethyl ether was collected and evaporated with warm air. The precipitate was redissolved into 1 mL of methanol. Free Put, Spd and Spm were quantified by HPLC (Waters 2695, USA). A C-18 reverse-phase separation column was used, with 1, 6-Hexanediamine as the internal standard and 254 nm as the detecting wavelength. At 25 °C, the PA sample was eluted from the separation column by a Perkin-Elmer Series 410 pump at 0.6 mL/min.
Extraction and quantification of conjugated PAs
The Extraction and quantification of conjugated PAs were conducted by the method of Quinet et al. (2010) with some adjustments. One millilitre of the supernatant of the free PA extraction mentioned above was mixed with 1 mL of 12 N HCl, sealed immediately in ampere glass bottles, and acid-hydrolysed at 110 °C for 15 h. The glass bottles were carefully crevassed to evaporate HCl at 80 °C. Finally, the residue was added with 10% PCA, benzoylated with benzoyl chloride and quantified by HPLC for quantification of conjugated PAs as described for the free PA assay.
Extraction and quantification of bound PAs
The extraction and quantification of bound PAs were conducted by the method of Quinet et al. (2010) with some modifications. The sediment of the free PA extraction mentioned above was added to 0.5 mL of 5% PCA, vortexed vigorously, and centrifuged at 21,500 × g for 30 min at 4 °C. The precipitate was resuspended in 0.5 mL of 1 N NaOH, vortexed, kept in an ice bath for 30 min, and centrifuged at 21,500 × g for 30 min at 4 °C. The sample was collected, acid-hydrolysed and quantified as described for the extraction and quantification of conjugated polyamines.
Data and statistical analysis
The experiment was performed three times. Three biological replicates and three technical replicates were carried out for every biological replicate. Therefore, the data shown in the paper were averages of 9 values ± S.E. The data were analysed by SPSS 16.0 and Microsoft Excel (SPSS Inc., Armonk, NY, USA). The deviation of the averages was statistically evaluated by two-way analysis of variance (ANOVA) and Duncan’s method was used to compare means at the P < 0.05 level. The significant differences among multiple groups are indicated by different letters above the columns in the figures and in the tables, which are shown as Supplementary Data 1 and 2.
Results
Dynamic changes in FLRWC and FLRPMP during wheat grain filling under drought, exogenous PAs and inhibitors
Drought resulted in decreased water content in wheat plants, especially in leaves. In the experiment, the FLRWC of the control group with a soil water potential of -0.15 MPa was regarded as 100%. As displayed in Fig. 2A, the FLRWC of wheat plants decreased with decreasing soil water potential. Furthermore, as the soil drought treatment lasted longer, the FLRWC decreased more severely. The FLRWC of the two wheat cultivars decreased over the whole drought period, and it decreased more significantly (P < 0.05) in drought-sensitive Wanmai No. 52 (Fig. 2B) than in drought-tolerant Longmai No. 079 (Fig. 2A). Especially at the end drought stage, it decreased to 71% in Wanmai No. 52, whereas in Longmai No. 079, it decreased only to 88%. Treatment with D-Arg, an ADC inhibitor of Put biosynthesis, aggravated the drought-induced decreases in FLRWC of both cultivars, and in Wanmai No. 52 and Longmai No. 079, the FLRWCs decreased to 65% and 67%, respectively. Applying exogenous Put reversed the effect of D-Arg on FLRWC. Exogenous Spd treatment markedly (P < 0.05) alleviated the drought-induced decreases in FLRWC of both cultivars, especially the drought-sensitive Wanmai No. 52, while treatment with the inhibitor MGBG or o-phenanthrolin markedly (P < 0.05) aggravated the drought-induced decreases in FLRWC of both cultivars, especially the drought-tolerant Longmai No. 079 (Fig. 2A, B).
Dynamic changes in FLRWC (A, B) and FLRPMP (C, D) during wheat grain filling under drought, exogenous PAs and inhibitors. The data shown here were averages of 9 values ± S.E. The deviation of the averages was statistically evaluated by two-way analysis of variance (ANOVA) and the multiple means of the groups were compared at P < 0.05 level by Duncan’s method. The significant differences among multiple groups were indicated by different letters above the columns in the figures
The FLRPMPs of the two wheat cultivars increased throughout the whole drought period, and the parameter increased more significantly (P < 0.05) in drought-sensitive Wanmai No. 52 (Fig. 2D) than in drought-tolerant Longmai No. 079 (Fig. 2C). The FLRPMPs increased to 38% and 22% at the end stage of treatment in Wanmai No. 52 and Longmai No. 079, respectively. D-Arg treatment aggravated the drought-induced increases in FLRPMPs of both cultivars, especially Longmai No. 079. The FLRPMPs of Wanmai No. 52 and Longmai No. 079 increased to 42% and 40%, respectively. Applying exogenous Put markedly (P < 0.05) reversed the effect of D-Arg treatment on FLRPMP. Exogenous Spd treatment markedly (P < 0.05) alleviated the drought-induced increases in FLRPMPs of the two cultivars, especially the drought-sensitive Wanmai No. 52, while the treatment with inhibitor, MGBG or o-phenanthrolin, markedly (P < 0.05) aggravated the drought-induced increases in FLRPMPs of both cultivars, especially the drought-tolerant Longmai No. 079 (Fig. 2C, D).
Effects of drought, exogenous PAs and inhibitors on wheat 1000-grain weight and grain number per spike
Drought treatment distinctly decreased the 1000-grain weight (Fig. 3A) and grain number per spike (Fig. 3B) of the two wheat cultivars compared with the control, and the two parameters of Wanmai No. 52 decreased by 25.9% and 32.3%, respectively, whereas they decreased by 6.9% and 8.8% in Longmai No. 079, respectively, indicating that they decreased more significantly in Wanmai No. 52 than in Longmai No. 079. Applying D-Arg, MGBG or o-phenanthrolin aggravated the drought-induced decreases in the two parameters of the two wheat cultivars, and the effects of the inhibitors were more marked (P < 0.05) on Longmai No. 079 than on Wanmai No. 52. Exogenous Put treatment obviously reversed the D-Arg effect. Exogenous Spd or Spm alleviated the effects of drought on the two parameters of both cultivars, especially the drought-sensitive Wanmai No. 52.
Effects of drought, exogenous PAs, and inhibitors on wheat 1000-grain weight (A) and grain number per spike (B). The data shown here were averages of 9 values ± S.E. The deviation of the averages was statistically evaluated by two-way analysis of variance (ANOVA) and the multiple means of the groups were compared at P < 0.05 level by Duncan’s method. The significant differences among multiple groups were indicated by different letters above the columns in the figures. Lower case letters discriminated treatment differences in Longmai No. 079, and capital letters discriminated treatment differences in Wanmai No. 052
Dynamic changes in ADC activity in grain embryos under drought and inhibitor D-Arg
The ADC activities in grain embryos of both cultivars rose continuously with the prolonged stress time (Fig. 4). At the end drought stage, the ADC activities were 329 and 320 nmol Agm g−1 FW h−1 in Longmai No. 079 (Fig. 4A) and Wanmai No. 52 (Fig. 4B), respectively. The results indicated that there was no marked difference in ADC activity between the two cultivars. D-Arg partially inhibited ADC activity over the whole drought period.
Dynamic changes in the levels of free PAs in grain embryos under drought, exogenous PAs, and inhibitors
The free Put contents in grain embryos of both cultivars increased drastically at the earlier drought stage, while on the 4th day, the contents began to decrease (Fig. 5). However, the decrease in free Put at the later drought stage was more significant in drought-tolerant Longmai No. 079 (Fig. 5A) than in Wanmai No. 52 (Fig. 5B). For example, at the end drought stage, the free Put contents reached 137 and 365 nmol g−1 FW in Longmai No. 079 (Fig. 5A) and Wanmai No. 52 (Fig. 5B), respectively. D-Arg inhibited the drought-induced increase in free Put in the two cultivars. Applying exogenous Put, Spd, Spm or the inhibitor MGBG enhanced the drought-induced increase in free Put and the MGBG effect was more significant than the others. Due to the similar effects to exogenous Spd, the data on the effects of exogenous Spm on endogenous free PAs are not shown here.
Dynamic change in free Put level in grain embryos under drought, exogenous PAs, and inhibitors. The data shown here were averages of 9 values ± S.E. The deviation of the averages was statistically evaluated by two-way analysis of variance (ANOVA) and the multiple means of the groups were compared at P < 0.05 level by Duncan’s method. The significant differences among multiple groups were indicated by different letters above the columns in the figures
The free Spd contents in the embryos of both cultivars increased continuously throughout the whole drought period (Fig. 6). However, the increase was more significant (P < 0.05) in drought-tolerant Longmai No. 079 (Fig. 6A) than in Wanmai No. 52 (Fig. 6B) during the later period (from the 4th to the 12th day). Applying exogenous Put and Spd enhanced the increase induced by drought in endogenous free Spd, while inhibitor D-Arg, MGBG and o-phenanthrolin treatment dramatically inhibited the increase. The trends of change in free Spm were similar to those of free Spd in grain embryos of both wheat cultivars under drought, exogenous PAs and inhibitors (Fig. 7).
Dynamic change in free Spd level in grain embryos under drought, exogenous PAs, and inhibitors. The data shown here were averages of 9 values ± S.E. The deviation of the averages was statistically evaluated by two-way variance (ANOVA) and the multiple means of the groups were compared at P < 0.05 level by Duncan’s method. The significant differences among multiple groups were indicated by different letters above the columns in the figures
Dynamic change in free Spm level in grain embryos under drought, exogenous PAs, and inhibitors. The data shown here were averages of 9 values ± S.E. The deviation of the averages was statistically evaluated by two-way variance (ANOVA) and the multiple means of the groups were compared at P < 0.05 level by Duncan’s method. The significant differences among multiple groups were indicated by different letters above the columns in the figures
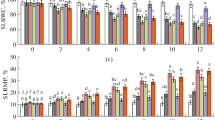
Dynamic changes in the levels of conjugated PAs in grain embryos under drought
Three conjugated PAs, Put (Fig. 8A, B), Spd (Fig. 8C, D) and Spm (Fig. 8E, F), were detected in this study. On the 6th day of drought treatment, the conjugated Put levels of both cultivars began to increase and reached the highest level at the end stage. However, there was no marked difference in the conjugated Put level between the two cultivars. Furthermore, the levels of conjugated Spd and conjugated Spm increased from the 8th to the 12th day of drought treatment, but it seemed that there was no regularity in dynamic changes in the two conjugated PA levels.
Dynamic changes in the levels of bound PAs in grain embryos under drought and the inhibitor o-phenanthrolin
Among the three bound PAs, the Put content was the highest (Fig. 9A, B), followed by Spd (Fig. 9C, D), and the Spm content was the lowest (Fig. 9E, F). The three bound PAs began to increase on the 4th day of drought treatment, and the increases in bound PAs were more significant (P < 0.05) in drought-tolerant Longmai No. 079 than in drought-sensitive Wanmai No. 52. In particular, the bound Put level in Longmai No. 079 rose from 75 to 235 nmol g−1 FW, while in Wanmai No. 52, it only rose from 62 to 83 nmol g−1 FW. Applying the inhibitor o-phenanthrolin markedly inhibited (P < 0.05) the drought-induced increases in bound PAs.
Dynamic changes in SAMDC activity in grain embryos under drought and the inhibitor MGBG
SAMDC activity in grain embryos of the control group was approximately invariable and began to increase slightly at the end stage of grain filling (Fig. 10). Nevertheless, drought obviously elevated SAMDC activity over the whole treatment period. More interestingly, the drought-induced increase in SAMDC activity was much more significant (P < 0.05) in drought-tolerant Longmai No. 079 (Fig. 10A) than in drought-sensitive Wanmai No. 52 (Fig. 10B), especially from the 4th to the 12th day of the drought treatment period. On the 12th day, it increased by 57.1% and 37.8% in Longmai No. 079 and Wanmai No. 52, respectively. MGBG treatment significantly inhibited (P < 0.05) the drought-induced increase in SAMDC activity.
Dynamic changes in TGase activity in grain embryos under drought and the inhibitor o-phenanthrolin
The change in TGase activity in grain embryos was indistinctive at the earlier drought stage (Fig. 11). However, on the 4th day, the activity began to increase in both Longmai No. 079 (Fig. 11A) and Wanmai No. 52 (Fig. 11B), and the increase in the former was more significant (P < 0.05) than that in the latter. On the 12th day, in Longmai No. 079, the activity was over 3 times as high as the value of the control group, while in Wanmai No. 52, it rose by merely 90%. Applying the inhibitor o-phenanthrolin markedly inhibited (P < 0.05) the drought-induced increase in TGase activity.
Discussion
Selecting wheat cultivars that differ in drought tolerance
Selecting wheat cultivars that differ in drought tolerance was useful to analyse cultivar differences in dynamic changes in different PA types and forms in grain embryos during the drought period. Two wheat cultivars, Longmai No. 079 and Wanmai No. 52, were selected as experimental materials since Longmai No. 079 is planted in dry ecotopes of Northwest China, such as the Gansu and Ningxia Provinces, while Wanmai No. 52 is distributed in rain-rich ecotopes of Central China, such as the Anhui, Jiangsu and Hubei Provinces. Secondly, drought inhibits growth, which is usually quantified by biomass accumulation (Bányai et al. 2017; Kaur and Asthir 2017); therefore, by analysing cultivar differences in important agronomic traits, such as 1000-grain weight (Fig. 3A) and grain number per spike (Fig. 3B), it could be inferred that Longmai No. 079 and Wanmai No. 52 were drought tolerant and drought sensitive, respectively. To further verify the two cultivar differences in drought tolerance, FLRPMP and FLRWC, which were closely associated with membrane injury and drought stress respectively, were assessed. Drought stress increased FLRPMP (Fig. 2C, D) and decreased FLRWC (Fig. 2A, B) much more markedly in Wanmai No. 52 than in Longmai No. 079. Therefore, we concluded that Wanmai No. 52 was drought sensitive and Longmai No. 079 was drought tolerant.
Significance of free Put accumulation in filling grain embryos at the earlier drought stage
NO unanimous conclusion can be drawn regarding the significance of free Put accumulation under abiotic stresses. Free Put build-up can cause injury and decrease plant tolerance to environmental stresses because oxidation and degradation products of Put, such as H2O2, amido aldehyde and propylene aldehyde, can be cross-linked with proteins and nucleic acids, leading to cell senescence and apoptosis (Di Tomaso et al. 1989; Pedrol et al. 2000; Rodriguez et al. 2001). For example, Jing et al. (2020) reported that the free Put content in grains was negatively correlated with the grain weight of wheat plants under heat stress. Furthermore, exogenous Put enhanced the inhibitory effect of drought on wheat grain filling (Liu et al. 2016). However, some recent studies have suggested that Put can alleviate the injury symptoms induced by stresses and improve plant tolerance to abiotic stresses (Ebeed et al. 2019; Ahmad and Ali 2019; Hassan et al. 2020; Upadhyay et al. 2021). These differences might be attributed to different treatment periods and experimental materials, especially cultivar variation in tolerance. To address this issue, dynamic changes in free Put were examined throughout the whole stress period, in two wheat cultivars differing in drought tolerance. Drought substantially increased free Put not only in the drought-sensitive wheat cultivar (Fig. 5B), but also in the drought-tolerant cultivar at the earlier stress stage (Fig. 5A). Furthermore, coupled with the significant decreases in the free Put level and ADC activity, which was inhibited by D-Arg (Fig. 4), drought tolerance of both cultivars decreased markedly, as judged by changes in FLRWC (Fig. 2A, B), FLRPMP (Fig. 2C, D), 1000-grain weight (Fig. 3A) and grain number per spike (Fig. 3B). Thus free Put accumulation during early drought might be essential for plant stress tolerance, as free Put could play a crucial role in signal transduction under abiotic stresses (Alcázar et al. 2020; Gondor et al. 2021; Gonzalez et al. 2021). Whether drought-induced increases in free Put levels could enhance stress tolerance would depend on whether free Put could be converted into free Spd and Spm, and other forms of PAs at the later drought stage.
Significance of free Put conversions into free Spd and Spm in filling grain embryos at the later drought stage
Since drought-induced increases in free Spd (Fig. 6) and Spm (Fig. 7) were more significant (P < 0.05) in the drought-tolerant cultivar than in the drought-sensitive cultivar during the later period, we concluded that free Spd and Spm might enhance drought tolerance. Furthermore, by analysing the dynamic changes in the three free PAs, increases in free Spd and Spm in the drought-tolerant cultivar agreed well with the decrease in free Put (Fig. 5A) and all the changes in free PAs began simultaneously after 4 days of drought. It was inferred that the drought-induced increases in free Spd and Spm were mainly from free Put conversion, which was confirmed by applying the inhibitors D-Arg and MGBG. D-Arg inhibited free Put biosynthesis, coupled with the decreases in free Spd and Spm; while MGBG inhibited the conversion of free Put into free Spd and Spm by inhibiting the drought-induced increase in SAMDC activity (Fig. 10), coupled with the decreased drought tolerance evidenced by the decreases in FLRWC (Fig. 2A, B), 1000-grain weight (Fig. 3A) and grain number per spike (Fig. 3B), and increased FLRPMP (Fig. 2C, D). Additionally, applying exogenous Spd increased endogenous free Spd and Spm in grain embryos, coupled with an increase in drought tolerance (Figs. 2 and 3), especially in the drought-sensitive cultivar due to the inherently high background levels of Spd and Spm in the tolerant cultivar. Correlation analysis further supported these findings. After 12 days of drought, the total content of free Spd + free Spm in grain embryos was positively significantly correlated with 1000-grain weight (r0.05 = 0.95, n = 10) (Fig. 12A) and grain number per spike (r0.05 = 0.90, n = 10) (Fig. 12B). Additionally, the ratio of free Spd + free Spm/free Put was also positively correlated with 1000-grain weight (r0.05 = 0.76, n = 10) (Fig. 12C) and grain number per spike (r0.05 = 0.59, n = 10) (Fig. 12D).
The results of statistical analysis for correlation (P < 0.05. A: between total content of free Spd + free Spm and 1000-grain weight; B: between total content of free Spd + free Spm grain number per spike; C: between ratio of free Spd + free Spm/free Put and 1000-grain weight; D: between ratio of free Spd + free Spm/free Put and grain number per spike; E: between bound Put content and 1000-grain weight; F: between bound Put content and grain number per spike; G: between total content of bound Put + Spd + Spm and 1000-grain weight; H: between total content of bound Put + Spd + Spm and grain number per spike). Triangular symbol and circular symbol represented Longmai No. 079 and Wanmai No. 52 cultivars, respectively. Furthermore, purple, blue, pink, green, orange and red were used to show drought, drought + D-Arg, drought + D-Arg + Put, drought + Spd, drought + MGBG and drought + o-phenanthrolin, respectively
Our findings agreed with previous studies (Yang et al. 2007; Gupta et al. 2013; Luo et al. 2019; Jing et al. 2020; Zarza et al. 2020). Since Spd and Spm carry more positive charges, they could be linked to biomacromolecules, such as acidic proteins and membrane phospholipids, more easily than Put, and could play important roles in drought responses (Yamaguchi et al. 2007; Kubis 2008; Dutra et al. 2013). Farooq et al. (2009) reported that Spm was the most effective in enhancing drought tolerance among PAs. Additionally, polyamines seem to regulate cell membrane proteins such as two major vacuolar cation channels and plasma membrane H+-ATPase (Janicka-Russak et al. 2010; Pottosin and Shabala 2014; Pottosin et al. 2021; Du et al. 2022). Thus Jing et al. (2020) found that the free Spd level in grains was positively correlated with wheat grain weight and that exogenous Spm/Spd enhanced heat tolerance of wheat plants. Furthermore, Jing et al. (2020) reported that exogenous Spm better alleviated heat stress injury in wheat plants than Spd, unlike our experiment where exogenous Spm and exogenous Spd had similar effects on enhancing wheat drought tolerance. Different abiotic stresses and wheat cultivars might account for this discrepancy. Additionally, our findings agreed with observations that the grain weight and filling rate were positively and significantly correlated with endogenous free Spd and Spm levels, and higher SAMDC activity in wheat grains enhanced the conversions of free Put into free Spd and Spm and increased the levels of free Spd and Spm (Liu et al. 2013a, b; 2016). Although Luo et al. (2019) used well-watered plants, their discovery also agreed with our findings in droughted wheat. The precise function of free Put in enhancing drought tolerance is still not clear, but our research revealed that under drought, the conversions of free Put into free Spd and Spm were the main pathway by which the Spd and Spm levels were elevated and therefore drought tolerance was enhanced.
Free Put conversion to conjugated Put in filling grain embryos at the later drought stage
Free PAs can be converted into acid-soluble conjugated PAs. Drought increased three conjugated PAs, especially the conjugated Put level. However, the two cultivars did not markedly differ in conjugated PA contents (Fig. 8), suggesting that this PA form in filling grain embryos might not be directly involved in drought tolerance, as in the study of Yang et al. (2007) in rice. In the present study, since conjugated PA biosynthesis inhibitors were not applied, more convincing data could not be supplied for further verification. However, Bais et al. (2000) showed that conjugated PAs could be regarded as temporary storage pools of free PAs and that there was no direct relationship between conjugated PAs and abiotic stress tolerance. While conjugated PA was important in callus proliferation under PEG osmotic stress (Kong et al. 1998), most previous studies suggested that conjugated PAs were correlated with organ development (Scaramagli et al. 2000; Shinozaki et al. 2000; Puga-Hermida et al. 2003) and disease resistance (Rabiti et al. 1998; Musetti et al. 1999). In any case, whether conjugated PAs might function in enhancing drought tolerance deserves further exploration.
Significance of free Put conversion to bound Put in filling grain embryos at the later drought stage
Bound PAs, especially bound Put, might be involved in drought tolerance because this PA form increased more significantly in the drought-tolerant cultivar than in the sensitive cultivar (Fig. 9). Furthermore, the bound Put content in grain embryos was positively significantly correlated with the 1000-grain weight (r0.05 = 0.99, n = 4) (Fig. 12E) and grain number per spike (r0.05 = 0.99, n = 4) (Fig. 12F). Additionally, the total content of bound Put + Spd + Spm was also positively and significantly correlated with the 1000-grain weight (r0.05 = 0.98, n = 4) (Fig. 12G) and grain number per spike (r0.05 = 0.98, n = 4) (Fig. 12H). Furthermore, analysing dynamic changes in bound Put determined that the increases in bound Put agreed with the decrease in free Put (Fig. 5), and both changes in bound and free Put occurred simultaneously on the 4th day of drought. Therefore, the drought-induced increase in bound Put was inferred to originate mainly from free Put conversion, as verified by applying the inhibitor o-phenanthrolin, that attenuated drought-induced increases in TGase activity (Fig. 11) and bound PA levels (Fig. 9) and decreased drought tolerance (Figs. 2 and 3). This notion was also supported by previous studies, with the conversion of free PAs into bound PAs functioning in posttranslational modification of chlorophyll proteins and stabilising protein conformation by preventing protein denaturation associated with senescence and programmed cell death (Del Duca et al. 1995, 2014; Serafini-Fracassini et al. 1995). In rice flag leaves, the drought-induced increase in insoluble conjugated Put, which was the same as bound Put in our experiment, was correlated with rice grain yield formation (Yang et al. 2007). Recently, it was reported that bound PAs might enhance salt stress tolerance in tomato and tobacco (Zhong et al. 2019, 2020) and chilling tolerance in plum fruit (Du et al. 2021). In particular, our previous research suggested that bound PAs in the plasma membrane could mitigate cell plasmolysis and thereby maintain the membrane conformation of maize grain embryos under drought (Du et al. 2022). By estimating the dynamic changes in PA levels, the present study revealed that the conversions of free Put into free Spd and Spm, and bound Put, rather than free Put itself, enhanced wheat drought tolerance.
Free Put conversion under well-watered and droughted conditions
In well-watered plants, PAs also play a significant role in grain developing and ripening (Hussain et al. 2011). Luo et al. (2019) have proposed that the direct synthetic pathway from free Put to free Spd in the grain is a key factor in promoting grain filling and thousand grain weight in well-watered wheat plants, and the promotive effect of free Spd on the grain filling is notably related to carbohydrate metabolism in that grain. Under well-watered condition, exogenous Spd, Spm and inhibitor MGBG can affect wheat grain filling by modulating endogenous PA biosynthesis (Liu et al. 2013a, b; Luo et al. 2019). However, whether or how PA levels are modulated during grain filling under drought is yet to be ascertained. All plant tissues and organs contain PAs, especially in the metabolically active embryos, indicating their indispensable role under adverse environmental conditions (Gupta et al. 2013). Free Put accumulation occurred during the earlier drought period and the conversions of free Put into free Spd and Spm, and bound Put occurred at the later drought stage. By analyzing the relationship between the conversions and the tolerance of the two cultivars with different drought tolerance, we conclude that the free Put conversions happen only under drought condition, but not under well-watered condition, thereby conferring drought tolerance to wheat plants. Furthermore, treating drought-stressed plants with exogenous PAs increased the levels of free Spd and Spm, and bound Put in embryos and enhanced the drought tolerance, while treating with inhibitors had contrary effects. Further research should analyze the effects of exogenous PAs and inhibitors in well-watered wheat plants.
Conclusions
In filling grain embryos, the conversions of free Put into free Spd and Spm, and bound Put, rather than free Put itself, function in enhancing the tolerance of wheat plants to drought. This finding might explain why exogenous Put application has different effects on different plant cultivars. In stress-sensitive cultivars, the excessive accumulation of free Put would be oxidized by polyamine oxidase, and the degradation products might have adverse effects on plant cells. Conversely, in stress-tolerant cultivars, exogenous Put could be converted into other forms of PAs, which function in enhancing tolerance. Future work should explore the application of conjugated PA biosynthesis inhibitors and/or use gene (related to ADC, SAMDC and TGase) knockout and recombination technology. Coupling 14C-isotope-labelled exogenous Put with electron microscope autoradiography techniques could examine where Put conversion occurs in the embryo. Additional data would help understand the mechanisms by which PA enhances plant drought tolerance.
Code availability
Not applicable.
Data Availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Ahmad A, Ali A (2019) Improvement of postharvest quality, regulation of antioxidants capacity and softening enzymes activity of cold-stored carambola in response to polyamines application. Postharvest Biol Tech 148:208–217. https://doi.org/10.1016/j.postharvbio.2018.10.017
Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Let 28:1867–1876. https://doi.org/10.1007/s10529-006-9179-3
Alcázar R, Bueno M, Tiburcio AF (2020) Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 9:2373. https://doi.org/10.3390/cells9112373
Bais HP, Sudha GS, Rarishankar GA (2000) Putrescine and silver nitrate influences shoot multiplication, in vitro flowering and endogenous titers of polyamines in Cichorium intybus L. cv. lucknow local. J Plant Growth Regul 19:238–248. https://doi.org/10.1007/s003440000012
Bányai J, Maccaferri M, Cané MA, Monostori I, Spitkó T, Kuti C, Mészáros K, Láng L, Pál M, Karsai I (2017) Phenotypical and physiological study of near-isogenic durum wheat lines under contrasting water regimes. S Afr J Bot 108: 248–255. https://doi.org/10.1016/j.sajb.2016.11.001
Beshamgan ES, Sharifi M, Zarinkamar F (2019) Crosstalk among polyamines, phytohormones, hydrogen peroxide, and phenylethanoid glycosides responses in Scrophularia striata to Cd stress. Plant Physiol Biochem 143: 129–141. https://doi.org/10.1016/j.plaphy.2019.08.028
Bradford MM (1976) A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binging. Anal Biochem 72:248–254. https://doi.org/10.1046/j.0960-7412.2001.01200.x
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1945. https://doi.org/10.3389/fpls.2018.01945
Del Duca S, Beninati S, Serafini-Fracassini D (1995) Polyamines in chloroplasts: identification of their glutamyl and acetyl derivatives. Biochem J 305:233–237. https://doi.org/10.1042/bj3050233
Del Duca S, Serafini-Fracassini D, Cai G (2014) Senescence and programmed cell death in plants: Polyamine action mediated by transglutaminase. Front Plant Sci 5:120. https://doi.org/10.3389/fpls.2014.00120
Di Tomaso JM, Shaff JE, Kochian LV (1989) Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings. Plant Physiol 90:988–995. https://doi.org/10.1104/pp.90.3.988
Du HY, Liu GT, Hua CM, Liu DX, He YY, Liu HP, Kurtenbach R, Ren DT (2021) Exogenous melatonin alleviated chilling injury in harvested plum fruit via affecting the levels of polyamines conjugated to plasma membrane. Postharvest Biol Tec 179:111585. https://doi.org/10.1016/j.postharvbio.2021.111585
Du HY, Liu DX, Liu GT, Liu HP, Sun HL, Li CH, Kurtenbach R (2022) Conjugated polyamines are involved in conformation stability of plasma membrane from maturing maize grain embryos under drought stress. Environ Exp Bot 194:104726. https://doi.org/10.1016/j.envexpbot.2021.104726
Dutra NT, Silveira V, Azevedo IG, Gomes-Neto LR, Facanha AR, Steiner N, Guerra MP, Floh EIS, Santa-Catarina C (2013) Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. Physiol Plant 148:121–132. https://doi.org/10.1111/j.1399-3054.2012.01695.x
Ebeed HT, Hassan NM, Keshta MM, Hassanin OS (2019) Comparative analysis of seed yield and biochemical attributes in different sunflower genotypes under different levels of irrigation and salinity. Egypt J Bot 59: 339–355. https://doi.org/10.21608/ejbo.2019.5043.1205
Ebmeyer H, Fiedler-Wiechers K, Hoffmann CM (2021) Drought tolerance of sugar beet–evaluation of genotypic differences in yield potential and yield stability under varying environmental conditions. Eur J Agron 125:126262. https://doi.org/10.1016/j.eja.2021.126262
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945. https://doi.org/10.1007/s11738-009-0307-2
Gardiner DM, Kazan K, Praud S, Torney FJ, Rusu A, Manners JM (2010) Early activation of wheat polyamine biosynthesis during Fusarium head blight implicates putrescine as an inducer of trichothecene mycotoxin production. BMC Plant Biol 10:289. https://doi.org/10.1186/1471-2229-10-289
Gondor OK, Tajti J, Hamow KÁ, Majláth I, Szalai G, Janda T, Pál M (2021) Polyamine metabolism under different light regimes in wheat. Int J Mol Sci 22:11717. https://doi.org/10.3390/ijms222111717
Gonzalez ME, Jasso-Robles FI, Flores-Hernández E, Rodríguez-Kessler M, Pieckenstain FL (2021) Current status and perspectives on the role of polyamines in plant immunity. Ann Appl Biol 178:244–255. https://doi.org/10.1111/aab.12670
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036. https://doi.org/10.1007/s11738-013-1239-4
Hashem AM, Moore S, Chen S, Hu C, Zhao Q, Elesawi IE, Feng Y, Topping JF, Liu J, Lindsey K (2021) Putrescine depletion affects arabidopsis root meristem size by modulating auxin and cytokinin signaling and ROS accumulation. Int J Mol Sci 22:4094. https://doi.org/10.3390/ijms22084094
Hassan N, Ebeed H, Aljaarany A (2020) Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol Mol Biol Plants 26:233–245. https://doi.org/10.1007/s12298-019-00744-7
Hussain SS, Ali M, Ahmad M, Siddique KHM (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311. https://doi.org/10.1016/j.biotechadv.2011.01.003
Icekson I, Apelbaum A (1987) Evidence for transglutaminase activity in plant tissue. Plant Physiol 84:972–974. https://doi.org/10.1104/pp.84.4.972
Jahan MS, Wang Y, Shu S, Zhong M, Chen Z, Wu J, Sun J, Guo S (2019) Exogenous salicylic acid increases the heat tolerance in tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci Hortic 247:421–429. https://doi.org/10.1016/j.scienta.2018.12.047
Janicka-Russak M, Kabala K, Mlodzinska E, Klobus G (2010) The role of polyamines in the regulation of the plasma membrane and the tonoplast proton pumps under salt stress. J Plant Physiol 167:261–269. https://doi.org/10.1016/j.jplph.2009.010
Jing JG, Guo SY, Li YF, Li WH (2020) The alleviating effect of exogenous polyamines on heat stress susceptibility of different heat resistant wheat (Triticum aestivum L.) varieties. Sci Rep 10:7467. https://doi.org/10.1038/s41598-020-64468-5
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Over-expression of spermidine synthase enhances tolerance to multiple environmental stress and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722. https://doi.org/10.1093/pcp/pch083
Kaur G, Asthir B (2017) Molecular responses to drought stress in plants. Biol Plant 61:201–209. https://doi.org/10.1007/s10535-016-0700-9
Kaur-Sawhney R, Shin M (1982) Relation of polyamines synthesized titer to aging and senescence in oat leaves. Plant Physiol 69:405–410. https://doi.org/10.1104/pp.69.2.405
Kong L, Attree SM, Fowke LC (1998) Effects of polyethylene glycol and methylglyoxal bis (guanylhydrazone) on endogenous polyamine levels and osmotic embryo maturation in white spruce. Plant Sci 133:211–220. https://doi.org/10.1016/S0168-9452(98)00040-5
Kubis J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406. https://doi.org/10.1016/j.jplph.2007.02.005
Lechowska K, Wojtyla Ł, Quinet M, Kubala S, Lutts S, Garnczarska M (2022) Endogenous polyamines and ethylene biosynthesis in relation to germination of osmoprimed brassica napus seeds under salt stress. Int J Mol Sci 23:349. https://doi.org/10.3390/ijms23010349
Liu Y, Gu D, Wu W, Wen X, Liao Y (2013a) The relationship between polyamines and hormones in the regulation of wheat grain filling. PLoS ONE 8:e78196. https://doi.org/10.1371/journal.pone.0078196
Liu Y, Wen XX, Gu DD, Guo Q, Zeng A, Li CJ, Liao YC (2013b) Effect of polyamine on grain filling of winter wheat and its physiological mechanism. Acta Agron Sin 39:712–719. https://doi.org/10.3724/SP.J.1006.2013.00712
Liu Y, Liang HY, Lv XK, Liu DD, Wen XX, Liao YC (2016) Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem 100:113–129. https://doi.org/10.1016/j.plaphy.2016.01.003
Luo J, Wei B, Han J, Liao Y, Liu Y (2019) Spermidine increases the sucrose content in inferior grain of wheat and thereby promotes its grain filling. Front Plant Sci 10:1309. https://doi.org/10.3389/fpls.2019.01309
Lutts S, Hausman J, Quinet M, Lefèvre I (2013) Polyamines and their roles in the alleviation of ion toxicities in plants. In: Ahmad P, Azooz M, Prasad M (Eds) Ecophysiology and Responses of Plants under Salt Stress. Springer: New York, NY, USA, pp 315–353.
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148. https://doi.org/10.1023/A:1013343106574
Musetti R, Scaramagli S, Vighi C, Pressaao L, Torrigiani P, Favali MA (1999) The involvement of polyamines in phytoplasma-infected periwinkle (Catharanthus roseus L.) plants. Plant Biosyst 133:37–45. https://doi.org/10.1080/11263509909381530
Nam KH, Lee SH, Lee JH (1997) Differential expression of ADC mRNA during development and upon acid stress in soybean (Glycine max) hypocotyls. Plant Cell Physiol 38:1156–1166. https://doi.org/10.1093/oxfordjournals.pcp.a029101
Ouyang W, Yin X, Yang J, Struik PC (2020) Comparisons with wheat reveal root anatomical and histochemical constraints of rice under water-deficit stress. Plant Soil 452:547–568. https://doi.org/10.1007/s11104-020-04581-6
Pál M, Szalai G, Gondor OK, Janda T (2021) Unfinished story of polyamines: role of conjugation, transport and light-related gegulation in the polyamine metabolism in plants. Plant Sci 308:110923. https://doi.org/10.1016/j.plantsci.2021.110923
Paschalidis KA, Roubelakis-Angelakis KA (2005) Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs / tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation. Plant Physiol 138:142–152. https://doi.org/10.1104/pp.104.055483
Pedrol N, Ramos P, Reigosa MJ (2000) Phenotypic plasticity and acclimation to water deficits in velvet-grass: A long-term greenhouse experiment. Changes in leaf morphology, photosynthesis and stress-induced metabolites. J Plant Physiol 157:383–393. https://doi.org/10.1016/S0176-1617(00)80023-1
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:154. https://doi.org/10.3389/fpls.2014.00154
Pottosin I, Olivas-Aguirre M, Dobrovinskaya O, Zepeda-Jazo I, Shabala S (2021) Modulation of ion transport across plant membranes by polyamines: Understanding specific modes of action under stress. Front Plant Sci 11:616077. https://doi.org/10.3389/fpls.2020.616077
Puga-Hermida MT, Gallardo M, Matilla AJ (2003) The zygotic embryogenesis and ripening of Brassica rapa seeds provokes important alterations in the levels of free and conjugated abscisic acid and polyamines. Physiol Plant 117:279–288. https://doi.org/10.1034/j.1399-3054.2003.00033.x
Quinet M, Ndayiragije A, Lefèvre I, Lambillotte B, Dupont-Gillain CC, Lutts S (2010) Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J Exp Bot 61:2719–2733. https://doi.org/10.1093/jxb/erq118
Rabiti AL, Betti L, Bortolotti G, Marini F, Canova A, Bagni N, Torrigiani P (1998) Shout-term polyamine response in TMV-inoculated hypersensitive and susceptible tobacco plants. New Phytol 139:549–553. https://doi.org/10.1046/j.1469-8137.1998.00205.x
Rakić T, Pešić M, Kostić N, Andrejić G, Fira D, Dželetović Ž, Stanković S, Lozo J (2021) Rhizobacteria associated with Miscanthus x Giganteus improve metal accumulation and plant growth in the flotation tailings. Plant Soil 462:349–363. https://doi.org/10.1007/s11104-021-04865-5
Rodriguez SDC, López B, Chaves AR (2001) Effect of different treatments on the evolution of polyamines during refrigerated storage of eggplants. J Agric Food Chem 49:4700–4705. https://doi.org/10.1021/jf0001031
Scaramagli S, Biondi S, Leone A, Grillo S, Torrigiani P (2000) Acclimation to low-water potential in potato cell suspension cultures leads to changes in putressine metabolism. Plant Physiol Biochem 38:345–351. https://doi.org/10.1016/S0981-9428(00)00750-6
Serafini-Fracassini D, Del Duca S, Beninati S (1995) Plant transglutaminase. Phytochemistry 40:355–365. https://doi.org/10.1016/0031-9422(95)00243-Z
Shinozaki S, Ogata T, Horiuchi S (2000) Endogenous polyamines in the pericarp and seed of the grape berry during development and ripening. Sci Hort 83:33–41. https://doi.org/10.1016/S0304-4238(99)00064-3
Sobieszczuk-Nowicka E, Paluch-Lubawa E, Mattoo AK, Arasimowicz-Jelonek M, Gregersen PL, Pacak A (2019) Polyamines—A new metabolic switch: Crosstalk with networks involving senescence, crop improvement, and mammalian cancer therapy. Front Plant Sci 10:859. https://doi.org/10.3389/fpls.2019.00859
Tiburcio AF, Campos JL, Figueras X (1993) Recent advances in the understanding of polyamines functions during plant development. Plant Growth Regul 12:331–340. https://doi.org/10.1007/BF00027215
Tomosugi M, Ichihara K, Saito K (2006) Polyamines are essential for the synthesis of 2-ricinoleoyl phosphatidic acid in developing seeds of castor. Planta 223:349–358. https://doi.org/10.1007/s00425-005-0083-1
Upadhyay RK, Fatima T, Handa AK, Mattoo AK (2021) Differential association of free, conjugated, and bound forms of polyamines and transcript abundance of their biosynthetic and catabolic genes during drought/salinity stress in tomato (Solanum lycopersicum L.) leaves. Front Plant Sci 12: 743568. https://doi.org/10.3389/fpls.2021.743568.
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T (2007) A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490. https://doi.org/10.1016/j.bbrc.2006.11.041
Yang JC, Zhang JH, Liu K, Wang ZQ, Liu LJ (2007) Involvement of polyamines in the drought resistance of rice. J Exp Bot 58:1545–1555. https://doi.org/10.1093/jxb/erm032
Zarza X, Wijk RV, Shabala L, Hunkeler A, Lefebvre M, Rodriguez-Villalón A, Shabala S, Tiburcio AF, Heilmann I, Munnik T (2020) Lipid kinases PIP5K7 and PIP5K9 are required for polyamine triggered K+ efflux in Arabidopsis roots. Plant J 104:416–432. https://doi.org/10.1111/tpj.14932
Zhong M, Wang Y, Zhang Y, Shu S, Sun J, Guo S (2019) Overexpression of transglutaminase from cucumber in tobacco increases salt tolerance through regulation of photosynthesis. Int J Mol Sci 20:894. https://doi.org/10.3390/ijms20040894
Zhong M, Song R, Wang Y, Shu S, Sun J, Guo S (2020) TGase regulates salt stress tolerance through enhancing bound polyamines-mediated antioxidant enzymes activity in tomato. Environ Exp Bot 179:104191. https://doi.org/10.1016/j.envexpbot.2020.104191
Funding
National Natural Science Foundation of China (Grant No.: 31271627) and Natural Science Foundation of Henan province (Grant No.: 222300420394).
Author information
Authors and Affiliations
Contributions
HPL and HYD conceived the project; HYD and GTL performed the experiment (except enzyme activity detection); DXL detected enzyme activity; HYD and GTL wrote original draft; HPL and RK reviewed and edited the MS. Authors have already read the MS and agreed to being published in the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
Authors declare no conflict of interest.
Additional information
Responsible Editor: Ian Dodd.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, H., Liu, G., Liu, D. et al. Significance of putrescine conversion in filling grain embryos of wheat plants subjected to drought stress. Plant Soil 484, 589–610 (2023). https://doi.org/10.1007/s11104-022-05823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05823-5